Mechanistic Understanding on Difluoromethane Absorption Thermodynamics on Novel Deep Eutectic Solvents by COSMO-Based Molecular Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. COSMO Simulation
2.2. Environmental Health and Safety
3. Results and Discussion
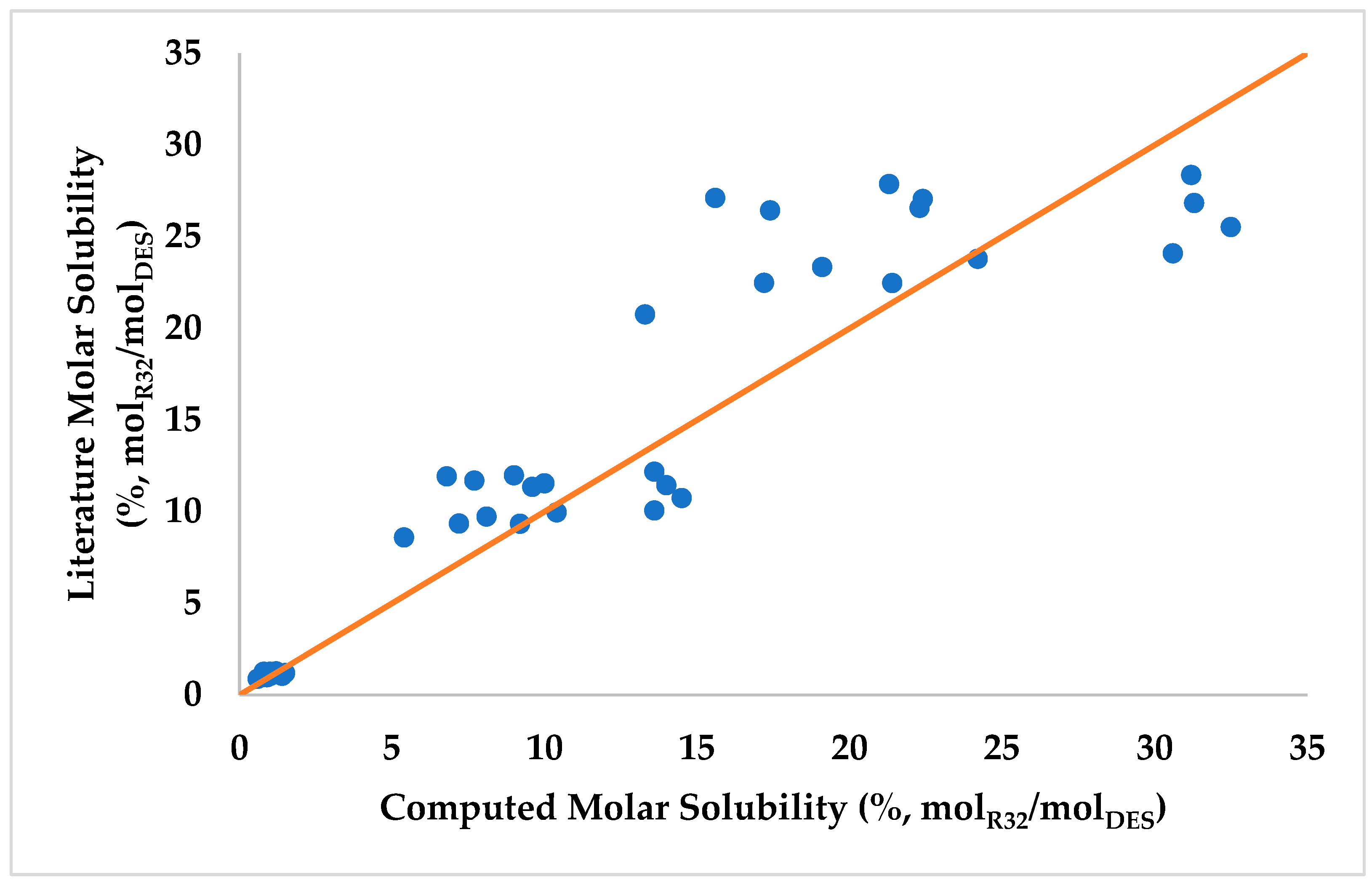
3.1. COSMO Validation
3.2. Evaluation of Novel HDES Solvent Combinations for R-32 Absorption
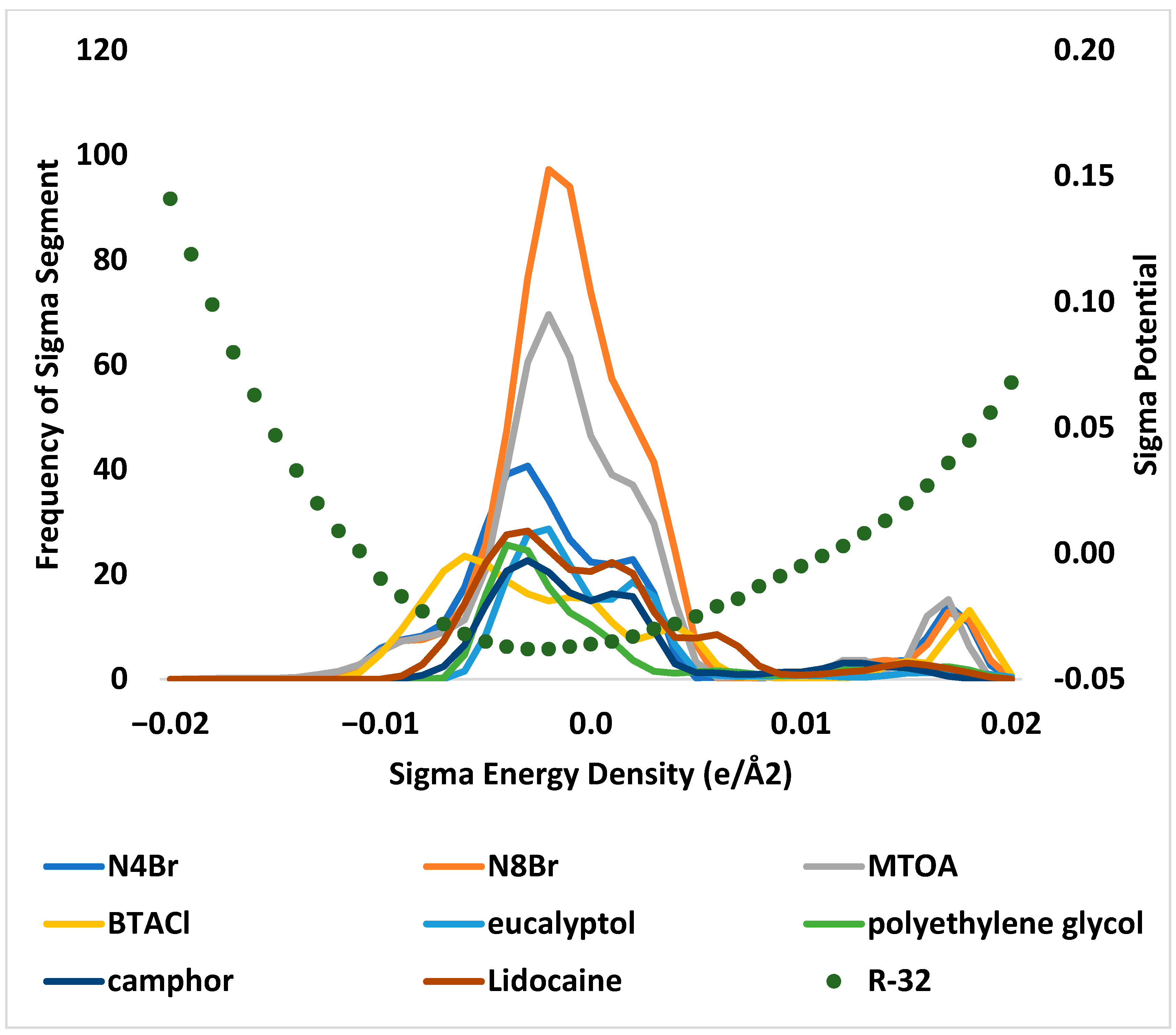
3.3. Absorption Mechanism through Enthalpy and Sigma Analysis
3.4. Effects of Change in Composition of DES
3.5. Effects of Varying System Pressure
3.6. EHS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EPA, USA. Fluorinated Greenhouse Gas Emissions and Supplies Reported to the GHGRP. 27 September 2015. Available online: https://www.epa.gov/ghgreporting/fluorinated-greenhouse-gas-emissions-and-supplies-reported-ghgrp (accessed on 9 October 2022).
- Fluorocarbon Refrigerants and Their Syntheses: Past to Present. Chemical Reviews. Available online: https://pubs.acs.org/doi/full/10.1021/acs.chemrev.9b00719?casa_token=as5gGhmVI0YAAAAA%3Ae_Dt0YfBX4Gv-mTRctdDnlrXpwewaRvD__7u6Z2nhiAf1SFXr8MGxNKO3SqYe5fcSbrkcX2QZv6b12uK (accessed on 9 October 2022).
- McLinden, M.O.; Huber, M.L. (R)Evolution of Refrigerants. J. Chem. Eng. Data 2020, 65, 4176–4193. [Google Scholar] [CrossRef] [PubMed]
- EPA, USA. Ozone-Depleting Substances. 17 July 2015. Available online: https://www.epa.gov/ozone-layer-protection/ozone-depleting-substances (accessed on 2 December 2022).
- EPA, USA. Overview of Greenhouse Gases. 23 December 2015. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 9 October 2022).
- What Are Hydrofluorocarbons?—EIA US. Available online: https://us.eia.org/campaigns/climate/what-are-hydrofluorocarbons/ (accessed on 9 October 2022).
- Refrigerant R32 as Lower GWP Working Fluid in Residential Air Conditioning Systems in Europe and the USA—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1364032117308559 (accessed on 2 December 2022).
- Pardo, F.; Zarca, G.; Urtiaga, A. Separation of Refrigerant Gas Mixtures Containing R32, R134a, and R1234yf through Poly(ether-block-amide) Membranes. ACS Sustain. Chem. Eng. 2020, 8, 2548–2556. [Google Scholar] [CrossRef]
- Liu, X.; Lv, N.; Su, C.; He, M. Solubilities of R32, R245fa, R227ea and R236fa in a phosphonium-based ionic liquid. J. Mol. Liq. 2016, 218, 525–530. [Google Scholar] [CrossRef]
- Asensio-Delgado, S.; Viar, M.; Pardo, F.; Zarca, G.; Urtiaga, A. Gas solubility and diffusivity of hydrofluorocarbons and hydrofluoroolefins in cyanide-based ionic liquids for the separation of refrigerant mixtures. Fluid Phase Equilibria 2021, 549, 113210. [Google Scholar] [CrossRef]
- Asensio-Delgado, S.; Pardo, F.; Zarca, G.; Urtiaga, A. Absorption separation of fluorinated refrigerant gases with ionic liquids: Equilibrium, mass transport, and process design. Sep. Purif. Technol. 2021, 276, 119363. [Google Scholar] [CrossRef]
- Quaid, T.; Reza, M.T. Carbon Capture from Biogas by Deep Eutectic Solvents: A COSMO Study to Evaluate the Effect of Impurities on Solubility and Selectivity. Clean Technol. 2021, 3, 2. [Google Scholar] [CrossRef]
- Castro, P.J.; Redondo, A.E.; Sosa, J.E.; Zakrzewska, M.E.; Nunes, A.V.M.; Araújo, J.M.M.; Pereiro, A.B. Absorption of Fluorinated Greenhouse Gases in Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2020, 59, 13246–13259. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- SciELO—Brazil—Use of Natural Deep Eutectic Solvents for Polymerization and Polymer Reactions Use of Natural Deep Eutectic Solvents for Polymerization and Polymer Reactions. Available online: https://www.scielo.br/j/jbchs/a/fSYnzjCc6Tg5b5KvY3F7NMJ/?lang=en (accessed on 8 August 2022).
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, Z.; Lin, X.; Ahmad, N.; Xu, J.; Xu, X. Efficient CO2 capture by a novel deep eutectic solvent through facile, one-pot synthesis with low energy consumption and feasible regeneration. Sci. Total Environ. 2020, 705, 135798. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś-Chełstowska, P.; Gębicki, J. Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process. Materials 2021, 14, 241. [Google Scholar] [CrossRef]
- Alioui, O.; Benguerba, Y.; Alnashef, I.M. Investigation of the CO2-solubility in deep eutectic solvents using COSMO-RS and molecular dynamics methods. J. Mol. Liq. 2020, 307, 113005. [Google Scholar] [CrossRef]
- Arenas, P.; Suárez, I.; Coto, B. Combination of molecular dynamics simulation, COSMO-RS, and experimental study to understand extraction of naphthenic acid. Sep. Purif. Technol. 2022, 280, 119810. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS from Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Anderson, J.L.; Clark, K.D. Ionic liquids as tunable materials in (bio)analytical chemistry. Anal. Bioanal. Chem. 2018, 410, 4565–4566. [Google Scholar] [CrossRef]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Krodkiewska, I.; Drummond, C.J. Protic Ionic Liquids: Solvents with Tunable Phase Behavior and Physicochemical Properties. J. Phys. Chem. B 2006, 110, 22479–22487. [Google Scholar] [CrossRef]
- Gonfa, G.; Bustam, M.A.; Sharif, A.M.; Mohamad, N.; Ullah, S. Tuning ionic liquids for natural gas dehydration using COSMO-RS methodology. J. Nat. Gas Sci. Eng. 2015, 27, 1141–1148. [Google Scholar] [CrossRef]
- Jeliński, T.; Cysewski, P. Application of a computational model of natural deep eutectic solvents utilizing the COSMO-RS approach for screening of solvents with high solubility of rutin. J. Mol. Model. 2018, 24, 180. [Google Scholar] [CrossRef]
- TURBOMOLE Documentation & How To. TURBOMOLE. Available online: https://www.turbomole.org/turbomole/turbomole-documentation/ (accessed on 26 June 2022).
- VEGA. Downloads|VEGA. Available online: https://www.vega.com/en-us/downloads (accessed on 4 August 2022).
- VEGA HUB—Virtual Models for Property Evaluation of Chemicals within a Global Architecture. Available online: https://www.vegahub.eu/ (accessed on 2 December 2022).
- Cheng, J.; Qin, H.; Cheng, H.; Song, Z.; Qi, Z.; Sundmacher, K. Rational Screening of Deep Eutectic Solvents for the Direct Extraction of α-Tocopherol from Deodorized Distillates. ACS Sustain. Chem. Eng. 2022, 10, 8216–8227. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J.; Leitner, W. An overview of the biphasic dehydration of sugars to 5-hydroxymethylfurfural and furfural: A rational selection of solvents using COSMO-RS and selection guides. Green Chem. 2020, 22, 2097–2128. [Google Scholar] [CrossRef]
- Linke, S.; McBride, K.; Sundmacher, K. Systematic Green Solvent Selection for the Hydroformylation of Long-Chain Alkenes. ACS Sustain. Chem. Eng. 2020, 8, 10795–10811. [Google Scholar] [CrossRef]
- Abedin, R.; Heidarian, S.; Flake, J.C.; Hung, F.R. Computational Evaluation of Mixtures of Hydrofluorocarbons and Deep Eutectic Solvents for Absorption Refrigeration Systems. Langmuir 2017, 33, 11611–11625. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Harmer, M.A.; Junk, C.P.; Yokozeki, A. Solubility and Diffusivity of Difluoromethane in Room-Temperature Ionic Liquids. J. Chem. Eng. Data 2006, 51, 483–495. [Google Scholar] [CrossRef]
- McGaughy, K.; Reza, M.T. Liquid–Liquid Extraction of Furfural from Water by Hydrophobic Deep Eutectic Solvents: Improvement of Density Function Theory Modeling with Experimental Validations. ACS Omega 2020, 5, 22305–22313. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Warrag, S.E.E.; Kroon, M.C. The Curious Case of Hydrophobic Deep Eutectic Solvents: A Story on the Discovery, Design, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 10591–10612. [Google Scholar] [CrossRef]
- Klein, J.M.; Squire, H.; Dean, W.; Gurkan, B.E. From Salt in Solution to Solely Ions: Solvation of Methyl Viologen in Deep Eutectic Solvents and Ionic Liquids. J. Phys. Chem. B 2020, 124, 6348–6357. [Google Scholar] [CrossRef]
- SDS Search. Available online: https://www.fishersci.com/us/en/catalog/search/sdshome.html (accessed on 18 August 2022).
- Airgas. Available online: https://www.airgas.com/search/nonproduct?text=R32&tabId=sds (accessed on 23 December 2022).
| N4Br | BTACl | N8Br | N81Br | |
|---|---|---|---|---|
| PEG | −1.39 | −1.28 | −1.11 | −1.04 |
| Camphor | −1.34 | −1.20 | −1.07 | −1.01 |
| Lidocaine | −1.27 | −1.16 | −1.06 | −1.00 |
| Eucolyptol | −1.27 | −1.11 | −1.02 | −0.94 |
| HBD | Property | N4Br (kJ/mol) | BTACl (kJ/mol) | N8Br (kJ/mol) | MTOA (kJ/mol) |
|---|---|---|---|---|---|
| PEG | Hint | −1.15158 | −1.12681 | −1.15158 | −1.12756 |
| PEG | Hmf | 1.4377 | 1.44851 | 1.4377 | 1.42068 |
| PEG | Hhb | −0.40207 | −0.43892 | −0.40207 | −0.37627 |
| PEG | Hvdw | −3.13471 | −3.08391 | −3.13471 | −3.11947 |
| Camphor | Hint | −1.12736 | −1.10647 | −1.15158 | −1.11496 |
| Camphor | Hmf | 1.45191 | 1.45861 | 1.4377 | 1.40033 |
| Camphor | Hhb | −0.39266 | −0.42837 | −0.40207 | −0.33878 |
| Camphor | Hvdw | −3.13412 | −3.0842 | −3.13471 | −3.12401 |
| Eucalyptol | Hint | −1.14948 | −1.13061 | −1.12736 | −1.09226 |
| Eucalyptol | Hmf | 1.46866 | 1.47271 | 1.45191 | 1.41422 |
| Eucalyptol | Hhb | −0.41278 | −0.4479 | −0.39266 | −0.33038 |
| Eucalyptol | Hvdw | −3.15286 | −3.10292 | −3.13412 | −3.1236 |
| Lidocaine | Hint | −1.12756 | −1.1175 | −1.14948 | −1.11091 |
| Lidocaine | Hmf | 1.42068 | 1.4228 | 1.46866 | 1.42843 |
| Lidocaine | Hhb | −0.37627 | −0.40817 | −0.41278 | −0.3468 |
| Lidocaine | Hvdw | −3.11947 | −3.07964 | −3.15286 | −3.14005 |
| HBD | Ratio | Pure | N4Br | N8Br | BTACl | MTOA |
|---|---|---|---|---|---|---|
| - | - | - | −1.61 | −1.43 | −1.61 | −1.16 |
| PEG | 1:1 | −0.71 | −1.39 | −1.39 | −1.27 | −1.03 |
| PEG | 1:2 | - | −1.27 | −1.27 | −1.2 | −0.97 |
| PEG | 1:3 | - | −1.19 | −1.19 | −1.14 | −0.93 |
| PEG | 2:1 | - | −1.48 | −1.33 | −1.48 | −1.08 |
| PEG | 3:1 | - | −1.52 | −1.36 | −1.52 | −1.10 |
| Camphor | 1:1 | −0.44 | −1.34 | −1.34 | −1.19 | −0.99 |
| Camphor | 1:2 | - | −1.18 | −1.18 | −1.08 | −0.89 |
| Camphor | 1:3 | - | −1.07 | −1.07 | −1.00 | −0.83 |
| Camphor | 2:1 | - | −1.45 | −1.28 | −1.45 | −1.06 |
| Camphor | 3:1 | - | −1.50 | −1.31 | −1.50 | −1.09 |
| Eucalyptol | 1:1 | −0.03 | −1.27 | −1.27 | −1.11 | −0.92 |
| Eucalyptol | 1:2 | - | −1.07 | −1.07 | −0.95 | −0.78 |
| Eucalyptol | 1:3 | - | −0.93 | −0.93 | −0.85 | −0.68 |
| Eucalyptol | 2:1 | - | −1.41 | −1.22 | −1.41 | −1.02 |
| Eucalyptol | 3:1 | - | −1.47 | −1.27 | −1.47 | −1.06 |
| Lidocaine | 1:1 | −0.58 | −1.27 | −1.27 | −1.16 | −0.98 |
| Lidocaine | 1:2 | - | −1.10 | −1.10 | −1.04 | −0.89 |
| Lidocaine | 1:3 | - | −1.00 | −1.00 | −0.96 | −0.83 |
| Lidocaine | 2:1 | - | −1.41 | −1.25 | −1.41 | −1.05 |
| Lidocaine | 3:1 | - | −1.46 | −1.29 | −1.46 | −1.08 |
| HBD | Pressure (Bar) | N4Br | BTACl | N8Br | MTOA |
|---|---|---|---|---|---|
| Camphor | 1 | 24.2 | 22.3 | 24.2 | 19.2 |
| Camphor | 3 | 49.8 | 48.3 | 49.8 | 43.9 |
| Camphor | 6 | 70.7 | 70.3 | 70.7 | 66.4 |
| Camphor | 9 | 85.8 | 85.6 | 84.7 | 86.2 |
| Eucalyptol | 1 | 22.9 | 20.9 | 22.9 | 18.0 |
| Eucalyptol | 3 | 47.8 | 46.2 | 47.8 | 41.8 |
| Eucalyptol | 6 | 68.9 | 68.6 | 68.9 | 64.2 |
| Eucalyptol | 9 | 86.0 | 85.6 | 84.5 | 86.2 |
| Lidocaine | 1 | 23.8 | 22.2 | 23.8 | 19.4 |
| Lidocaine | 3 | 50.2 | 48.8 | 50.2 | 44.8 |
| Lidocaine | 6 | 71.6 | 71.2 | 71.6 | 67.7 |
| Lidocaine | 9 | 83.7 | 83.1 | 81.4 | 84.0 |
| Persistence Air | Persistence Water | Persistence Soil | Mutagenicity | Acute Toxicity | BCF | Carcinogenicity | |
|---|---|---|---|---|---|---|---|
| N4Br | g | g | b | g | g | b | b |
| BTACl | g | g | g | g | g | b | b |
| N8Br | b | b | b | g | g | b | b |
| MTOA | g | g | g | g | r | b | b |
| PEG | g | g | g | g | g | g | g |
| Camphor | b | g | g | g | g | g | g |
| Eucalyptol | b | g | g | g | g | g | g |
| Lidocaine | b | b | g | g | g | g | g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaid, T.; Reza, M.T. Mechanistic Understanding on Difluoromethane Absorption Thermodynamics on Novel Deep Eutectic Solvents by COSMO-Based Molecular Simulation. Appl. Sci. 2023, 13, 6182. https://doi.org/10.3390/app13106182
Quaid T, Reza MT. Mechanistic Understanding on Difluoromethane Absorption Thermodynamics on Novel Deep Eutectic Solvents by COSMO-Based Molecular Simulation. Applied Sciences. 2023; 13(10):6182. https://doi.org/10.3390/app13106182
Chicago/Turabian StyleQuaid, Thomas, and M. Toufiq Reza. 2023. "Mechanistic Understanding on Difluoromethane Absorption Thermodynamics on Novel Deep Eutectic Solvents by COSMO-Based Molecular Simulation" Applied Sciences 13, no. 10: 6182. https://doi.org/10.3390/app13106182
APA StyleQuaid, T., & Reza, M. T. (2023). Mechanistic Understanding on Difluoromethane Absorption Thermodynamics on Novel Deep Eutectic Solvents by COSMO-Based Molecular Simulation. Applied Sciences, 13(10), 6182. https://doi.org/10.3390/app13106182






