Microfabrication Bonding Process Optimization for a 3D Multi-Layer PDMS Suspended Microfluidics
Abstract
:1. Introduction
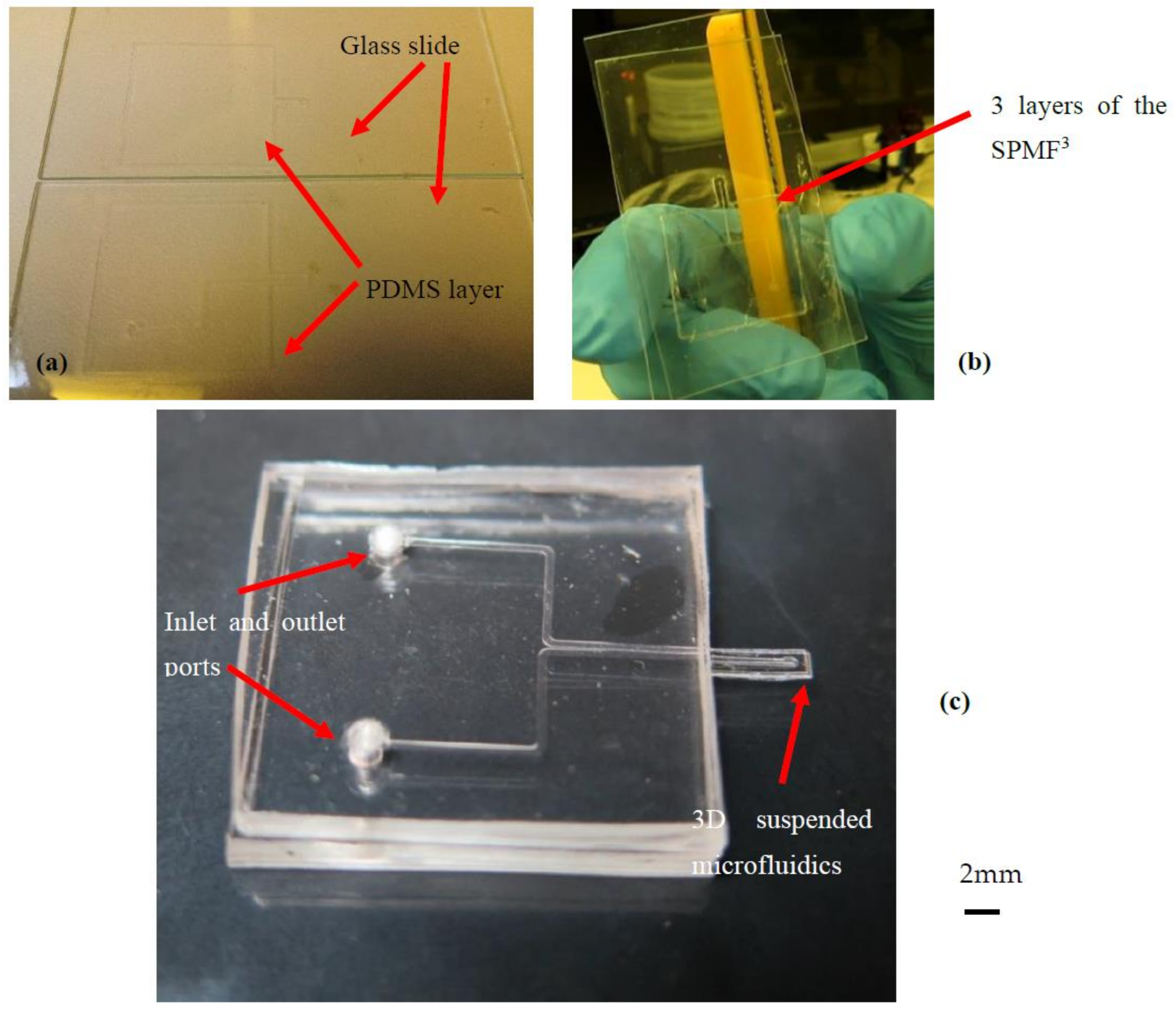
2. 3D Suspended Polymeric Microfluidic Resonator Fabrication (SPMF3)
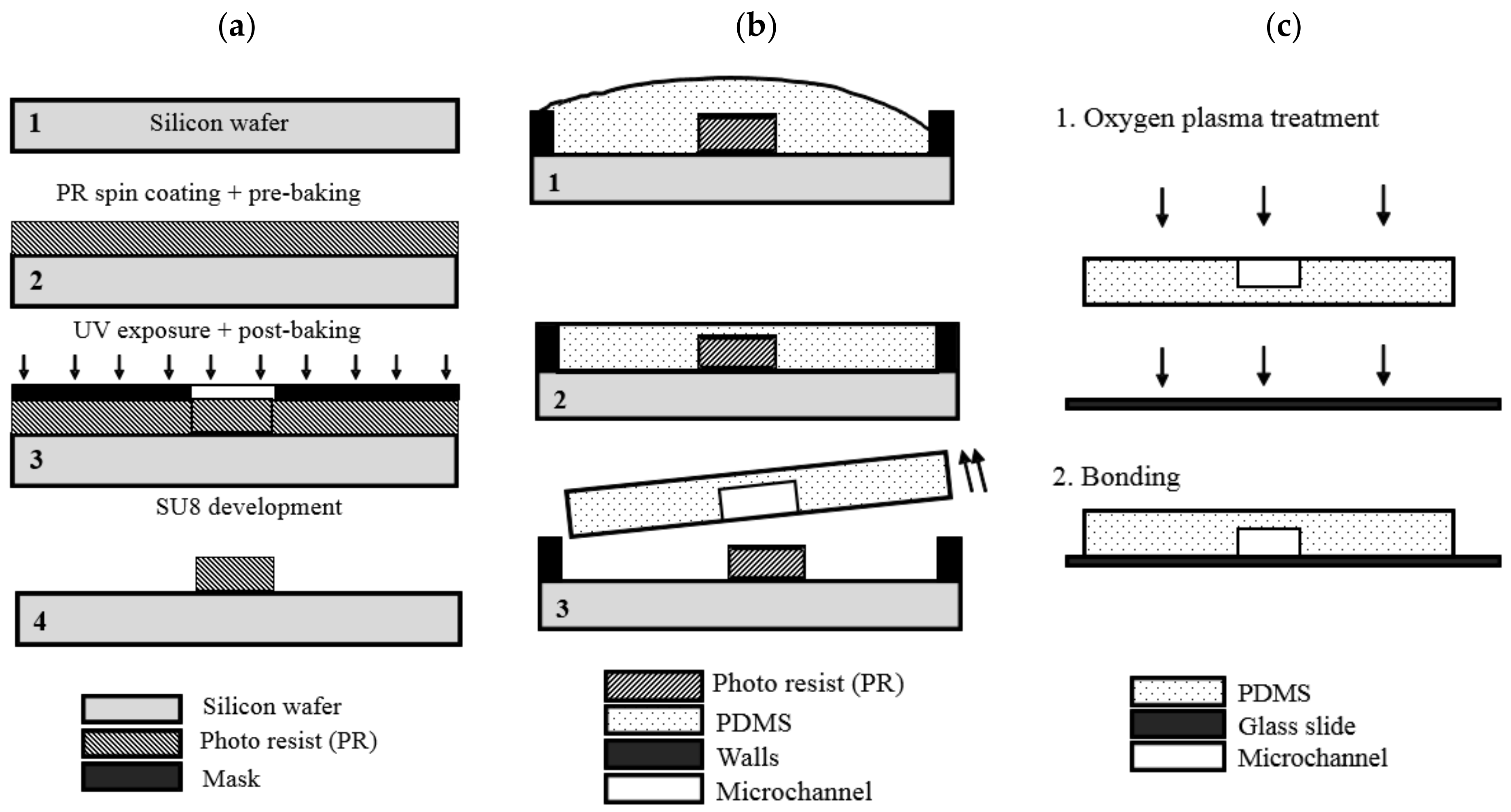
2.1. Fabrication Method
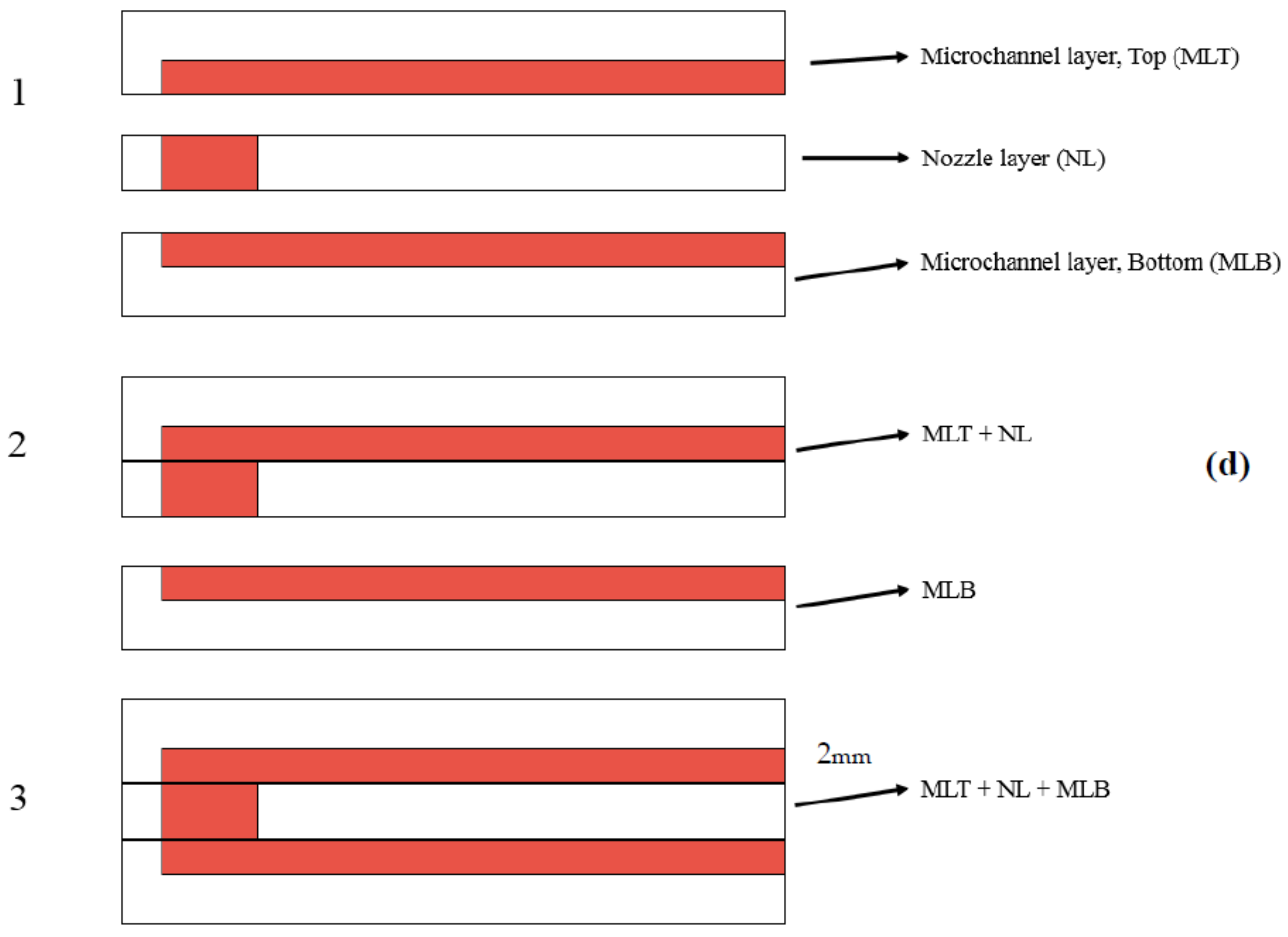
2.2. Fabrication Steps
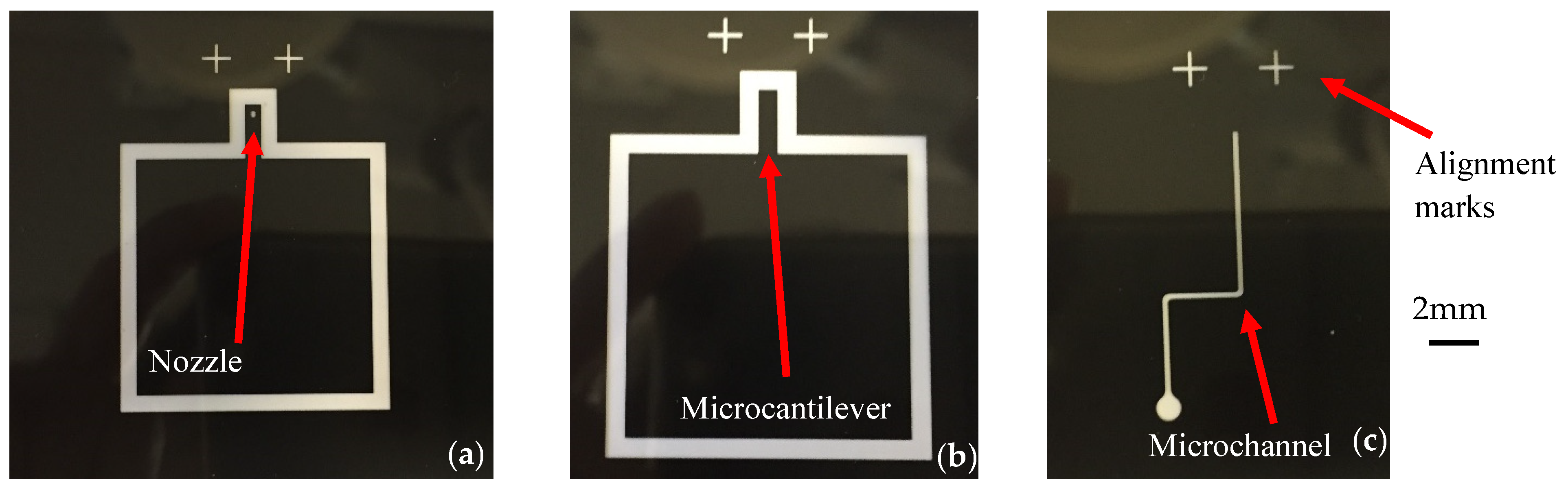
2.2.1. Mold Fabrication
2.2.2. PDMS Layers Fabrication Procedure
2.2.3. Bonding Procedure
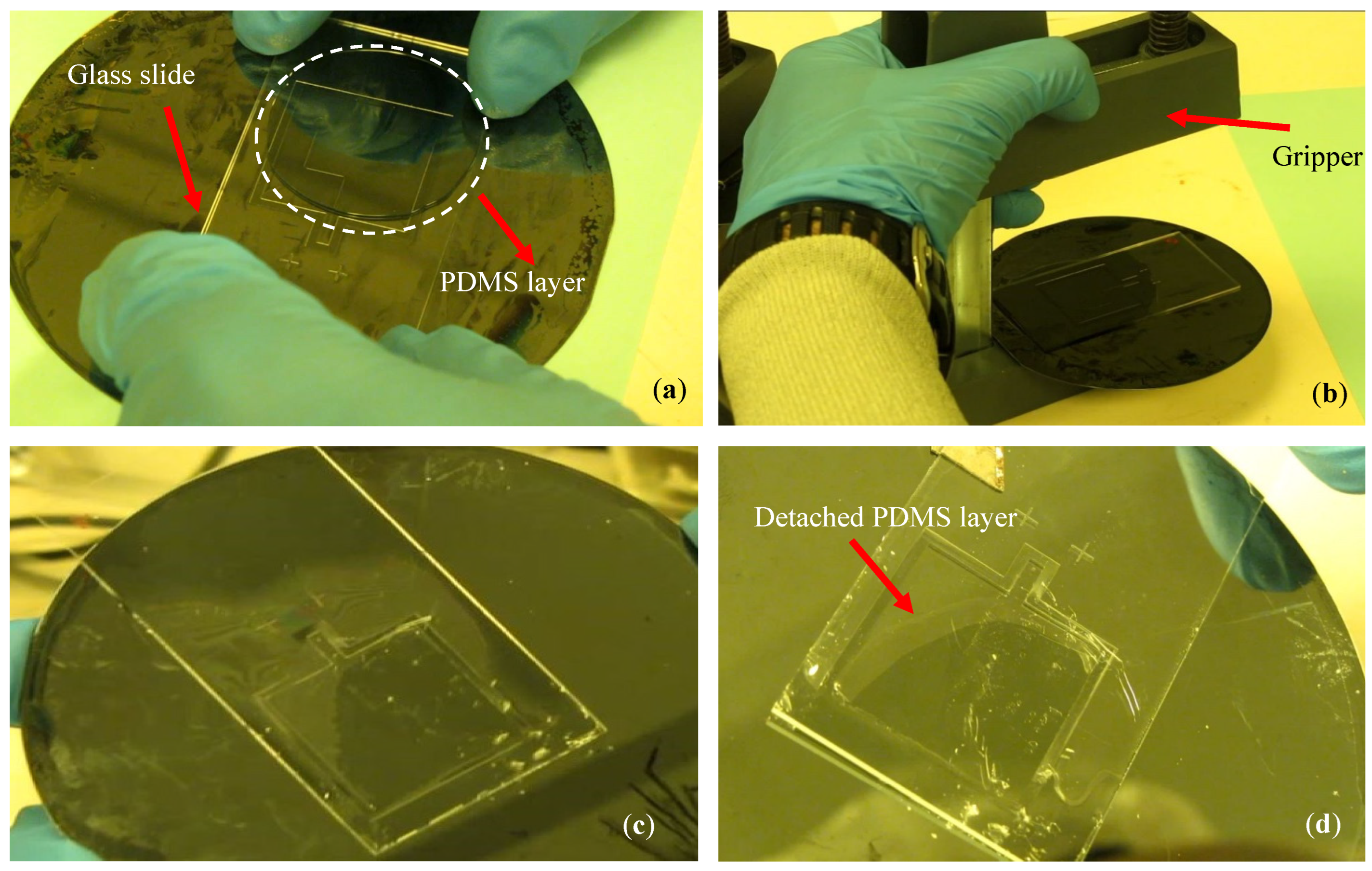
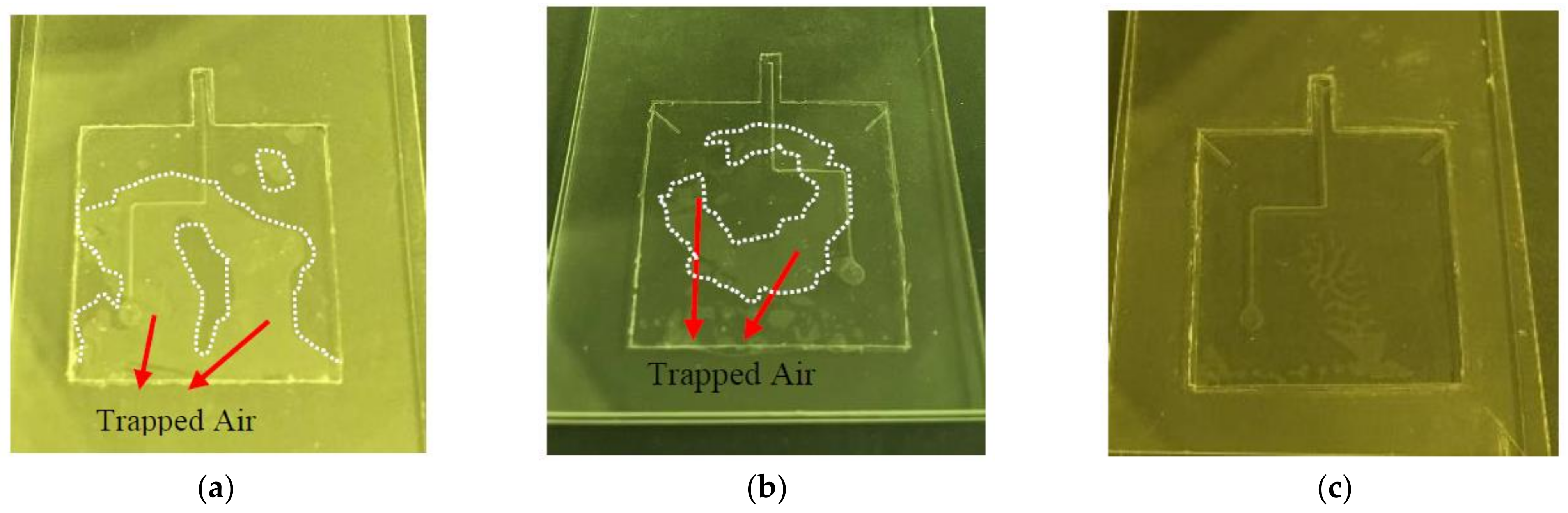
2.3. Fabrication Issues
2.3.1. Air-Vent PDMS Fabrication Method
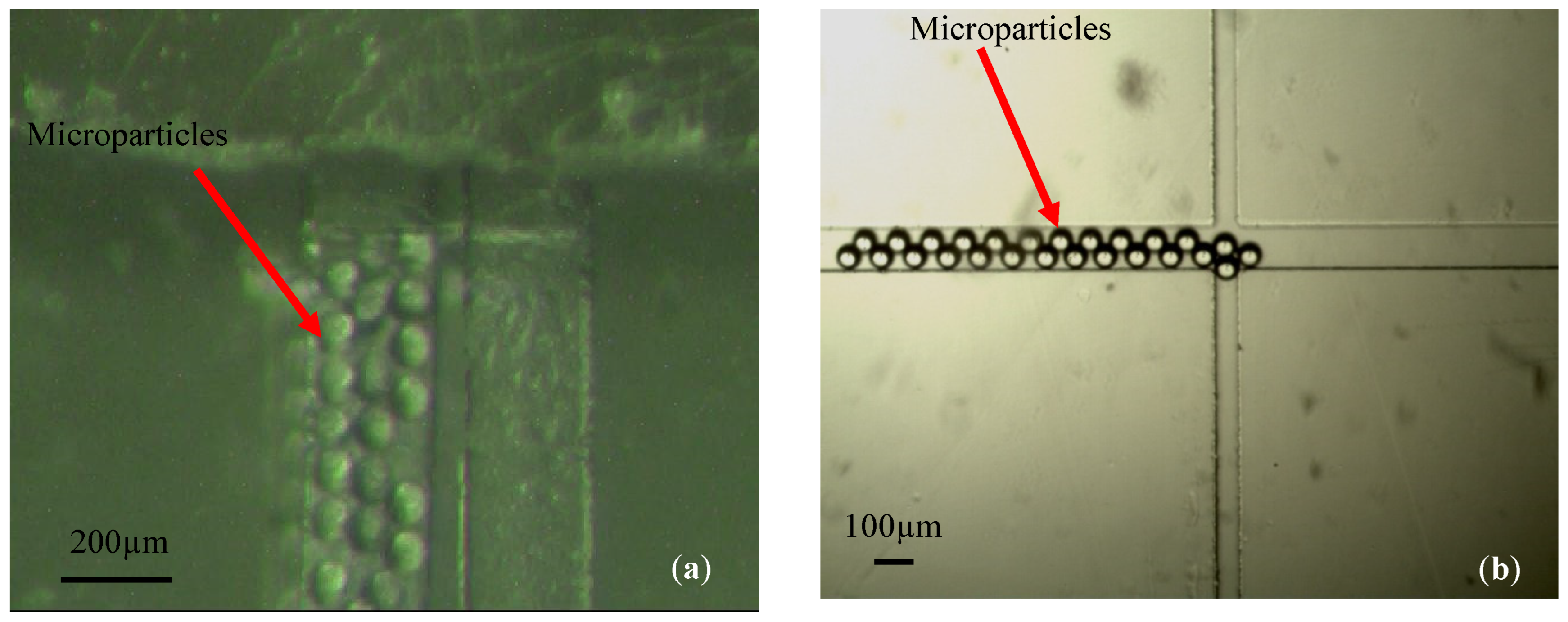
2.3.2. Particle Stickiness
2.3.3. Bonding Strength
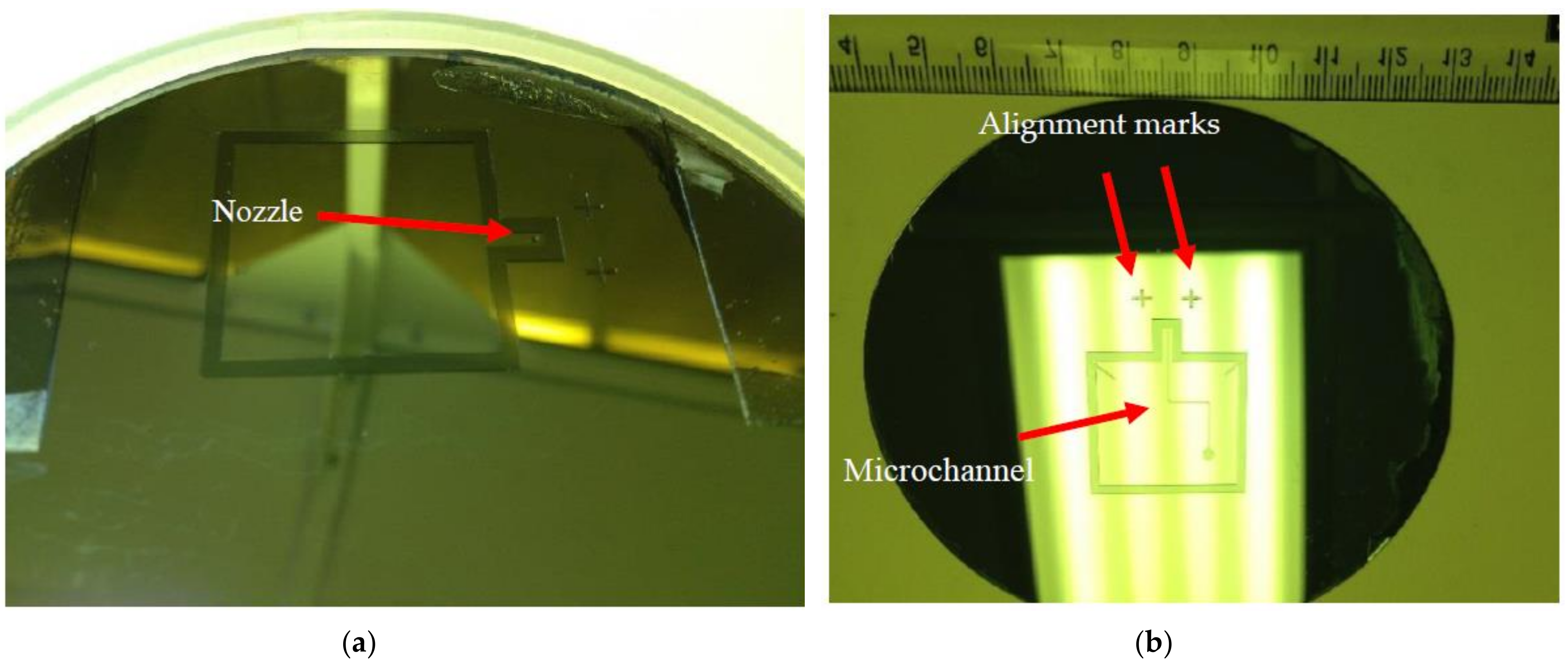
2.3.4. Alignment between PDMS Layers
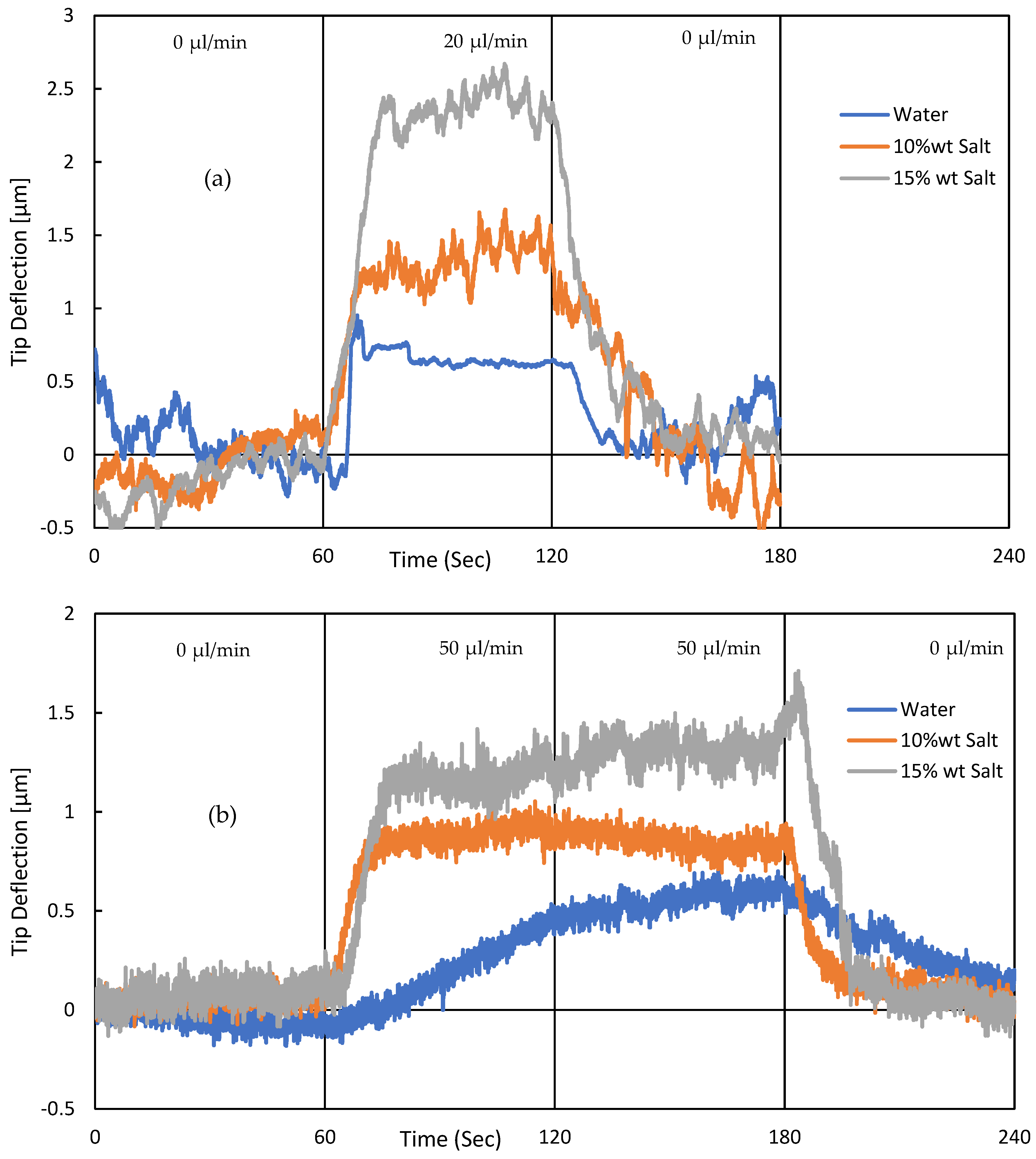
3. Experimental Validation of the SPMF3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRos, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, S.; Pinti, M.; Bhushan, B. Theory, fabrication and applications of microfluidic and nanofluidic biosensors. Phil. Trans. R. Soc. A 2012, 370, 2269–2303. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Manz, A.; Fan, Z.; Ludi, H.; Widmer, H.M. Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal. Chem. 1992, 64, 1926–1932. [Google Scholar] [CrossRef]
- Jacobson, S.C.; Hergenroder, R.; Koutny, L.B.; Warmack, R.J.; Ramsey, J.M. Effects of injection schemes and column geometry on the performance of microchip electrophoresis devices. Anal. Chem. 1994, 66, 1107–1113. [Google Scholar] [CrossRef]
- Tong, Q.-Y.; Gosele, U. Semiconductor Wafer Bonding: Science and Technology; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Sparks, D.; Queen, G.; Weston, R.; Woodward, G.; Putty, M.; Jordan, L.; Zarabadi, S.; Jayakar, K. Wafer-to-wafer bonding of nonplanarized MEMS surfaces using solder. J. Micromech. Microeng. 2001, 11, 630–634. [Google Scholar] [CrossRef]
- Iyer, S.S.; Auberton-Herve, A.J. Silicon Wafer Bonding Technology for VLSI and MEMS; INSPEC: London, UK, 2002. [Google Scholar]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Quake, S.R.; Scherer, A. From micro- to nanofabrication with soft materials. Science 2000, 290, 1536–1540. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Odom, T.W.; Chiu, D.T.; Whitesides, G.M. Fabrication of complex three-dimensional microchannel systems in PDMS. J. Am. Chem. Soc. 2003, 125, 554–559. [Google Scholar] [CrossRef]
- Samel, B.; Chowdhury, M.K.; Stemme, G. The fabrication of microfluidic structuresby means of full-wafer adhesive bondingusing a poly(dimethylsiloxane) catalyst. J. Micromech. Microeng. 2007, 17, 1710–1714. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricatingmicrofluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.M.; Rodrigues, R.O.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A.; Minas, G. Recent trends of biomaterials and biosensors for organ-on-chip platforms. Bioprinting 2022, 26, e00202. [Google Scholar] [CrossRef]
- Femmer, T.; Jans, A.; Eswein, R.; Anwar, N.; Moeller, M.; Wessling, M.; Kuehne, A.J. High-Throughput Generation of Emulsions and Microgels in Parallelized Microfluidic Drop-Makers Prepared by Rapid Prototyping. ACS Appl. Mater. Interfaces 2015, 7, 12635–12638. [Google Scholar] [CrossRef] [PubMed]
- Femmer, T.; Kuehne, A.J.; Wessling, M. Print your own membrane: Direct rapid prototyping of polydimethylsiloxane. Lab Chip 2014, 14, 2610–2613. [Google Scholar] [CrossRef] [PubMed]
- Jans, A.; Lölsberg, J.; Omidinia-Anarkoli, A.; Viermann, R.; Möller, M.; De Laporte, L.; Wessling, M.; Kuehne, A.J. High-throughput production of micrometer sized double emulsions and microgel capsules in parallelized 3D printed microfluidic devices. Polymers 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grilli, S.; Coppola, S.; Nasti, G.; Vespini, V.; Gentile, G.; Ambrogi, V.; Carfagna, C.; Ferraro, P. Hybrid ferroelectric–polymer microfluidic device for dielectrophoretic self-assembling of nanoparticles. RSC Adv. 2014, 4, 2851–2857. [Google Scholar] [CrossRef]
- Coppola, S.; Nasti, G.; Todino, M.; Olivieri, F.; Vespini, V.; Ferraro, P. Direct writing of microfluidic footpaths by pyro-EHD printing. ACS Appl. Mater. Interfaces 2017, 9, 16488–16494. [Google Scholar] [CrossRef]
- Wu, H.; Huang, B.; Zare, R.N. Construction of microfluidic chips using polydimethylsiloxane for adhesive bonding. Lab Chip 2005, 5, 1393–1398. [Google Scholar] [CrossRef]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Marzban, M.; Packirisamy, M.; Dargahi, J. 3D Suspended Polymeric Microfluidics (SPMF3) with Flow Orthogonal to Bending (FOB) for Fluid Analysis through Kinematic Viscosity. Appl. Sci. 2017, 7, 1048. [Google Scholar] [CrossRef] [Green Version]
- Marzban, M.; Dargahi, J.; Packirisamy, M. Flow force augmented 3D suspended polymeric microfluidic (SPMF3) platform. Electrophor. Microfluid. Miniat. 2019, 40, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Marzban, M.; Dargahi, J.; Packirisamy, M. Rigid and Elastic Microparticles Detection Using 3D Suspended Polymeric Microfluidics (SPMF3) Sensor. IEEE Sens. 2018, 18, 5674–5684. [Google Scholar] [CrossRef]
- Marzban, M.; Packirisamy, M.; Dargahi, J. Parametric study on fluid structure interaction of a 3D suspended polymeric microfluidics (SPMF3). Micorsyst. Technol. 2018, 24, 2549–2559. [Google Scholar] [CrossRef]
- Moghadam, E.Y.; Packirisamy, M. Increase of Sensitivity in 3D Suspended Polymeric Microfluidic Platform through Lateral Misalignment. Waset Acad. Sci. 2017, 11, 1896–1901. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Dendukuri, D.; Doyle, P.S. The Synthesis and Assembly of Polymeric Microparticles Using Microfluidics. Adv. Mater. 2009, 21, 4071–4086. [Google Scholar] [CrossRef]
- Tan, W.H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.; Haraldsson, T.; Sandström, N.; Stemme, G.; Russom, A.; van der Wijngaart, W. On-Chip Liquid Degassing with Low Water Loss. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherlands, 3–7 October 2010. [Google Scholar]
- Wyss, H.M.; Blair, D.L.; Morris, J.F.; Stone, H.A.; Weitz, D.A. Mechanism for clogging of micro-channels. Phys. Rev. 2006, 74, 061402. [Google Scholar]
- Beaulieu, L.; Godin, M.; Laroche, O.; Tabard-Cossa, V.; Grutter, P. A complete analysis of the laser beam deflection systems used in cantilever-based systems. Ultramicroscopy 2007, 107, 422–430. [Google Scholar] [CrossRef]
- Vliet, T.V.; Walstra, P. Relationship between viscosity and fat content of milk and cream. Texture Stud. 1980, 11, 65–68. [Google Scholar] [CrossRef]



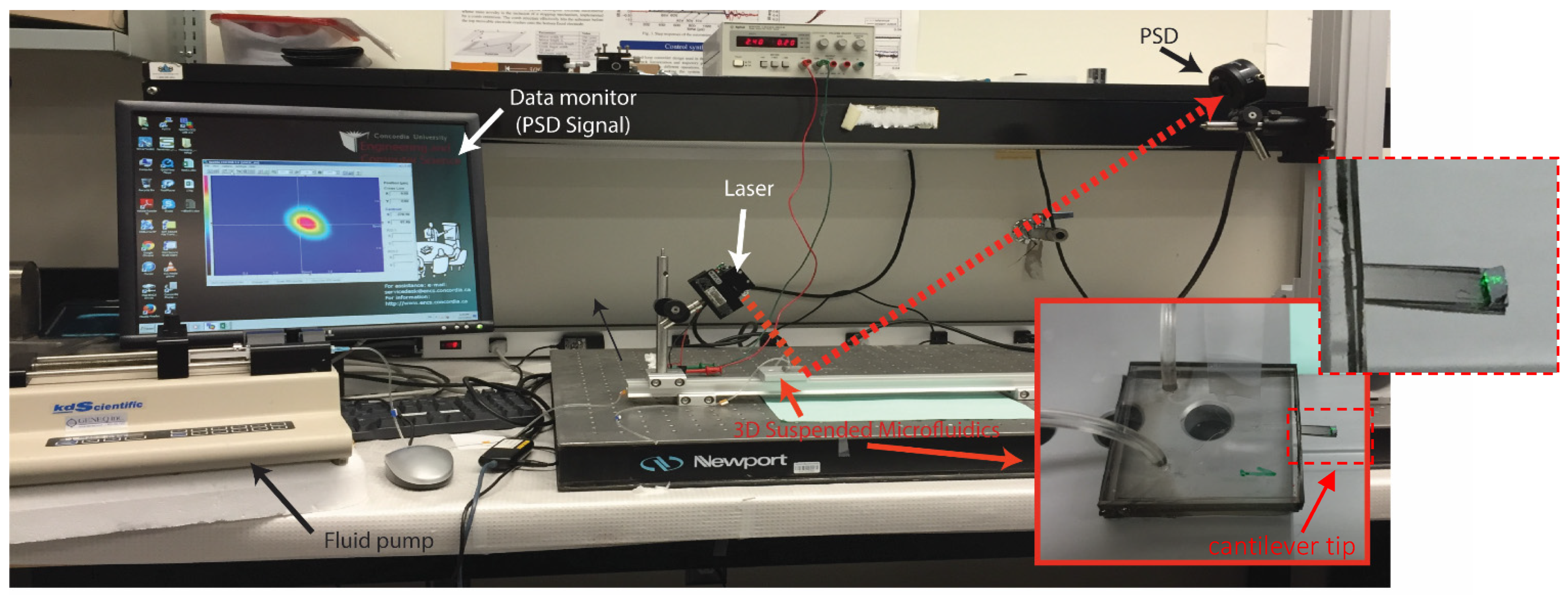
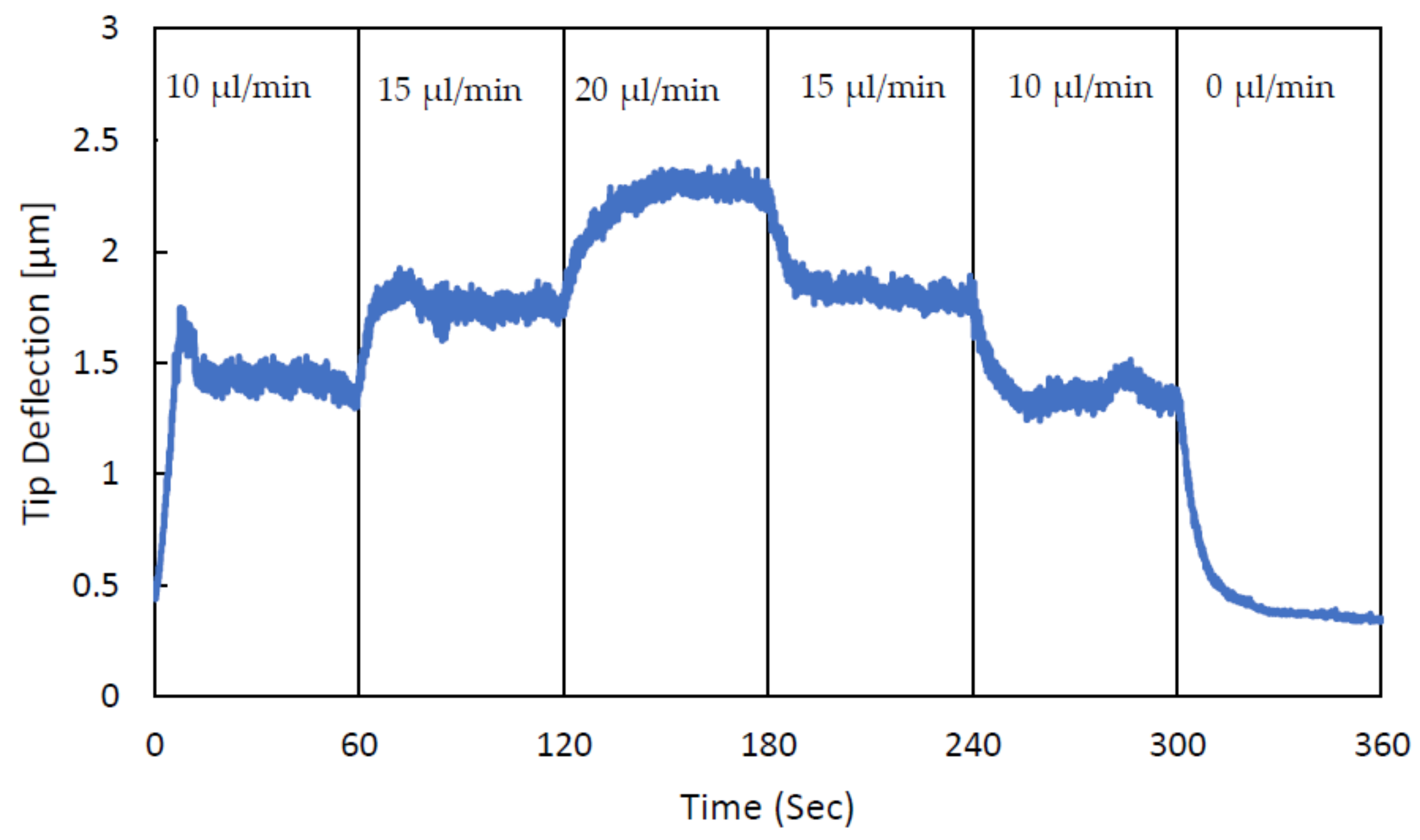
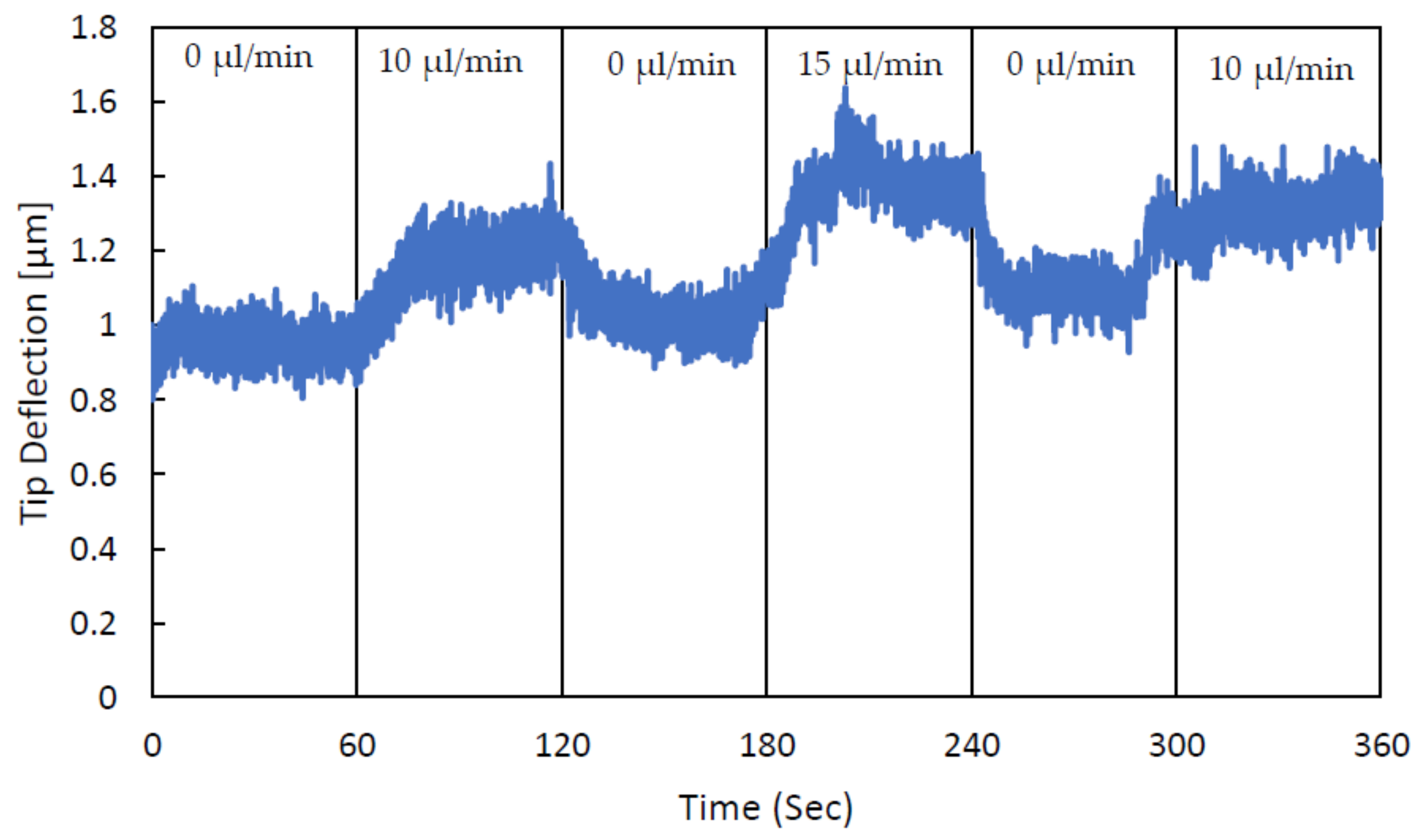
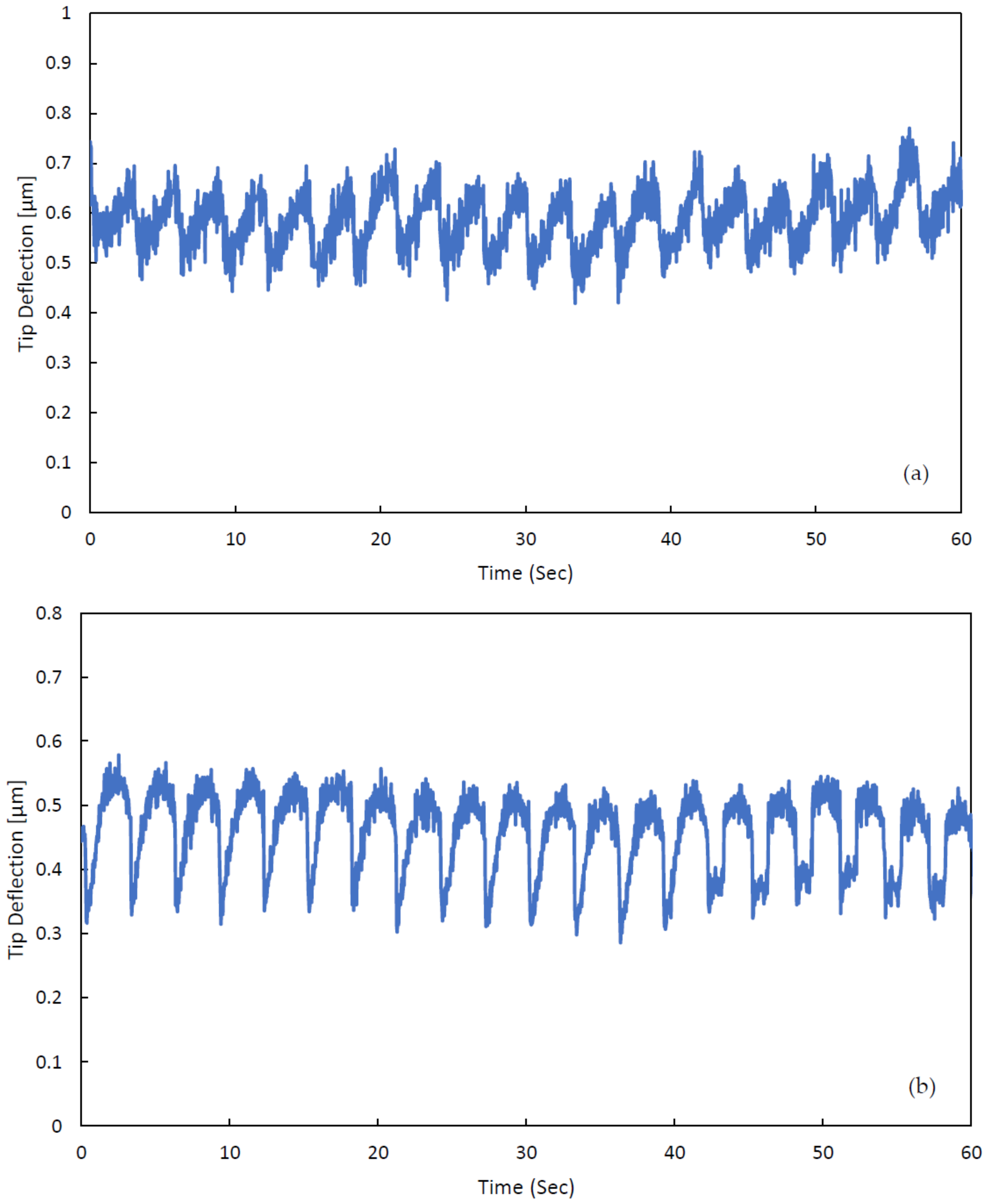
| Cantilever Size, L, W, T (µm) | Microchannel Size, W, T (µm) | Nozzle Size, W, L (µm) |
|---|---|---|
| 6000 × 2000 × 600 | 200 × 100 | 200 × 400 |
| Salt wt % | Density (kg/m3) | Viscosity (cP) |
|---|---|---|
| 0% | 999 | 1.002 |
| 10% | 1070 | 1.193 |
| 15% | 1110 | 1.350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzban, M.; Yazdanpanah Moghadam, E.; Dargahi, J.; Packirisamy, M. Microfabrication Bonding Process Optimization for a 3D Multi-Layer PDMS Suspended Microfluidics. Appl. Sci. 2022, 12, 4626. https://doi.org/10.3390/app12094626
Marzban M, Yazdanpanah Moghadam E, Dargahi J, Packirisamy M. Microfabrication Bonding Process Optimization for a 3D Multi-Layer PDMS Suspended Microfluidics. Applied Sciences. 2022; 12(9):4626. https://doi.org/10.3390/app12094626
Chicago/Turabian StyleMarzban, Mostapha, Ehsan Yazdanpanah Moghadam, Javad Dargahi, and Muthukumaran Packirisamy. 2022. "Microfabrication Bonding Process Optimization for a 3D Multi-Layer PDMS Suspended Microfluidics" Applied Sciences 12, no. 9: 4626. https://doi.org/10.3390/app12094626
APA StyleMarzban, M., Yazdanpanah Moghadam, E., Dargahi, J., & Packirisamy, M. (2022). Microfabrication Bonding Process Optimization for a 3D Multi-Layer PDMS Suspended Microfluidics. Applied Sciences, 12(9), 4626. https://doi.org/10.3390/app12094626






