Pharmaceuticals in Coastal Waters: An UHPLC-TOF-MS Multi-Residue Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sample Treatment
2.3. Instrumentation
2.4. Validation Procedure
3. Results and Discussion
3.1. Method Development
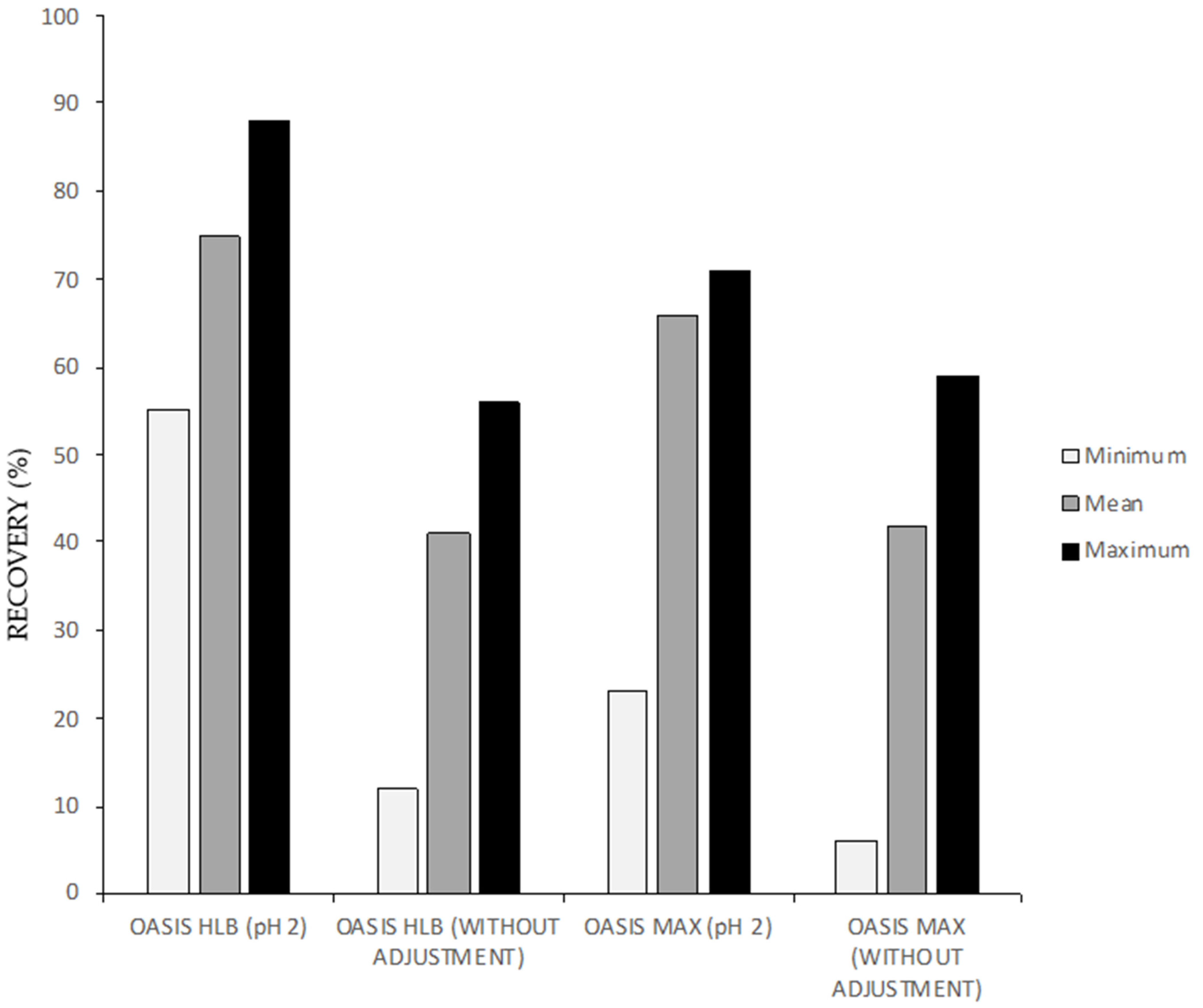
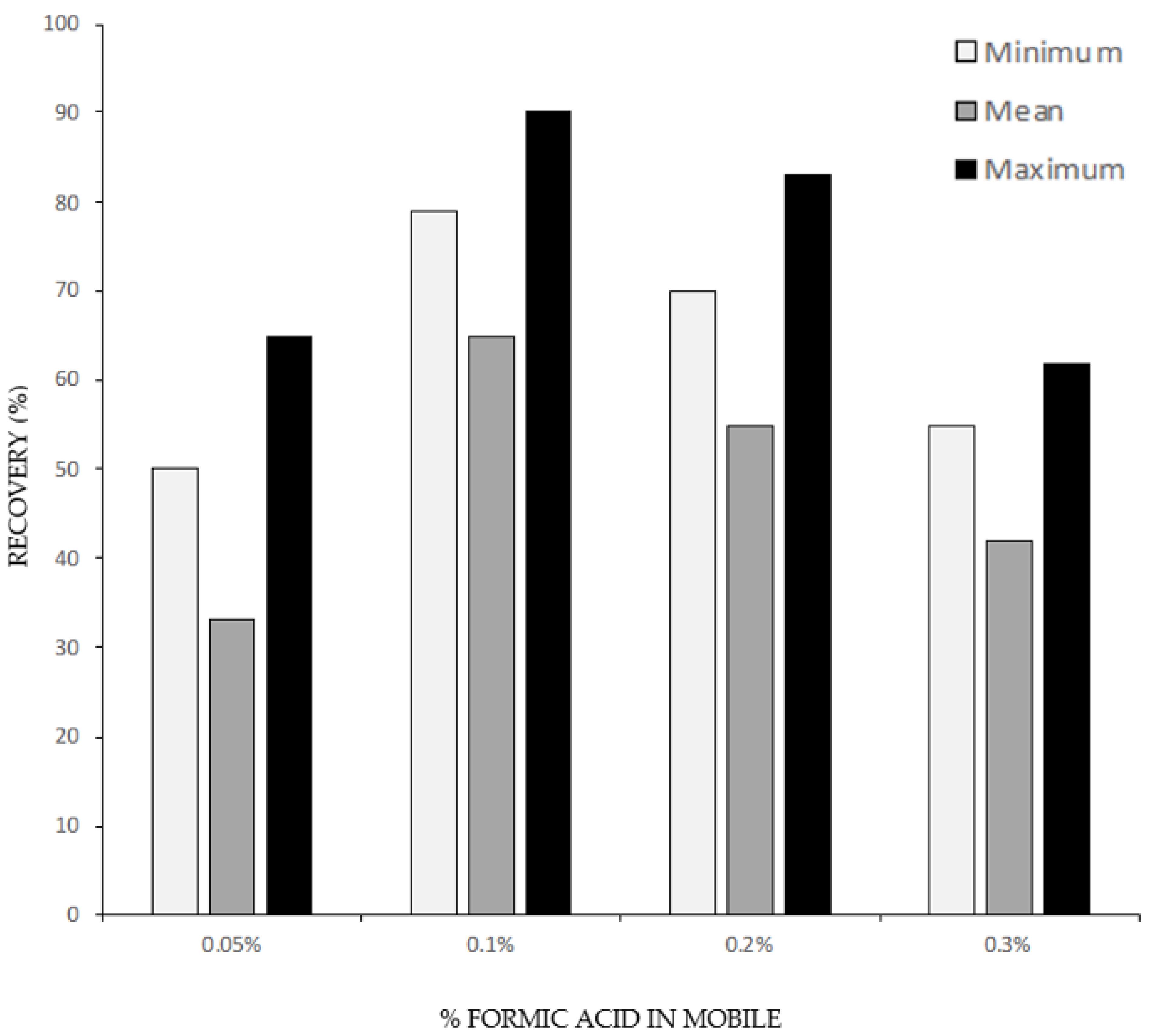
3.1.1. Solid Phase Extraction
3.1.2. UHPLC-TOF-MS Performance
3.2. Validation
3.3. Application to Field Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias-Campos, A.; Meiner, A.; Bowen, K.; Onwona Ansong, J. Chapter 3—Coastal Population and Land Use Changes in Juan; Baztan, J., Chouinard, O., Jorgensen, B., Tett, P., Vanderlinden, J.-P., Vasseur, L., Eds.; Europe: Challenges for a Sustainable Future in Coastal Zones; Elsevier: Amsterdam, The Netherlands, 2015; pp. 29–49. [Google Scholar] [CrossRef]
- Nunes, M.; Leston, S. Coastal Pollution: An Overview. In Life Below Water. Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Riera, R.; Menci, C.; Sanabria-Fernández, J.A.; Becerro, M.A. Do recreational activities affect coastal biodiversity? Estuar. Coast. Shelf Sci. 2016, 178, 129–136. [Google Scholar] [CrossRef]
- United Nations. The Second World Ocean Assessment II; Cambridge University Press: Cambridge, UK, 2021.
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the marine environment: What are the present challenges in their monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef] [PubMed]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, P. Occurrence and ecotoxicological assessment of pharmaceuticals: Is there a risk for the Mediterranean aquatic environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Leston, S.; Freitas, A.; Rosa, J.; Barbosa, J.; Lemos, M.F.L.; Pardal, M.A.; Ramos, F. A multiresidue approach for the simultaneous quantification of antibiotics in macroalgae by ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2016, 1033, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, W.; Li, H.; Xu, N.; Zhang, R.; Chen, X.; Sun, W.; Wen, D.; He, S.; Pan, J.; et al. Antibiotics in water and sediments of rivers and coastal area of Zhuhai City, Pearl River estuary, South China. Sci. Total Environ. 2018, 636, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Marinov, D.; Sanseverino, I.; Napiersk, D.; Lettieri, T. Review of the 1st Watch List under the Water Framework Directive and Recommendations for the 2nd Watch List, EUR 29173 EN; JRC; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-81839-4. [Google Scholar] [CrossRef]
- OSPAR Commission. JAMP Guidelines for Monitoring of Contaminants in Seawater. 2013. Available online: https://mcc.jrc.ec.europa.eu/documents/OSPAR/Guidelines_forMonitoring_of_ContaminantsSeawater.pdf (accessed on 3 February 2023).
- INFARMED. Medicine and Healthcare Products Statistics. 2017. Available online: https://www.infarmed.pt/web/infarmed/entidades/medicamentos-uso-humano/monitorizacao-mercado/estatistica-anual/relatorios-anuais (accessed on 3 February 2023).
- Sousa, M.A.; Gonçalves, C.; Cunha, E.; Hajšlová, J.; Alpendurada, M.F. Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE–LC–MS/MS. Anal. Bioanal. Chem. 2011, 399, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: Geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Decision (EU) 2020/1161. Official Journal of the European Union, L 257/32. 2020. Available online: http://data.europa.eu/eli/dec_impl/2020/1161/oj (accessed on 3 February 2023).
- Commission Implementing Regulation (EU) Official Journal of the European Union, L 180/84. 2021. Available online: http://data.europa.eu/eli/reg_impl/2021/808/oj (accessed on 3 February 2023).
- Magalhães, D.; Freitas, A.; Vila Pouca, A.S.; Barbosa, J.; Ramos, F. The use of ultra-high-pressure-liquid-chromatography tandem time-of-flight mass spectrometry as a confirmatory method in drug residue analysis: Application to the determination of antibiotics in piglet liver. J. Chromatogr. B 2020, 1153, 122264. [Google Scholar] [CrossRef] [PubMed]
- Reis-Santos, P.; Pais, M.; Duarte, B.; Caçador, I.; Freitas, A.; Vila Pouca, A.S.; Barbosa, J.; Leston, S.; Rosa, J.; Ramos, F.; et al. Screening of human and veterinary pharmaceuticals in estuarine waters: A baseline assessment for the Tejo estuary. Mar. Pollut. Bull. 2018, 135, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.F.; Duarte, I.A.; Duarte, B.; Freitas, A.; Pouca AS, V.; Barbosa, J.; Gillanders, B.M.; Reis-Santos, P. Environmental risk assessment and bioaccumulation of pharmaceuticals in a large urbanized estuary. Sci. Total Environ. 2021, 783, 147021. [Google Scholar] [CrossRef] [PubMed]
- Moyo, B.; Tawanda Tavengwa, N. Modern Extraction and Cleanup Methods of Veterinary Drug Residues in Food Samples of Animal Origin. Recent Adv. Anal. Chem. 2019, 1, 21. [Google Scholar] [CrossRef]
- Nurmi, J.; Pellinen, J. Multiresidue method for the analysis of emerging contaminants in wastewater by ultra performance liquid chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 6712–6719. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Gros, M.; Barcelo, D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography–quadrupole–time-of-flight mass spectrometry. J. Chromatogr. A 2006, 1124, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Kiontke, A.; Oliveira-Birkmeier, A.; Opitz, A.; Birkemeyer, C. Electrospray Ionization Efficiency Is Dependent on Different Molecular Descriptors with Respect to Solvent pH and Instrumental Configuration. PLoS ONE 2016, 11, e0167502. [Google Scholar] [CrossRef] [PubMed]


| Compound | Molecular Formula | LogP | Molecular Weight | [M + H]+ | ΔM (ppm) | LoD (ng/L) | LoQ (ng/L) | Repeatability (%) | Reproducibility (%) | Recovery (%) | RT (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analgesic | |||||||||||
| Acetaminophen | C8H9NO2 | 0.51 | 151.0633 | 152.0706 | −0.8 | 1.89 | 6.30 | 3.0% | 4.5% | 99.8% | 2.21 |
| Antibiotics | |||||||||||
| Amoxicillin | C16H19N3O5S | 0.75 | 365.1045 | 366.1118 | 2.3 | 0.11 | 0.38 | 16.2% | 20.9% | 107.2% | 6.91 |
| Azithromycin | C38H72N2O12 | 3.03 | 748.5085 | 749.5158 | −0.9 | 0.01 | 0.04 | 11.5% | 14.4% | 96.0% | 5.04 |
| Benzylpenicillin | C16H18N2O4S | 1.83 | 334.0987 | 335.1060 | 0.1 | 0.75 | 2.52 | 3.1% | 4.7% | 88.7% | 4.49 |
| Ceftiofur | C19H17N5O7S3 | 1.22 | 523.0290 | 524.0363 | 1.3 | 0.03 | 0.09 | 10.5% | 16.8% | 94.7% | 5.85 |
| Cephalexin | C16H17N3O4S | 0.55 | 347.0939 | 348.1013 | 0.5 | 0.04 | 0.10 | 16.6% | 18.5% | 98.6% | 4.85 |
| Chlortetracycline | C22H23ClN2O8 | −0.13 | 478.1142 | 479.1216 | 0.7 | 1.73 | 5.77 | 2.2% | 3.2% | 99.5% | 4.78 |
| Cinoxacin | C12H10N2O5 | 1.25 | 262.0589 | 263.0663 | −0.8 | 0.03 | 0.09 | 14.9% | 16.9% | 92.5% | 5.25 |
| Ciprofloxacin | C17H18FN3O3 | −0.57 | 331.1332 | 332.1405 | 0.6 | 3.47 | 11.56 | 3.2% | 4.7% | 110.5% | 4.47 |
| Danofloxacin | C19H20FN3O3 | 0.33 | 357.1488 | 358.1562 | 0.5 | 3.25 | 10.82 | 6.5% | 9.7% | 110.1% | 4.59 |
| Doxycyclin | C22H24N2O8 | −0.72 | 444.1532 | 445.1605 | −0.6 | 0.28 | 0.94 | 6.7% | 9.5% | 101.5% | 5.23 |
| Enoxacin | C15H17FN4O3 | −0.97 | 320.1284 | 321.1358 | 0.9 | 3.33 | 11.09 | 3.9% | 5.8% | 109.5% | 4.33 |
| Enrofloxacin | C19H22FN3O3 | 0.58 | 359.1645 | 360.1718 | 0.6 | 2.27 | 7.58 | 2.5% | 3.8% | 101.4% | 4.66 |
| Epi-Chlortetracycline | C22H23ClN2O8 | −0.13 | 478.1142 | 479.1216 | 0.6 | 1.00 | 3.33 | 2.2% | 3.3% | 99.0% | 4.54 |
| epi-Tetracycline | C22H24N2O8 | −0.56 | 444.1532 | 445.1605 | 1.0 | 0.40 | 1.32 | 6.5% | 7.2% | 104.7% | 4.27 |
| Flumequine | C14H12FNO3 | 1.62 | 261.0801 | 262.0874 | −0.3 | 0.01 | 0.04 | 8.2% | 10.1% | 80.6% | 6.18 |
| Marbofloxacin | C17H19FN4O4 | −0.53 | 362.1390 | 363.1463 | 0.6 | 2.51 | 8.35 | 3.1% | 4.6% | 108.6% | 4.29 |
| Nalidixic acid | C12H12N2O3 | 0.95 | 232.0847 | 233.0921 | −0.9 | 0.92 | 3.07 | 4.2% | 6.3% | 106.0% | 6.07 |
| Norfloxacin | C16H18FN3O3 | −0.47 | 319.1332 | 320.1405 | −0.4 | 1.81 | 6.04 | 9.0% | 10.2% | 109.6% | 4.41 |
| Ofloxacin | C18H20FN3O4 | −0.02 | 361.1437 | 362.1511 | 0.6 | 0.99 | 3.31 | 1.4% | 2.6% | 91.5% | 4.43 |
| Oxolinic acid | C13H11NO5 | 0.86 | 261.0637 | 262.0710 | 0.6 | 3.08 | 10.26 | 3.4% | 7.1% | 92.3% | 5.50 |
| Oxytetracycline | C22H24N2O9 | −0.99 | 460.1481 | 461.1555 | 0.3 | 0.24 | 0.79 | 7.6% | 11.4% | 104.3% | 4.40 |
| Spiramycin | C43H74N2O14 | 2.99 | 842.5140 | 843.5213 | −0.2 | 0.01 | 0.04 | 5.6% | 7.1% | 87.7% | 5.02 |
| Sulfachloropyridazine | C10H9ClN4O2S | 0.97 | 284.0134 | 285.0208 | −1.0 | 0.43 | 1.43 | 6.0% | 7.0% | 103.9% | 4.95 |
| Sulfadiazine | C10H10N4O2S | 0.25 | 250.0524 | 251.0597 | −1.1 | 0.01 | 0.02 | 5.5% | 8.2% | 107.5% | 3.81 |
| Sulfadimethoxine | C12H14N4O4S | 1.08 | 310.0735 | 311.0809 | −0.9 | 0.30 | 0.98 | 2.7% | 4.1% | 100.3% | 5.70 |
| Sulfadimidin | C12H14N4O2S | 0.43 | 278.0837 | 279.091 | −0.9 | 0.14 | 0.47 | 1.9% | 2.5% | 100.9% | 4.51 |
| Sulfadoxine | C12H14N4O4S | 0.72 | 310.0735 | 311.0809 | −0.7 | 0.03 | 0.10 | 6.6% | 7.7% | 98.3% | 4.94 |
| Sulfamethizole | C9H10N4O2S2 | 0.53 | 270.0245 | 271.0318 | −1.3 | 0.32 | 1.06 | 12.7% | 15.2% | 100.0% | 4.52 |
| Sulfamethoxazole | C10H11N3O3S | 0.79 | 253.0521 | 254.0594 | −0.7 | 0.03 | 0.09 | 2.5% | 3.7% | 99.5% | 5.12 |
| Sulfapyridine | C11H11N3O2S | 0.84 | 249.0572 | 250.0645 | −0.7 | 0.46 | 1.52 | 3.3% | 3.3% | 104.4% | 3.80 |
| Sulfaquinoxaline | C14H12N4O2S | 1.24 | 300.0681 | 301.0754 | −0.7 | 0.03 | 0.09 | 4.5% | 6.7% | 88.3% | 5.71 |
| Sulfathiazole | C9H9N3O2S2 | 0.88 | 255.0136 | 256.0209 | −1.3 | 0.56 | 1.87 | 6.6% | 10.6% | 108.6% | 3.65 |
| Sulfisomidine | C12H14N4O2S | 0.84 | 278.0837 | 279.091 | −0.2 | 0.10 | 0.32 | 9.8% | 14.6% | 87.0% | 3.42 |
| Sulfisoxazole | C11H13N3O3S | 1.14 | 267.0677 | 268.075 | 0.1 | 0.02 | 0.07 | 9.8% | 13.5% | 101.1% | 5.04 |
| Tetracycline | C22H24N2O8 | −0.56 | 444.1532 | 445.1605 | 0.2 | 0.48 | 1.61 | 5.5% | 8.2% | 112.6% | 4.57 |
| Tilmicosin | C46H80N2O13 | 3.34 | 868.5660 | 869.5733 | −0.9 | 0.01 | 0.05 | 3.7% | 4.5% | 95.9% | 5.40 |
| Trimethoprim | C14H18N4O3 | 1.26 | 290.1378 | 291.1452 | 0.3 | 0.80 | 2.68 | 4.3% | 6.4% | 109.5% | 4.24 |
| Tylosin A | C46H77NO17 | 1.46 | 915.5191 | 916.5264 | −0.9 | 0.36 | 1.21 | 4.6% | 6.1% | 86.7% | 5.90 |
| Anticonvulsants | |||||||||||
| Carbamazepine | C15H12N2O | 2.10 | 237.1022 | 237.1022 | −0.8 | 0.01 | 0.05 | 12.5% | 18.7% | 102.9% | 6.08 |
| Gabapentin | C9H17NO2 | −1.90 | 172.1332 | 172.1332 | −0.5 | 0.81 | 2.69 | 4.1% | 6.1% | 80.6% | 3.61 |
| Topiramate | C12H21NO8S | 1.29 | 340.1061 | 340.1061 | 0.6 | 0.03 | 0.11 | 2.9% | 3.7% | 104.7% | 5.82 |
| Antidepressants | |||||||||||
| Alpha-Hydroxyalprazolam | C17H13ClN4O | 1.53 | 324.0777 | 325.0851 | −0.3 | 0.02 | 0.08 | 5.6% | 8.3% | 84.9% | 6.12 |
| Fluoxetine | C17H18F3NO | 4.09 | 309.1340 | 310.1413 | −0.2 | 0.01 | 0.03 | 5.2% | 7.8% | 109.3% | 4.88 |
| Lorazepam | C15H10Cl2N2O2 | 2.98 | 320.0119 | 321.0192 | 3.1 | 2.98 | 9.94 | 7.6% | 8.7% | 108.9% | 4.53 |
| Sertraline | C17H17Cl2N | 5.06 | 305.0738 | 306.0811 | 0.4 | 0.03 | 0.09 | 3.2% | 4.0% | 109.6% | 6.13 |
| Venlafaxine | C17H27NO2 | 2.69 | 277.2041 | 278.2115 | −0.9 | 0.02 | 0.08 | 6.0% | 7.3% | 99.6% | 5.24 |
| Antihypertensives | |||||||||||
| Furosemide | C12H11ClN2O5S | 2.71 | 331.015 | 331.015 | −1.0 | 0.62 | 2.07 | 7.7% | 11.6% | 109.3% | 6.04 |
| Indapamide | C16H16ClN3O3S | 2.52 | 366.0674 | 366.0674 | −0.3 | 0.03 | 0.09 | 3.6% | 4.0% | 100.4% | 6.27 |
| Irbesartan | C25H28N6O | 4.51 | 429.2397 | 429.2397 | −0.7 | 0.03 | 0.17 | 10.5% | 15.7% | 96.0% | 6.31 |
| Losartan | C22H23ClN6O | 4.50 | 423.1695 | 423.1695 | −0.5 | 0.05 | 0.09 | 12.3% | 18.4% | 107.1% | 5.77 |
| β-Blockers | |||||||||||
| Atenolol | C14H22N2O3 | 0.57 | 266.1630 | 267.1703 | −0.7 | 0.01 | 0.03 | 3.0% | 4.2% | 104.4% | 3.47 |
| Bisoprolol | C18H31NO4 | 2.30 | 325.2253 | 326.2326 | −1.0 | 0.14 | 0.46 | 8.3% | 9.1% | 94.7% | 5.29 |
| Carvedilol | C24H26N2O4 | 3.05 | 406.1892 | 407.1965 | −0.9 | 0.83 | 2.76 | 13.4% | 19.7% | 108.7% | 5.87 |
| Propranolol | C16H21NO2 | 3.03 | 259.1572 | 260.1645 | −0.6 | 0.06 | 0.21 | 4.5% | 6.5% | 100.9% | 4.39 |
| Lipid regulators | |||||||||||
| Atorvastatin | C33H35FN2O5 | 4.24 | 559.2603 | 559.2603 | 0.6 | 8.92 | 29.73 | 7.9% | 10.2% | 88.6% | 8.46 |
| Bezafibrate | C19H20ClNO4 | 3.97 | 362.1154 | 362.1154 | −0.8 | 0.07 | 0.24 | 7.4% | 8.6% | 85.7% | 6.91 |
| Fenofibrate | C20H21ClO4 | 4.86 | 361.1201 | 361.1201 | 0.7 | 0.02 | 0.06 | 17.2% | 18.1% | 103.5% | 9.41 |
| Gemfibrozil | C15H22O3 | 3.61 | 251.1642 | 251.1642 | −0.8 | 0.21 | 0.71 | 4.5% | 6.8% | 110.4% | 4.99 |
| Simvastatin | C25H38O5 | 4.51 | 419.2792 | 419.2792 | −1.6 | 2.80 | 9.34 | 11.7% | 12.2% | 110.3% | 6.20 |
| NSAID’s | |||||||||||
| Diclofenac | C14H11Cl2NO2 | 4.98 | 295.0166 | 296.024 | −0.5 | 0.02 | 0.05 | 5.9% | 8.8% | 106.0% | 7.70 |
| Ibuprofen | C13H18O2 | 3.50 | 206.1306 | 207.138 | −0.4 | 2.72 | 9.08 | 3.5% | 5.3% | 108.9% | 6.22 |
| Nimesulide | C13H12N2O5S | 2.56 | 308.0466 | 309.054 | 0.3 | 0.03 | 0.08 | 7.4% | 11.1% | 104.5% | 7.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leston, S.; Freitas, A.; Rosa, J.; Vila Pouca, A.S.; Barbosa, J.; Reis-Santos, P.; Fonseca, V.F.; Pardal, M.A.; Ramos, F. Pharmaceuticals in Coastal Waters: An UHPLC-TOF-MS Multi-Residue Approach. Appl. Sci. 2023, 13, 5975. https://doi.org/10.3390/app13105975
Leston S, Freitas A, Rosa J, Vila Pouca AS, Barbosa J, Reis-Santos P, Fonseca VF, Pardal MA, Ramos F. Pharmaceuticals in Coastal Waters: An UHPLC-TOF-MS Multi-Residue Approach. Applied Sciences. 2023; 13(10):5975. https://doi.org/10.3390/app13105975
Chicago/Turabian StyleLeston, Sara, Andreia Freitas, João Rosa, Ana Sofia Vila Pouca, Jorge Barbosa, Patrick Reis-Santos, Vanessa F. Fonseca, Miguel A. Pardal, and Fernando Ramos. 2023. "Pharmaceuticals in Coastal Waters: An UHPLC-TOF-MS Multi-Residue Approach" Applied Sciences 13, no. 10: 5975. https://doi.org/10.3390/app13105975
APA StyleLeston, S., Freitas, A., Rosa, J., Vila Pouca, A. S., Barbosa, J., Reis-Santos, P., Fonseca, V. F., Pardal, M. A., & Ramos, F. (2023). Pharmaceuticals in Coastal Waters: An UHPLC-TOF-MS Multi-Residue Approach. Applied Sciences, 13(10), 5975. https://doi.org/10.3390/app13105975










