Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. OLE
2.3. Protocol
2.4. Biochemical Tests
2.4.1. Measurement of Fasting Blood Sugar (FBS) and Postprandial Sugar (PPS) Levels
2.4.2. Measurement of Serum Insulin Level
2.4.3. Serum Lipid Profile
2.5. Muscle Biopsies
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Body Weight
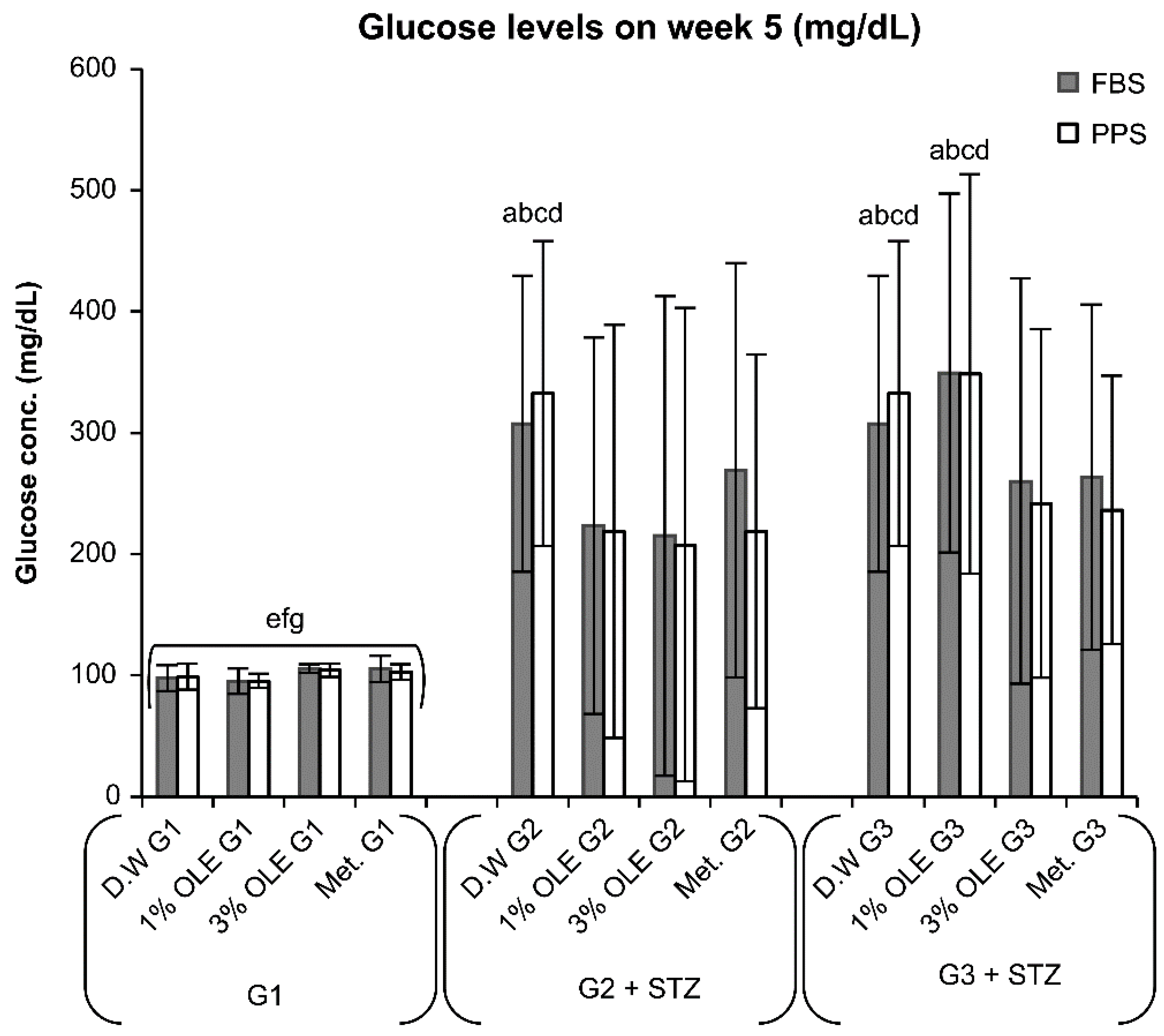
3.2. Effects of Various Treatments (1% OLE, 3% OLE, and Met.) on Glucose Levels
3.3. STZ Induces T1D
3.3.1. Protective Effect of OLE and Met. against STZ Toxicity in the Beta Cells
3.3.2. Effect of STZ on Insulin Levels
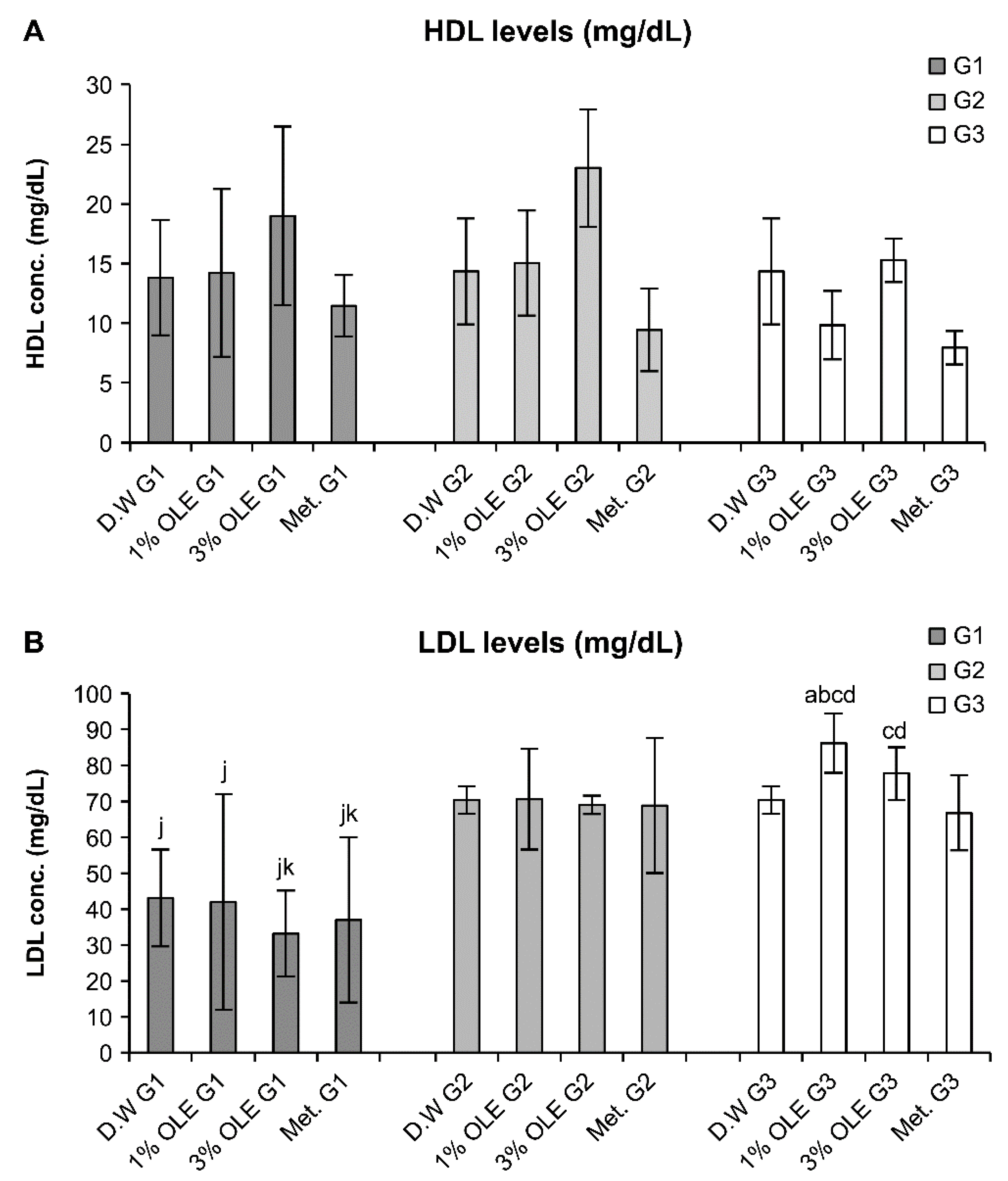
3.4. Effects of Various Treatments on the Lipid Profile Levels
3.5. AMPK α2 Expression
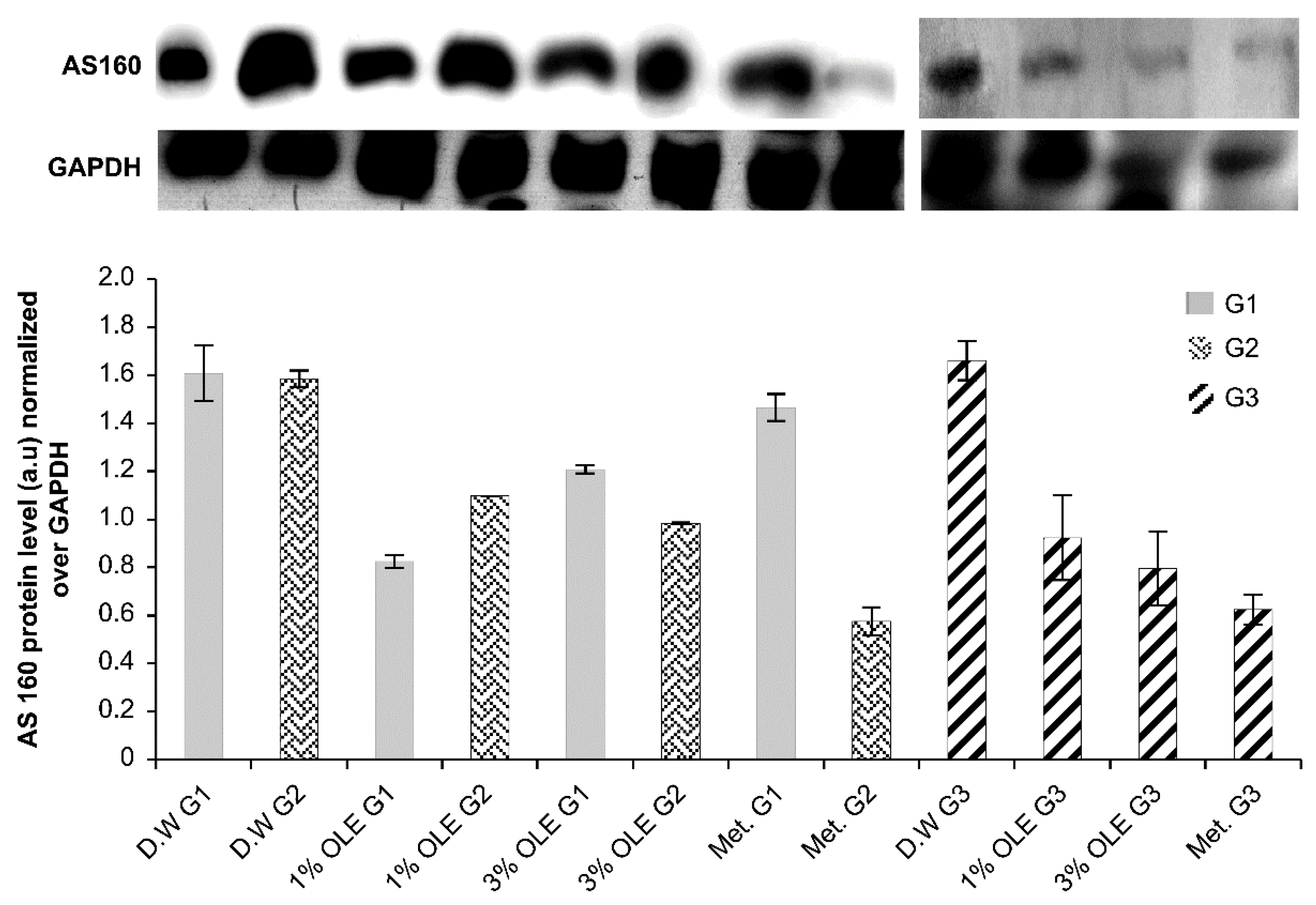
3.6. AS160 Expression
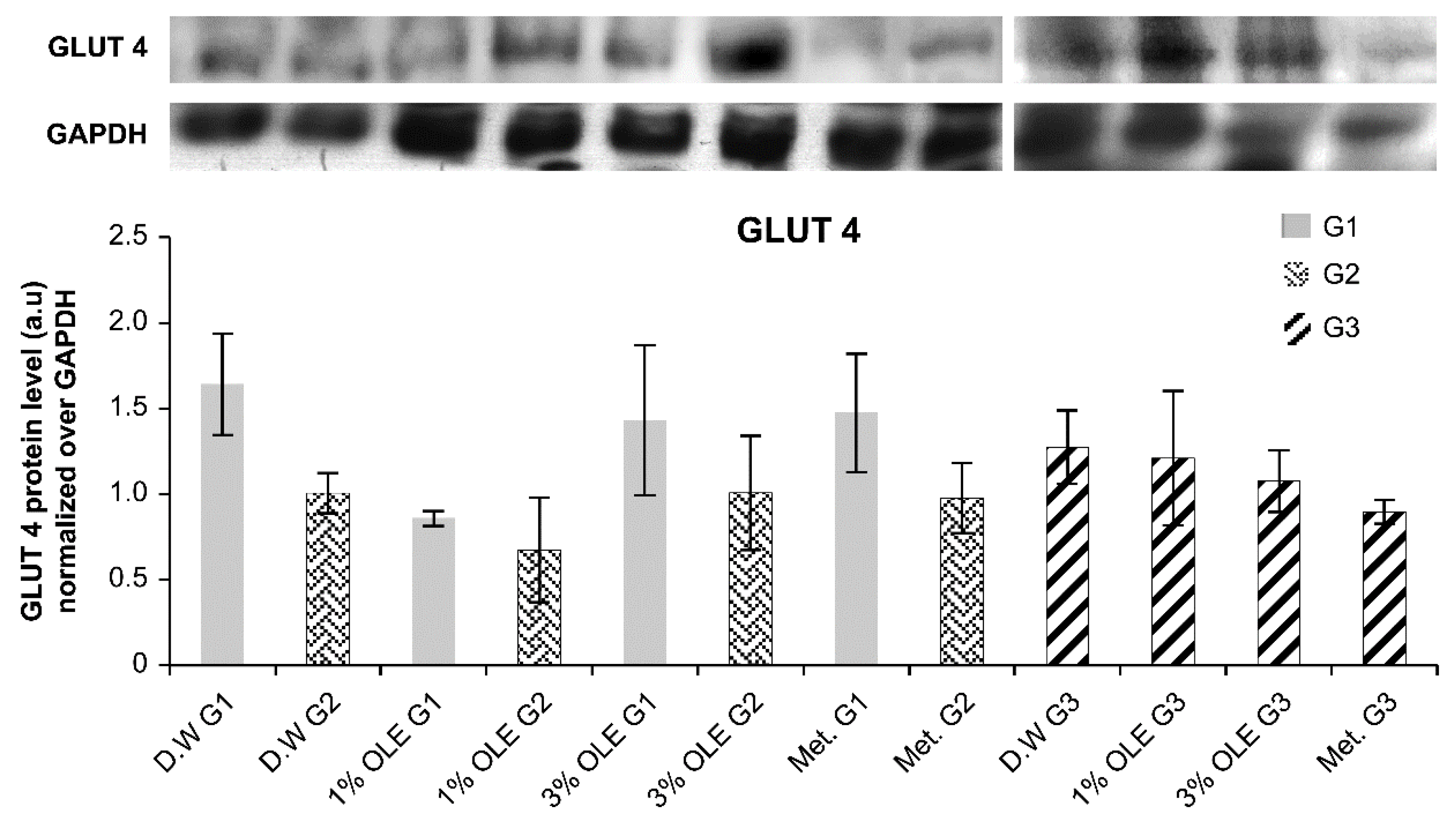
3.7. GLUT4 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez Mejia, C. Molecular basis of type-2 diabetes. Mol. Endocrinol. 2006, 87, 87–108. [Google Scholar]
- World Health Organization. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 20 April 2022).
- World Health Organization. Guidelines for the Prevention, Management and Care of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Alyaarubi, S. Diabetes care in Oman: Obstacles and solutions. Sultan Qaboos Univ. Med. J. 2011, 11, 343. [Google Scholar] [PubMed]
- Kahanovitz, L.; Sluss, P.M.; Russell, S.J. Type 1 diabetes–A clinical perspective. Point Care 2017, 16, 37. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S. Diabetes mellitus and its treatment. Int. J. Diabetes Metab. 2005, 13, 111–134. [Google Scholar] [CrossRef]
- Mohammed, S.; Yaqub, A.; Nicholas, A.; Arastus, W.; Muhammad, M.; Abdullahi, S. Review on diabetes, synthetic drugs and glycemic effects of medicinal plants. J. Med. Plants Res. 2013, 7, 2628–2637. [Google Scholar]
- Shane-McWhorter, L. Biological complementary therapies: A focus on botanical products in diabetes. Diabetes Spectr. 2001, 14, 199–208. [Google Scholar] [CrossRef]
- Liu, C.X.; Xiao, P.G. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992, 36, 27–38. [Google Scholar]
- Dey, L.; Attele, A.S.; Yuan, C.-S. Alternative therapies for type 2 diabetes. Altern. Med. Rev. 2002, 7, 45–58. [Google Scholar]
- Sauvaire, Y.; Baccou, J.-C.; Valette, G.; Chenon, D.; Trimble, E.; Loubatières-Mariani, M.-M. Effects of fenugreek seeds on endocrine pancreatic secretions in dogs. Ann. Nutr. Metab. 1984, 28, 37–43. [Google Scholar]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef]
- Baysal, G.; Kasapbaşı, E.E.; Yavuz, N.; Hür, Z.; Genç, K.; Genç, M. Determination of theoretical calculations by DFT method and investigation of antioxidant, antimicrobial properties of olive leaf extracts from different regions. J. Food Sci. Technol. 2021, 58, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Brewer, P.D.; Romenskaia, I.; Kanow, M.A.; Mastick, C.C. Loss of AS160 Akt substrate causes Glut4 protein to accumulate in compartments that are primed for fusion in basal adipocytes. J. Biol. Chem. 2011, 286, 26287–26297. [Google Scholar] [CrossRef] [PubMed]
- Webster, I.; Friedrich, S.O.; Lochner, A.; Huisamen, B. AMP kinase activation and glut4 translocation in isolated cardiomyocytes: Cardiovascular topics. Cardiovasc. J. Afr. 2010, 21, 72–78. [Google Scholar] [PubMed]
- Winder, W. AMP-activated protein kinase: Possible target for treatment of type 2 diabetes. Diabetes Technol. Ther. 2000, 2, 441–448. [Google Scholar] [CrossRef]
- Henin, N.; Vincent, M.F.; Gruber, H.E.; Van Den Berghe, G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995, 9, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.F.; Kurth-Kraczek, E.; Winder, W. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 1999, 87, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Nishino, Y.; Miura, T.; Miki, T.; Sakamoto, J.; Nakamura, Y.; Ikeda, Y.; Kobayashi, H.; Shimamoto, K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc. Res. 2004, 61, 610–619. [Google Scholar] [CrossRef]
- Song, H.; Guan, Y.; Zhang, L.; Li, K.; Dong, C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochem. Biophys. Res. Commun. 2010, 396, 961–966. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.O.; Lee, S.K.; Moon, J.W.; You, G.Y.; Kim, S.J.; Park, S.-H.; Park, J.M.; Lim, S.Y.; Suh, P.-G. The glutamate agonist homocysteine sulfinic acid stimulates glucose uptake through the calcium-dependent AMPK-p38 MAPK-protein kinase C ζ pathway in skeletal muscle cells. J. Biol. Chem. 2011, 286, 7567–7576. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.M.; Kim, E.K.; Lee, J.O.; Lee, S.K.; Jung, J.H.; You, G.Y.; Park, S.H.; Suh, P.G.; Kim, H.S. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J. Cell. Physiol. 2010, 223, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.P.; Krishna, M.S.; Padmakumari, K.P.; Raghu, K.G.; Sundaresan, A. Zingiber officinale extract exhibits antidiabetic potential via modulating glucose uptake, protein glycation and inhibiting adipocyte differentiation: An in vitro study. J. Sci. Food Agric. 2012, 92, 1948–1955. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Koolaji, N.; Duke, C.; Roufogalis, B.D. (S)-[6]-Gingerol enhances glucose uptake in L6 myotubes by activation of AMPK in response to [Ca2+]i. J. Pharm. Pharm. Sci. 2013, 16, 304–312. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Darzi, R. Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 347–350. [Google Scholar]
- El-Amin, M.; Virk, P.; Elobeid, M.; Almarhoon, Z.M.; Hassan, Z.K.; Omer, S.A.; Merghani, N.M.; Daghestani, M.H.; Al-Olayan, E.M. Anti-diabetic effect of Murraya koenigii (L.) and Olea europaea (L.) leaf extracts on streptozotocin induced diabetic rats. Pak. J. Pharm. Sci. 2013, 26, 359–365. [Google Scholar] [PubMed]
- Kahn, C.R.; White, M. The insulin receptor and the molecular mechanism of insulin action. J. Clin. Investig. 1988, 82, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M. The effect of oral consumption of olive leaves on serum glucose level and lipid profile of diabetic rats. J. Basic Clin. Pathophysiol. 2013, 1, 39–44. [Google Scholar]
- Oloyede, H.; Bello, T.; Ajiboye, T.; Salawu, M. Antidiabetic and antidyslipidemic activities of aqueous leaf extract of Dioscoreophyllum cumminsii (Stapf) Diels in alloxan-induced diabetic rats. J. Ethnopharmacol. 2015, 166, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Al-Rimawi, F. Development and validation of a simple reversed-phase HPLC-UV method for determination of oleuropein in olive leaves. J. Food Drug Anal. 2014, 22, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Clewell, A.E.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Murbach, T.S.; Szakonyiné, I.P. A comprehensive toxicological safety assessment of an extract of Olea europaea L. leaves (bonolive™). Int. J. Toxicol. 2016, 35, 208–221. [Google Scholar] [CrossRef]
- Guex, C.G.; Reginato, F.Z.; Figueredo, K.C.; da Silva, A.R.H.; Pires, F.B.; da Silva Jesus, R.; Lhamas, C.L.; Lopes, G.H.H.; de Freitas Bauermann, L. Safety assessment of ethanolic extract of Olea europaea L. leaves after acute and subacute administration to Wistar rats. Regul. Toxicol. Pharmacol. 2018, 95, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, N.H.; Bagayoko, N.D.; Antwi, M.E. The effects of dietary caloric restriction on antioxidant status and lipid peroxidation in mild and severe streptozotocin-induced diabetic rats. Clin. Chim. Acta 2004, 348, 121–129. [Google Scholar] [CrossRef]
- Carter, L.G.; Qi, N.R.; De Cabo, R.; Pearson, K.J. Maternal exercise improves insulin sensitivity in mature rat offspring. Med. Sci. Sport. Exerc. 2013, 45, 832. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Copray, S.; Liem, R.; Brouwer, N.; Greenhaff, P.; Habens, F.; Fernyhough, P. Contraction-induced muscle fiber damage is increased in soleus muscle of streptozotocin-diabetic rats and is associated with elevated expression of brain-derived neurotrophic factor mRNA in muscle fibers and activated satellite cells. Exp. Neurol. 2000, 161, 597–608. [Google Scholar] [CrossRef]
- Fewell, J.G.; Moerland, T.S. Responses of mouse fast and slow skeletal muscle to streptozotocin diabetes: Myosin isoenzymes and phosphorous metabolites. Mol. Cell. Biochem. 1995, 148, 147–154. [Google Scholar] [CrossRef]
- Minamide, L.; Bamburg, J. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal. Biochem. 1990, 190, 66–70. [Google Scholar] [CrossRef]
- Pournaghi, P.; Sadrkhanlou, R.-A.; Hasanzadeh, S.; Foroughi, A. An investigation on body weights, blood glucose levels and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet. Res. Forum 2012, 3, 79–84. [Google Scholar] [PubMed]
- Kanazawa, I. A Novel Attribute of Insulin Secretion via Incretin Axis by Metformin. Int. J. Endocrinol. Metab. 2011, 9, 420–421. [Google Scholar] [CrossRef]
- Somova, L.; Shode, F.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Baig, S.G.; Siddiq, A. Comparative effects of metformin and pioglitazone on lipid profile of rabbits. Pak. J. Pharm. Sci. 2015, 28, 553–555. [Google Scholar]
- Al-neaimy, K.S.A. Comparative effect of Metformin and Glibenclamide on Lipid profile in type 2 Diabetic Patients. Tikrit J. Pharm. Sci. 2011, 7, 46–50. [Google Scholar]
- Diamanti-Kandarakis, E.; Christakou, C.D.; Kandaraki, E.; Economou, F.N. Metformin: An old medication of new fashion: Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur. J. Endocrinol. 2010, 162, 193. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluations of cytotoxicity of eight antidiabetic medicinal plants and their effect on GLUT4 translocation. Evid. Based Complement. Altern. Med. 2013, 2013, 549345. [Google Scholar] [CrossRef]
- Lee, J.O.; Lee, S.K.; Jung, J.H.; Kim, J.H.; You, G.Y.; Kim, S.J.; Park, S.H.; Uhm, K.O.; Kim, H.S. Metformin induces Rab4 through AMPK and modulates GLUT4 translocation in skeletal muscle cells. J. Cell. Physiol. 2011, 226, 974–981. [Google Scholar] [CrossRef]
- Almeida, D.A.T.d.; Braga, C.P.; Novelli, E.L.B.; Fernandes, A.A.H. Evaluation of lipid profile and oxidative stress in STZ-induced rats treated with antioxidant vitamin. Braz. Arch. Biol. Technol. 2012, 55, 527–536. [Google Scholar] [CrossRef]
- Andallu, B.; Kumar, A.V.; Varadacharyulu, N.C. Lipid abnormalities in streptozotocin-diabetes: Amelioration by Morus indica L. cv Suguna leaves. Int. J. Diabetes Dev. Ctries. 2009, 29, 123. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Lee, S.K.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J. Biol. Chem. 2012, 287, 44121–44129. [Google Scholar] [CrossRef]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Hällsten, K.; Bjornholm, M.; Tsuchida, H.; Chibalin, A.V.; Virtanen, K.A.; Heinonen, O.J.; Lonnqvist, F.; Nuutila, P.; Zierath, J.R. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: A randomized controlled study. Diabetes 2005, 54, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Muhvić, D.; Grubić-Kezele, T.; Nikolić, M.; Šoić-Vranić, T.; Bajek, S. Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 8981. [Google Scholar] [CrossRef] [PubMed]



| Week | G1 | G2 | G3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D.W | 1% OLE | 3% OLE | Met. | D.W | 1% OLE | 3% OLE | Met. | N.T. | N.T. | N.T. | N.T. |
| 2 | D.W | 1% OLE | 3% OLE | Met. | D.W | 1% OLE | 3% OLE | Met. | N.T. | N.T. | N.T. | N.T. |
| 3 | D.W | 1% OLE | 3% OLE | Met. | STZ treatment + previous treatment. | STZ treatment only. | ||||||
| 4 | D.W | 1% OLE | 3% OLE | Met. | D.W | 1% OLE | 3% OLE | Met. | D.W | 1% OLE | 3% OLE | Met. |
| 5 | D.W | 1% OLE | 3% OLE | Met. | D.W | 1% OLE | 3% OLE | Met. | D.W | 1% OLE | 3% OLE | Met. |
| Experimental Groups | Initial Body Weight (g) | Final Body Weight (g) | Weight Changes (g) | |
|---|---|---|---|---|
| G1 | D.W G1 | 199.28 ± 15.39 | 255 ± 37.74 | 55.71 ± 27.14 e |
| 1% OLE G1 | 258.33 ± 18.34 | 319.16 ± 10 ef | 60.833 ± 21.77 e | |
| 3% OLE G1 | 220 ± 18.70 | 291 ± 28.80 f | 71 ± 16.73 e | |
| Met. G1 | 244.5 ± 30.97 | 280.83 ± 35.55 | 36.33 ± 10.80 e | |
| G2 | D.W G2 | 254 ± 51.64 | 252 ± 77.91 | −2 ± 50.81 |
| 1% OLE G2 | 265 ± 14.71 | 287.5 ± 45.73 | 22.5 ± 55.45 | |
| 3% OLE G2 | 283 ± 19.55 | 279 ± 63.38 | −4 ± 56.94 | |
| Met. G2 | 268.75 ± 11.81 | 285 ± 26.77 | 16.25 ± 18.90 | |
| G3 | D.W G3 | 254 ± 51.64 | 252 ± 77.91 | −2 ± 50.81 |
| 1% OLE G3 | 245 ± 14.71 | 196 ± 26.31 b | −49 ± 25.83 abcd | |
| 3% OLE G3 | 226.66 ± 36.56 | 235 ± 40.49 bc | 8.33 ± 26.39 | |
| Met. G3 | 258 ± 43.09 | 246 ± 32.86 | −12 ± 59.85 |
| % of Diabetic Rats | % of Nondiabetic Rats | |
|---|---|---|
| G2 Subgroups (1% OLE, 3% OLE) n = 14 | 35% a Five rats were not protected from STZ toxicity | 65% a Nine rats were protected from STZ toxicity |
| G3 Subgroups (1% OLE, 3% OLE) n = 14 | 57% b Eight rats were not protected from STZ toxicity | 43% b Six rats were protected from STZ toxicity |
| Experimental Groups | AS160 |
|---|---|
| D.W G1 | 1.60 ± 0.11 bcfghjkl |
| D.W G2 | 1.58 ± 0.033 bcfghjkl |
| 1% OLE G1 | 0.824 ± 0.025 acdefghi |
| 1% OLE G2 | 1.097 ± 0.0005 abcdeghil |
| 3% OLE G1 | 1.20 ± 0.017 abdefghikl |
| 3% OLE G2 | 0.98 ± 0.0038 abcdefhil |
| Met. G1 | 1.46 ± 0.055 bcfghjkl |
| Met. G2 | 0.57 ± 0.082 abcdefgil |
| D.W G3 | 1.66 ± 0.08 bcfghjkl |
| 1% OLE G3 | 0.92 ± 0.17 adehi |
| 3% OLE G3 | 0.79 ± 0.153 acdefi |
| Met. G3 | 0.62 ± 0.06 acdefgi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shudiefat, A.A.-R.; Alturk, H.; Al-Ameer, H.J.; Zihlif, M.; Alenazy, M. Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats. Appl. Sci. 2023, 13, 5939. https://doi.org/10.3390/app13105939
Al-Shudiefat AA-R, Alturk H, Al-Ameer HJ, Zihlif M, Alenazy M. Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats. Applied Sciences. 2023; 13(10):5939. https://doi.org/10.3390/app13105939
Chicago/Turabian StyleAl-Shudiefat, Abd Al-Rahman, Hadeel Alturk, Hamzeh J. Al-Ameer, Malek Zihlif, and Maha Alenazy. 2023. "Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats" Applied Sciences 13, no. 10: 5939. https://doi.org/10.3390/app13105939
APA StyleAl-Shudiefat, A. A.-R., Alturk, H., Al-Ameer, H. J., Zihlif, M., & Alenazy, M. (2023). Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats. Applied Sciences, 13(10), 5939. https://doi.org/10.3390/app13105939






