A Review Study on Total Ankle Replacement
Abstract
1. Introduction
1.1. Background
1.2. Contribution
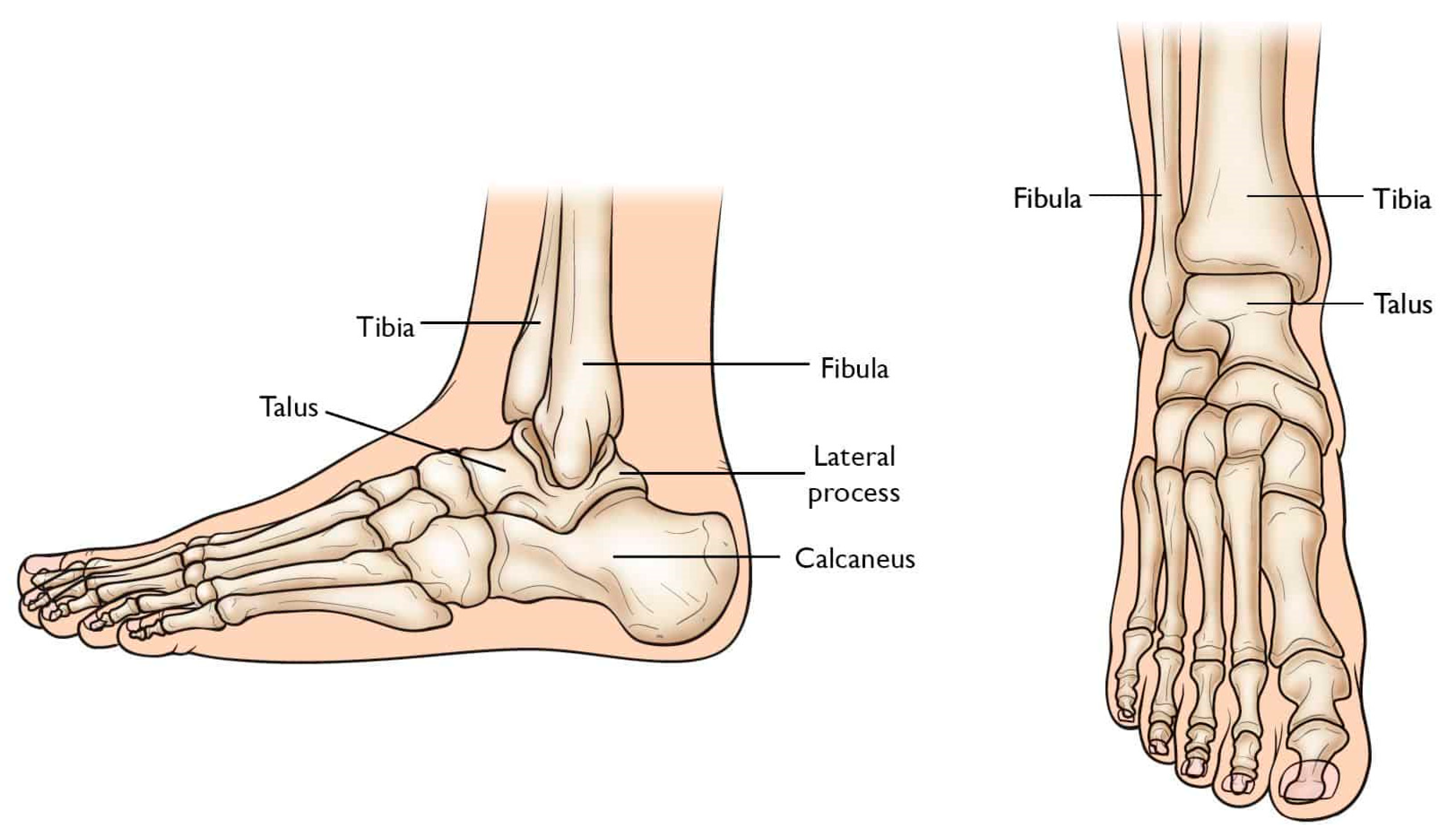
2. TAR System Study
2.1. Indications
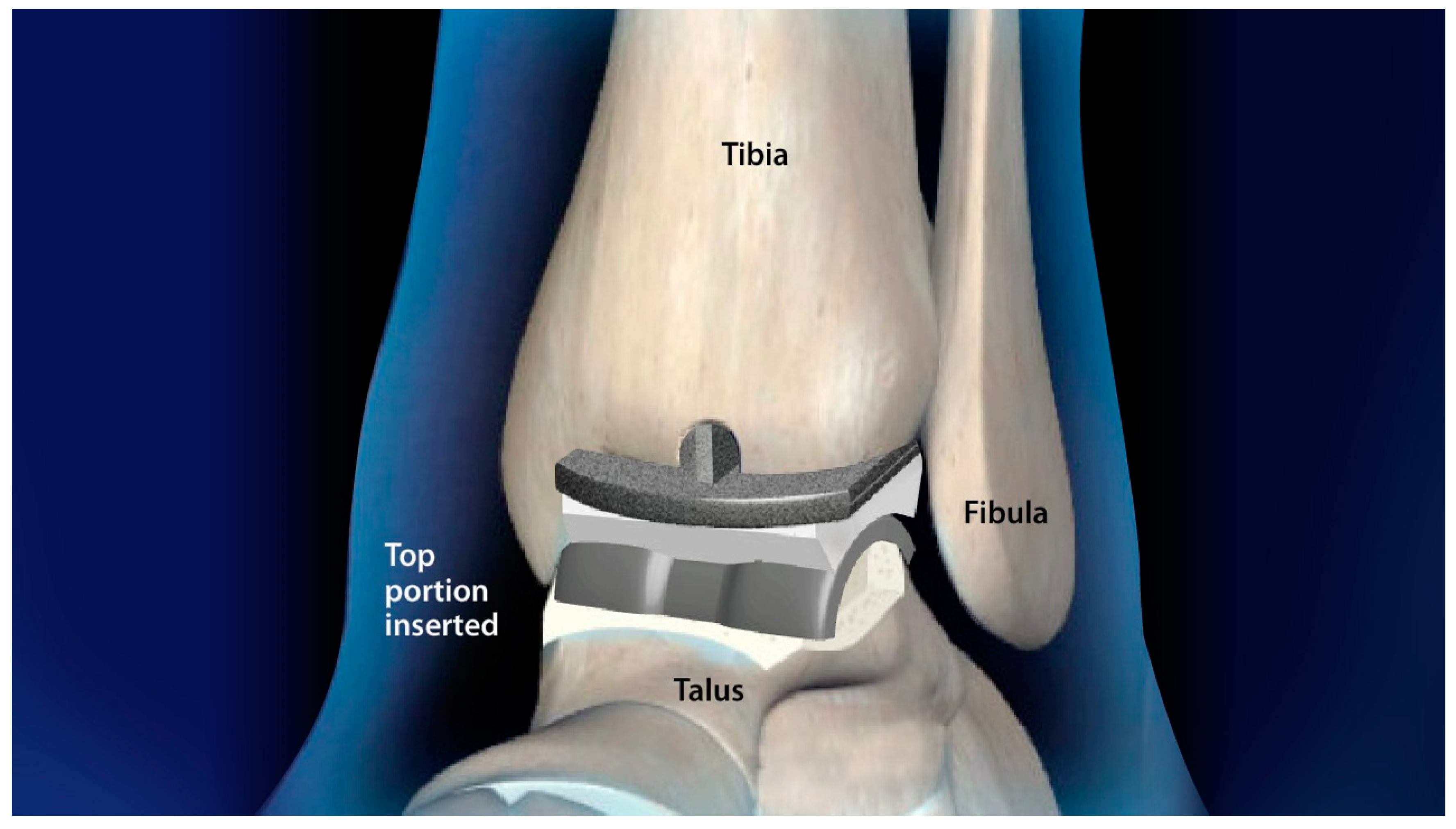
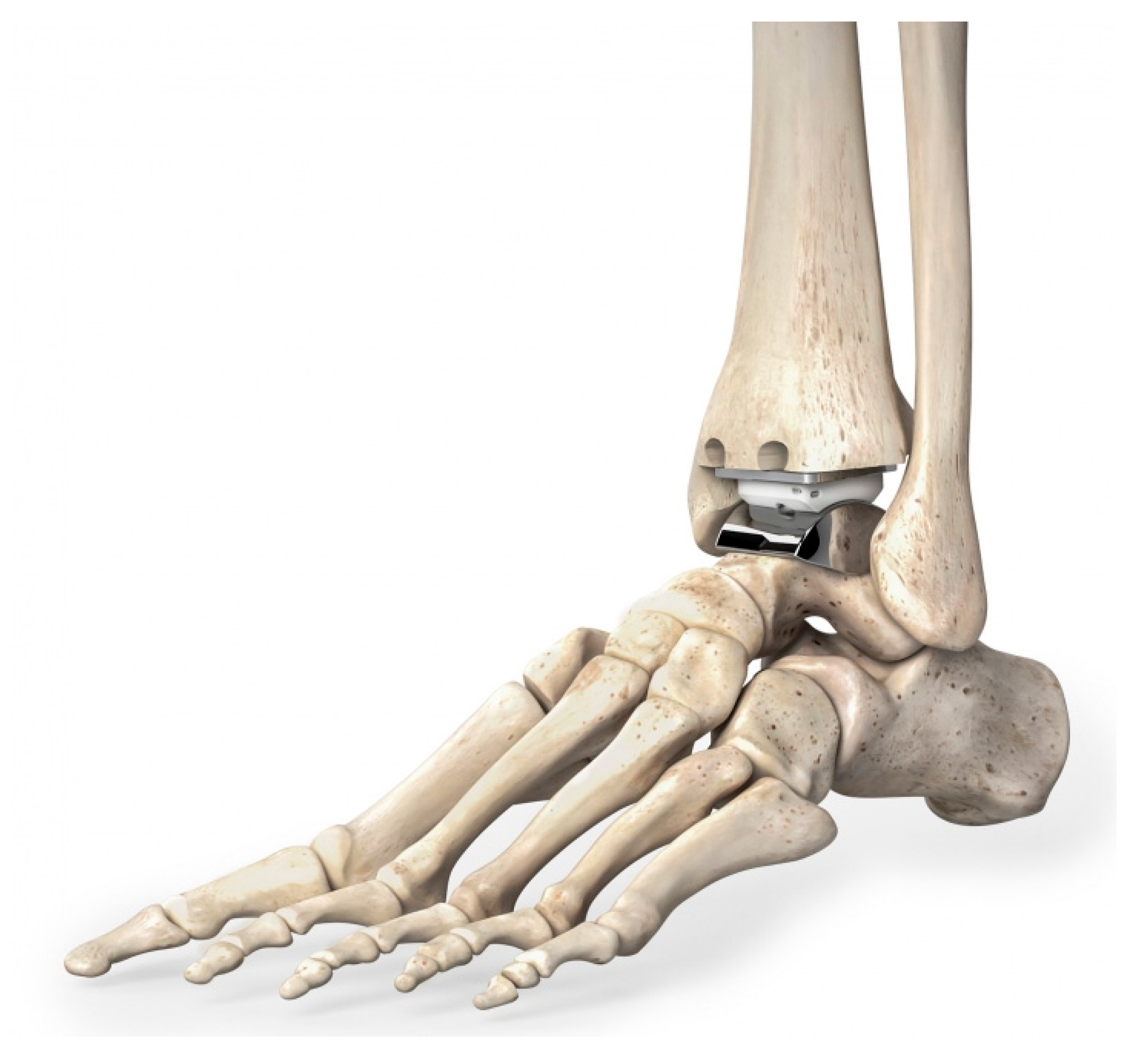
2.2. Component Number
2.3. Bearing Type
3. Revision TAR
3.1. Finite Element Modeling
3.2. Current Prostheses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blockett, C.L.; Graham, J.C. Biomechanics of the ankle. Orthop. Trauma 2016, 30, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, P.L.; Hamilton, W. Changes in tibiotalar area of contact caused by lateral talar shift. J. Bone Jt. Surg. Am. 1976, 58, 356–357. [Google Scholar] [CrossRef]
- Muir, D.C.; Amendola, A.; Saltzman, C.L. Long-term outcome of ankle arthrodesis. Foot Ankle Clin. 2002, 7, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Coester, L.M.; Saltzman, C.L.; Leupold, J.; Pontarelli, W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J. Bone Jt. Surg. Am. 2001, 83, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Heuvel, A.; van bouwel, S.; Dereymaeker, G. Total ankle replacement Design evolution and results. Acta Orthop. Belg. 2010, 76, 150–161. [Google Scholar]
- Nathan, C.H.; Sang-Hyun, P.; Patricia, C.; Douglas, W.V.C.; Edward, E.; Sophia, S. Damage patterns in polyethylene fixed bearings of retrieved total ankle replacements. Foot Ankle Surg. 2021, 27, 316–320. [Google Scholar] [CrossRef]
- Hordyk, P.J.; Fuerbringer, B.A.; Roukis, T.S. Sagittal Ankle and Midfoot Range of Motion Before and After Revision Total Ankle Replacement: A Retrospective Comparative Analysis. Foot Ankle Surg. 2018, 57, 521–526. [Google Scholar] [CrossRef]
- Brodsky, J.W.; Kane, J.M.; Coleman, S.; Bariteau, J.; Tenenbaum, S. Abnormalities of gait caused by ankle arthritis are improved by ankle arthrodesis. Bone Jt. J. 2016, 98, 1369–1375. [Google Scholar] [CrossRef]
- Flavin, R.; Coleman, S.C.; Tenenbaum, S.; Brodsky, J.W. Comparison of gait after total ankle arthroplasty and ankle arthrodesis. Foot Ankle Int. 2013, 34, 1340–1348. [Google Scholar] [CrossRef]
- Henne, T.D.; Anderson, J.G. Total ankle arthroplasty: A historical perspective. Foot Ankle Clin. 2002, 7, 695–702. [Google Scholar] [CrossRef]
- Gougoulias, N.; Khanna, A.; Maffulli, N. History and evolution in total ankle arthroplasty. Br. Med. Bull. 2008, 89, 111–151. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, D.E.; Dettoni, F.; Femino, J.E.; Phisitkul, P.; Germano, M.; Amendola, A. Total ankle replacement: Why, when and how? Iowa Orthop. J. 2010, 30, 119–130. [Google Scholar] [PubMed]
- Usuelli, F.G.; Maccario, C.; D’Ambrosi, R.; Surace, M.F.; Vulcano, E. Age-related outcome of mobile- bearing total ankle replacement. Orthopedics 2017, 40, e567–e573. [Google Scholar] [CrossRef] [PubMed]
- Easley, M.E.; Vertullo, C.J.; Urban, W.C.; Nunley, J.A. Total ankle arthroplasty. J. Am. Acad. Orthop. Surg. 2002, 10, 157–167. [Google Scholar] [CrossRef]
- Tenenbaum, S.; Bariteau, J.; Coleman, S.; Brodsky, J. Functional and clinical outcomes of total ankle arthroplasty in elderly compared to younger patients. Foot Ankle Surg. 2017, 23, 102–107. [Google Scholar] [CrossRef]
- Demetracopoulos, C.A.; Adams, S.B., Jr.; Queen, R.M.; DeOrio, J.K.; Nunley, J.M.; Easley, M.E. Effect of age on outcomes in total ankle arthroplasty. Foot Ankle Int. 2015, 36, 871–880. [Google Scholar] [CrossRef]
- Queen, R.M.; Franck, C.T.; Schmitt, D.; Adams, S.B. Are there differences in gait mechanics in patients with a fixed versus mobile bearing total ankle arthroplasty? A randomized trial. Clin. Orthop. Relat. Res. 2017, 475, 2599–2606. [Google Scholar] [CrossRef]
- Martinelli, N.; Baretta, S.; Pagano, J.; Bianchi, A.; Villa, T.; Casaroli, G.; Galbusera, F. Contact stresses, pressure and area in a fixed-bearing total ankle replacement: A finite element analysis. BMC Musculoskelet Disord. 2017, 18, 493. [Google Scholar] [CrossRef]
- Protheroe, D.; Mulroy, M. An update on Total Ankle Replacement survivorship rates and future directions for patient selection. Sport. Orthop. Traumatol. 2020, 36, 60–69. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Muras, J.; Oliva, X.M.; Amado, P. Total ankle replacement in patients under the age of 50. Should the indications be revised? Foot Ankle Surg. 2013, 19, 229–233. [Google Scholar] [CrossRef]
- Wansbrough, G.; Sharp, R.; Cooke, P. Total ankle replacement in juvenile chronic arthritis. Orthop. Proc. 2012, 94, 32. [Google Scholar]
- Roxa, R.; Nicola, K.; Roman, S.; Tamara, H.; Alexej, B.; Beat, H. Ankle range of motion following 3-component total ankle arthroplasty. Foot Ankle Int. 2021, 42, 31–37. [Google Scholar]
- James, W.B.; Justin, M.K.; Akira, T.; Scott, C.; Yahya, D. Role of total ankle arthroplasty in stiff ankles. Foot Ankle Int. 2017, 38, 1070–1077. [Google Scholar]
- James, W.B.; Justin, M.K.; Andrew, W.P.; David, D.V.; Scott, C.; Yahya, D. Role of Total Ankle Arthroplasty in Stiff Ankles–Long Term Follow-Up. Foot Ankle Int. 2019, 4, 2473011419S00013. [Google Scholar]
- David, E.D.; Scott, A.M.; Christopher, N.C.; Natalie, A.G.; Timothy, S.B.; Nicholas, A.B. What is the Impact of Body Mass Index Cutoffs on Total Knee Arthroplasty Complications? Arthroplasty 2022, 37, 683–687. [Google Scholar]
- Jaiben, G.; Nicolas, S.P.; Mitchell, N.; Nipun, S.; Anton, A.K.; Michael, A.M. Association between body mass index and thirty-day complications after total knee arthroplasty. Arthroplasty 2018, 33, 865–871. [Google Scholar]
- Oliver, N.S.; Sahitya, K.D.; Ying, Z.; Steven, L.H. The impact of obesity on the outcome of total ankle replacement. Foot Ankle Int. 2015, 97, 904–910. [Google Scholar]
- Alexej, B.; Markus, K.; Andrew, E.A.; Beat, H. Total ankle replacement in obese patients: Component stability, weight change, and functional outcome in 118 consecutive patients. Foot Ankle Int. 2011, 32, 925–932. [Google Scholar]
- Haskell, A.; Mann, R.A. Ankle arthroplasty with preoperative coronal plane deformity: Short-term results. Clin. Orthop. Relat. Res.® 2004, 424, 98–103. [Google Scholar]
- Hanselman, A.E.; Powell, B.D.; Santrock, R.D. Total ankle arthroplasty with severe preoperative varus deformity. Orthopedics 2015, 38, e343–e346. [Google Scholar] [CrossRef]
- Sung, K.S.; Ahn, J.; Lee, K.H.; Chun, T.H. Short-term results of total ankle arthroplasty for end-stage ankle arthritis with severe varus deformity. Foot Ankle Int. 2014, 35, 225–231. [Google Scholar] [CrossRef]
- Queen, R.M.; Adams, S.B., Jr.; Viens, N.A.; Friend, J.K.; Easley, M.E.; Deorio, J.K.; Nunley, J.A. Differences in outcomes following total ankle replacement in patients with neutral alignment compared with tibiotalar joint malalignment. Bone Jt. Surg Am. 2013, 95, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Roberto, A.B.; Mark, A.P.; Christopher, F.H. Current and Emerging Insights on Total Ankle Replacement. Podiatry Today. HMP Glob. Learn. Netw. 2018. Available online: https://www.hmpgloballearningnetwork.com/site/podiatry/current-and-emerging-insights-total-ankle-replacement. (accessed on 29 November 2022).
- Mann, J.A.; Mann, R.A.; Horton, E. STAR™ ankle: Long-term results. Foot Ankle Int. 2011, 32, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Jastifer, J.R.; Coughlin, M.J. Long-term follow-up of mobile bearing total ankle arthroplasty in the United States. Foot Ankle Int. 2015, 36, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; DeOrio, J. The INBONE Total Ankle Replacement. Oper. Tech. Orthop. 2010, 20, 201–210. [Google Scholar] [CrossRef]
- Giada, L.; Paolo, C.; Alberto, L.; Maurizio, O.; Antonio, M.; Sandro, G.; Lisa, B. Retrospective comparison between a two- and three-component ankle arthroplasty: Clinical and functional evaluation via gait analysis. Clin. Biomech. 2020, 80, 105180. [Google Scholar] [CrossRef]
- Leardini, A.; O’Connor, J.J.; Giannini, S. Biomechanics of the natural, arthritic, and replaced human ankle joint. Foot Ankle Res. 2014, 7, 8. [Google Scholar] [CrossRef]
- Helka, K.; Sami, K.; Ia, K.; Hannu, T. The motion between components in a mobile-bearing total ankle replacement measured by cone-beam CT scanning. Foot Ankle Surg. 2022, 28, 324–330. [Google Scholar] [CrossRef]
- Stephen, A.B.; Garrett, M.W.; Melissa, M.G.; Scott, T.B.; Nicole, M.P. Evaluating Component Migration After Modular Stem Fixed-Bearing Total Ankle Replacement. Foot Ankle Surg. 2015, 54, 326–331. [Google Scholar] [CrossRef]
- Paul-André, D.; Alexandre, N.; Laurence, C.; Raphaël, D.; Bernhard, D.B.; Ivan, B.; Jean-Luc, B.; Thibaut, L. Changes in ankle and foot kinematic after fixed-bearing total ankle replacement. Biomechanics 2022, 136, 111060. [Google Scholar] [CrossRef]
- Anders, H.; Stanislav, P.; Urban, R. Six-year results of the Rebalance mobile bearing total ankle replacement. Foot Ankle Surg. 2021, 27, 66–69. [Google Scholar] [CrossRef]
- Bradley, J.E.; Dinesh, G.; Tarun, G. Finite element analysis of stress and wear characterization in total ankle replacements. Mech. Behav. Biomed. Mater. 2014, 34, 134–145. [Google Scholar] [CrossRef]
- Johnson-Lynn, S.; Siddique, M. The Effect of Sagittal and Coronal Balance on Patient-Reported Outcomes Following Mobile-Bearing Total Ankle Replacement. Foot Ankle Surg. 2019, 58, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Lee, J.; Choi, W.J.; Lee, J.W. Periprosthetic osteolysis after total ankle arthroplasty. Foot Ankle Int. 2014, 35, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nunley, J.A.; Adams, S.B.; Easley, M.E.; DeOrio, J.K. Prospective Randomized Trial Comparing Mobile-Bearing and Fixed-Bearing Total Ankle Replacement. Foot Ankle Int. 2019, 40, 1239–1248. [Google Scholar] [CrossRef]
- Matthew, G.S.; Cindy, L.G.; Samuel, B.A.; James, K.D.; Mark, E.E.; James, A.N. Midterm results of the Salto Talaris total ankle arthroplasty. Foot Ankle Int. 2017, 38, 1215–1221. [Google Scholar] [CrossRef]
- Pagenstert, G.; Wimmer, M.D.; Jacxsens, M.; Saltzman, C.L.; Barg, A. Aseptic loosening of total ankle replacement: One-stage revision ankle arthroplasty. Oper. Orthop. Traumatol. 2017, 29, 220–235. [Google Scholar] [CrossRef]
- Georg, H.; Reinhard, H.; Markus, K.; Jan, L.; Lukas, L.; Roman, R.; Andreas, L.; Patrick, S. Revision Rates After Total Ankle Replacement: A Comparison of Clinical Studies and Arthroplasty Registers. Foot Ankle Int. 2022, 43, 176–185. [Google Scholar]
- Zaidi, R.; Macgregor, A.J.; Goldberg, A. Quality measures for total ankle replacement, 30-day readmission and reoperation rates within 1 year of surgery: A data linkage study using the NJR data set. BMJ Open 2016, 6, e011332. [Google Scholar] [CrossRef]
- Jung, W.L.; Woo-Young, I.; Si, Y.; Jae-Young, C.; Sung, J.K. Analysis of early failure rate and its risk factor with 2157 total ankle replacements. Sci. Rep. 2022, 11, 1901. [Google Scholar]
- Christian, S.; Matthias, L.; Stella, S.; Manuel, G.; Lorenz, P.; Tobias, G.; Antonio, K.; Matthias, C.K. Revision rate of total ankle replacement in patients younger than 50 years. Foot Ankle Surg. 2017, 23, 28. [Google Scholar] [CrossRef]
- Paul, M.R.; Michael, W.D.; David, F.; John, K. Dynamic Ultrasonography: A Cadaveric Model for Evaluating Aseptic Loosening of Total Ankle Arthroplasty. Foot Ankle Surg. 2013, 52, 311–314. [Google Scholar] [CrossRef]
- Rohit, D.; Jake, T.; Vikas, S.; Ramesh, K.N. Tri-Component, Mobile Bearing, Total Ankle Replacement: Mid-term Functional Outcome and Survival. Foot Ankle Surg. 2012, 51, 566–569. [Google Scholar] [CrossRef]
- Roukis, T.; Prissel, M. Management of Extensive Talar Osteolysis with Agility™ Total Ankle Replacement Systems Using Geometric Metal-reinforced Polymethylmethacrylate Cement Augmentation. Foot Ankle Surg. 2014, 53, 108–113. [Google Scholar] [CrossRef]
- Dharia, M.A.; Snyder, S.; Bischoff, J.E. Computational Model Validation of Contact Mechanics in Total Ankle Arthroplasty. Orthop Res. 2020, 38, 1063–1069. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, R. Experimental and finite element investigation of total ankle replacement: A review of literature and recommendations. Orthopaedics 2020, 18, 41–49. [Google Scholar] [CrossRef]
- Alexandre, T.; Xabier, L.; Jonas, G.; Xavier, C. Development and experimental validation of a finite element model of total ankle replacement. Biomechanics 2014, 47, 742–745. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wong, D.W.; Cheng, C.K.; Zhang, M. Finite element analysis of biomechanical effects of total ankle arthroplasty on the foot. Orthop Translat. 2017, 12, 55–65. [Google Scholar] [CrossRef]
- Federico, G.U.; Luigi, M.; Francesco, G.; Camilla, M.; Christian, I.; Alexander, M.C.; Christopher, E.G. Previous Surgery and the Temporal Evolution of Functional Outcomes and Complication Rates in Total Ankle Replacement. Foot Ankle Surg. 2022, 61, 572–576. [Google Scholar] [CrossRef]
- Wood, P.; Clough, T.; Smith, R. The present state of ankle arthroplasty. Foot Ankle Surg. 2008, 14, 115–119. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Chen, W.M.; Zhao, D.; Chu, P.; Wang, S.; Huang, J.; Wang, X.; Ma, X. Finite-element analysis of the influence of tibial implant fixation design of total ankle replacement on bone-implant interfacial biomechanical performance. Orthop. Surg. 2020, 28, 2309499020966125. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, B.; Leardini, A.; Corazza, F.; Taylor, M. Finite element analysis of a total ankle replacement during the stance phase of gait. Biomechanics 2006, 39, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Paul-André, D.; Alexandre NThibaut, L.; Raphaël, D.; Bernhard, D.B.; Jean-Luc, B.; Xavier, C.; Laurence, C. Impact of foot modeling on the quantification of the effect of total ankle replacement: A pilot study. Gait Posture 2021, 84, 308–314. [Google Scholar] [CrossRef]
- Collet, A. Top Ankle Replacement Implants in the United States. IData Res. 2021. Available online: https://idataresearch.com/top-ankle-replacement-implants/ (accessed on 29 November 2022).
- Choi, J.H.; Coleman, S.C.; Tenenbaum, S.; Polo, F.E.; Brodsky, J.W. Prospective study of the effect on gait of a two-component total ankle replacement. Foot Ankle Int. 2013, 34, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Cottom, J.; Plemmons, B.; Douthett, S. Relationship Between Body Mass Index and Complications in Total Ankle Arthroplasty: A Single Surgeon’s Experience in Ninety-Seven Replacements. Foot Ankle Surg. 2019, 58, 687–691. [Google Scholar] [CrossRef]
- Seth, A. A review of the STAR prosthetic system and the biomechanical considerations in total ankle replacements. Foot Ankle Surg. 2011, 17, 64–67. [Google Scholar] [CrossRef]
- Daniels, T.R.; Mayich, D.J.; Penner, M.J. Intermediate to long-term outcomes of total ankle replacement with the Scandinavian Total Ankle Replacement (STAR). Bone Jt. Surg Am. 2015, 97, 895–903. [Google Scholar] [CrossRef]
- Koivu, H.; Kohonen, I.; Mattila, K.; Loyttyniemi, E.; Tiusanen, H. Long-term results of Scandinavian total ankle replacement. Foot Ankle Int. 2017, 38, 723–731. [Google Scholar] [CrossRef]
- Scott, R.T.; Witt, B.L.; Hyer, C.F. Design comparison of the INBONE I versus INBONE II total ankle system. Foot Ankle Spec. 2013, 6, 137–140. [Google Scholar] [CrossRef]
- Hsu, A.R.; Davis, W.H.; Cohen, B.E.; Jones, C.P.; Ellington, J.K.; Anderson, R.B. Radiographic outcomes of preoperative CT scan-derived patient-specific total ankle arthroplasty. Foot Ankle Int. 2015, 36, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Daigre, J.; Berlet, G.; Van Dyke, B.; Peterson, K.S.; Santrock, R. Accuracy and reproducibility using patient-specific instrumentation in total ankle arthroplasty. Foot Ankle Int. 2017, 38, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.S.; Matson, A.P.; Nwachukwu, B.U.; Scott, D.J.; Mather, R.C.; DeOrio, J.K. Determining the cost-savings threshold and alignment accuracy of patient-specific instrumentation in total ankle replacements. Foot Ankle Int. 2017, 38, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Basile, P.; Miner, S.; Crafton, J.; McKenna, B. Preliminary Report of a Hybrid Total Ankle Arthroplasty Combining a Stemmed Intramedullary Tibial Component With Chamfer-Cut Talar Dome. Foot Ankle Surg. 2021, 61, E25–E33. [Google Scholar] [CrossRef]
- Assaf, A.; Susan, M.G.; Patrick, P.; Dan, C.; Laura, E.; Ruth, E.C.; Monika, V. Total ankle arthroplasty results using fixed bearing CT-guided patient specific implants in posttraumatic versus nontraumatic arthritis. Foot Ankle Surg. 2022, 28, 222–228. [Google Scholar] [CrossRef]
- Barg, A.; Wiewiorski, M.; Valderrabano, V. Aseptic loosening of total ankle replacement: Two-stage revision with bone augmentation of osseous defects and secondary prosthesis implantation. Oper. Orthop. Traumatol. 2017, 29, 236–252. [Google Scholar] [CrossRef]
- Karl, M.S.; Samuel, B.A.; Mark, E.E.; James, K.D.; James, A.N. Total ankle arthroplasty with a modern fixed-bearing system: The Salto Talaris prosthesis. JBJS Essent. Surg. Tech. 2013, 3, e18. [Google Scholar] [CrossRef]
- Jonathan, D.; Jaeyoung, K.; Martin, J.O.; Constantine, A.D.; Jonathan, G.; Austin, S.; Andrew, R.; Jonathan, T.D.; David, S.L.; Scott, J.E. Radiographic and clinical outcomes of the Salto Talaris total ankle arthroplasty. Foot Ankle Int. 2020, 41, 1519–1528. [Google Scholar]
- Bischoff, J.E.; Schon, L.; Saltzman, C. Influence of geometry and depth of resections on bone support for total ankle replacement. Foot Ankle Int. 2017, 38, 1026–1034. [Google Scholar] [CrossRef]
- King, M.; Vesely, B.; Scott, A. Early outcomes with the vantage total ankle prosthesis. Foot Ankle Surg. Tech. Rep. Cases 2022, 2, 100121. [Google Scholar] [CrossRef]
- Brianna, F.; Daniel, O.C.; Ryan, G.R.; David, I.P.; Justin, T. Short-Term Complications and Outcomes of the Cadence Total Ankle Arthroplasty. Foot Ankle Int. 2022, 43, 371–377. [Google Scholar] [CrossRef]
- DeOrio, J.K.; Nunley, J.A.; Easley, M.; Valerrabano, V. Vantage Total Ankle Replacement. In Primary and Revision Total Ankle Replacement; Springer: Cham, Switzerland, 2021; pp. 151–163. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noori, N.B.; Ouyang, J.Y.; Noori, M.; Altabey, W.A. A Review Study on Total Ankle Replacement. Appl. Sci. 2023, 13, 535. https://doi.org/10.3390/app13010535
Noori NB, Ouyang JY, Noori M, Altabey WA. A Review Study on Total Ankle Replacement. Applied Sciences. 2023; 13(1):535. https://doi.org/10.3390/app13010535
Chicago/Turabian StyleNoori, Naudereh B., Jessica Yi Ouyang, Mohammad Noori, and Wael A. Altabey. 2023. "A Review Study on Total Ankle Replacement" Applied Sciences 13, no. 1: 535. https://doi.org/10.3390/app13010535
APA StyleNoori, N. B., Ouyang, J. Y., Noori, M., & Altabey, W. A. (2023). A Review Study on Total Ankle Replacement. Applied Sciences, 13(1), 535. https://doi.org/10.3390/app13010535





