Featured Application
Antioxidant signaling mutants, namely mitogen-activated protein kinase mutants, can be adopted as risk assessment tools during antimicrobial compound screening, which can eliminate compounds triggering microbial tolerance.
Abstract
Antimicrobial efficacy of the water or methanolic extracts of three medicinal mushrooms Taiwanofungus camphoratus, Agaricus blazei Murrill, and Ganoderma lucidum (Curtis) P. Karst were investigated against yeast and filamentous fungal pathogens as well as against commensal and pathogenic bacteria. The methanolic extract of T. camphoratus (TcM) exhibited both potent antifungal and antibacterial activity, while the water extract of T. camphoratus (TcW) showed limited antibacterial activity against Listeria monocytogenes. Neither the methanolic nor water extracts of A. blazei and G. lucidum exhibited antimicrobial activity. In the risk assessment testing monitoring the development of fungal tolerance to mushroom extracts in food matrices, two P. expansum mitogen-activated protein kinase (MAPK) mutants exhibited a tolerance to TcM. In a proof-of-concept bioassay using the natural benzoic salicylaldehyde (SA), P. expansum and A. fumigatus MAPK antioxidant mutants showed similar tolerance to SA, suggesting that natural ingredients in TcM such as benzoic derivatives could negatively affect the efficacy of TcM when antioxidant mutants are targeted. Conclusion: TcM could be developed as a food ingredient having antimicrobial potential. The antimicrobial activity of TcM operates via the intact MAPK antioxidant signaling system in microbes, however, mutants lacking genes in the MAPK system escape the toxicity triggered by TcM. Therefore, caution should be exercised in the use of TcM so as to not adversely affect food safety and quality by triggering the resistance of antioxidant mutants in contaminated food.
1. Introduction
The identification of foodborne pathogens or contaminants (fungi, bacteria) with increased resistance to conventional food-preservation methods or disinfectants is a recurring food safety and security concern. Studies have shown that invasive microbial infection including pulmonary aspergillosis, fungemia (fungal infection in blood), chorioamnionitis (inflammation of the fetal membranes), etc. could be acquired from contaminated food sources, indicating human infections also involve food safety issues [1,2,3]. Moreover, outbreaks in commodity-specific food sources such as wheat, corn, or tree nut contamination by fungi-produced mycotoxins directly affect public health. For instance, aflatoxins are hepatotoxic and/or nephrotoxic carcinogens/mutagens highly detrimental to human or animal health, thus are a serious threat to food safety, security, and food/crop marketability [4,5]. Therefore, the development of new antimicrobial agents or intervention methods for the control of foodborne pathogens, especially those resistant to conventional antimicrobials, is continually needed.
Many antimicrobial agents have been developed from mushroom ingredients [6,7]. For crop protection, as an example, the natural fungicides strobilurins have been isolated from the basidiomycete Strobilurus tenacellus, an agaric fungus known as pinecone cap. Strobilurins are quinone outside inhibitor (QoI) fungicides that interrupt fungal mitochondrial respiration by specifically binding to the quinol oxidation (Qo) site of cytochrome b in the respiratory chain. While strobilurin-A is the original, natural form of the compound, other synthetic derivatives of strobilurins such as azoxystrobin, kresoxim-methyl, pyraclostrobin, trifloxystrobin, etc., have been developed and commercialized for their higher efficacy, low volatility, and enhanced resistance to UV breakdown [8]. Nevertheless, there have been several reports describing the development of fungal resistance to strobilurins. It has been determined that the function of the intrinsic alternative oxidase (a strobilurin-insensitive terminal oxidase enabling electrons from ubiquinol to bypass Complex III), the point mutation G143A in the cytochrome b complex, efflux pumps, etc., contributed to fungal insensitivity or resistance to the strobilurin fungicides [9,10].
Recently, mushrooms have also been potential sources of antimicrobials for food preservation and food safety control [11]. For example, the long-chain glycolipids isolated from the jelly mushroom Dacryopinax spathularia have been classified as Generally Regarded As Safe (GRAS) molecules by the United States Food and Drug Administration [12], for proposed use as food preservatives against microbial contaminants in select non-alcoholic beverages. Additionally, the methanolic extract of industrially grown basidiomycete Coriolus versicolor exhibited potent antibiotic activity against foodborne bacterium Salmonella enterica serovar Enteritidis [13]. However, despite the increasing consumer demands for safe, natural alternatives to the conventional food preservatives, antimicrobial agents from edible mushrooms have not been fully explored to date for their application in food production, processing, and preservation [14].
Edible mushrooms have been a sustainable source of bioactive compounds, and constituents of certain medicinal mushrooms have emerged as integral ingredients of dietary supplements [15,16]. For instance, the folk medicinal mushroom Taiwanofungus camphoratus exerted resistance against obesity, where the mushroom reduced obesity caused by leptin-deficiency and restored intestinal barrier integrity [16]. Several studies have further determined that T. camphoratus ethanolic extracts possess broad-spectrum bioactivities including (a) anti-inflammatory/ameliorative effects to prevent diabetes-induced male reproductive dysfunction [17]; (b) synergistic adjuvant effects to downregulate cancer stem genes and to enhance the antitumor ability of 5-fluorouracil [18]; and (c) inhibitory effects on lung tumor growth and metastasis by inducing apoptosis and inhibiting the “Signal Transducer and Activator of Transcription 3 (STAT3)” signaling pathway [19]. To date, over seventy compounds in T. camphoratus have been characterized as exhibiting hepatoprotective, antihypertensive, antihyperlipidemic, anti-inflammatory, antioxidant, antitumor, and immunomodulatory activities [15,20].
The basidiomycete Agaricus blazei has also been used for medicinal purposes. Recent studies have determined that A. blazei contained hydrophilic small molecules such as phenolics, lipophilic small molecules such as agarol, and macromolecules such as β-glucans, which exhibited: (a) immunomodulation, cell signaling, anti-inflammatory, antidiabetic, antiparasitic, and antimicrobial activities; (b) antimutagenic, anticancer/tumor-growth inhibitory effects; and (c) hepatoprotection activity against chemical or viral infection [21]. In addition, several studies have reported the antioxidant potential of A. blazei. The A. blazei ethyl acetate extract exhibited hepatic antioxidant activity, whereby it helped the recovery of impaired pancreatic tissues [22]. A. blazei in the dried and powdered mycelial form also presented antigenotoxic and hydroxyl radical (OH) scavenging activities [23], while the A. blazei methanolic extract showed antioxidant/anti-inflammatory properties in Parkinsonic mice [24]. Other investigators have reported that an A. blazei hot water extract inhibited the proliferation of pancreatic cancer cells via the induction of G0/G1 cell cycle arrest and caspase-dependent apoptosis [25], and that A. blazei polysaccharides inhibited schistosome infection as well as improved pathological effects associated with granulomas [26].
The polypore fungi Ganoderma spp. are also one of the most studied edible mushrooms. They possess active ingredients such as polysaccharides, mono-/tri-terpenoids, ganoderic acids, alkaloids, fatty acids, organic germanium, ergosterol, mannitol, and other bioactive compounds [27,28,29,30]. Ganoderma spp. ingredients exerted: (a) modulation of neurogenesis, amelioration of Alzheimer’s disease, protection on neural cells in stroke injury, treatment of diabetes and insulin resistance [29,30]; (b) antifibrotic activity or inhibition of pancreatic lipase or T-type voltage-gated calcium channels [27]; and (c) antiviral, antibacterial, and antifungal activities [28]. Of note, certain immunomodulatory proteins or polysaccharides identified in Ganoderma spp. have been used in cosmetics for their antioxidant and antibacterial activities, inhibition of melanin, and regulation of inflammatory mediators [31]. Ganoderma spp. also appear useful for cancer treatment through the regulation of the immune system [32].
However, reports on the risk assessment of mushroom extracts per se or the purified mushroom ingredients toward food preservation are still very scarce. Except for the limited studies on the toxicity mushrooms may exert [33,34], risks such as the development of microbial resistance to mushroom derivatives have not been fully investigated.
In this study, the antimicrobial activity of alcoholic and water extracts from T. camphoratus, A. blaze, and Ganoderma lucidum powders were investigated against foodborne fungal and bacterial contaminants in defined medium or commercial food matrices (apple and grape juice agar). The efficacy as well as the resistance risks of mushroom extracts were examined by including the antioxidant signaling mutants of the mycotoxin (patulin)-producing Penicillium expansum and the pathogenic Aspergillus fumigatus to monitor their resistance to the mushroom extracts.
2. Materials and Methods
2.1. Chemicals and Microorganisms
Chemicals and media, namely, fludioxonil (FLU), salicylaldehyde (SA), gentamycin, levofloxacin, Luria broth, brain heart infusion agar, tryptic soy agar, potato dextrose agar (PDA), and select agar were purchased from Sigma Aldrich Co., St. Louis, MO, USA, except for dimethyl sulfoxide (DMSO; AMRESCO Co., Solon, OH, USA) and methanol (Thermo Fisher Scientific, Waltham, MA, USA). Microorganisms used in this study are described in Table 1. All fungi were maintained at 35 °C on PDA except for P. expansum, which were grown at 28 °C on PDA [35]. All bacteria were cultured on a designated medium (see Section 2.4) at 37 °C.

Table 1.
Microbial strains used in this study 1.
2.2. Preparation of Extracts from Mushrooms
Mushroom samples (T. camphoratus, A. blazei, G. lucidum) were graciously obtained from Dr. David Ojcius, University of the Pacific School of Dentistry (San Francisco, CA, USA) in a fine granular form (powder) as ready-to-eat foods procured from vendors. Each sample (250 g, 162 g and 132 g for T. camphoratus, A. blazei, G. lucidum, respectively) was extracted with methanol (2 L) for 3 min using a sonic generator with 1 cm horn (Branson Ultrasonics Co., Danbury, CT, USA) based on the modified methods described by Liu et al. [39]. The extracts were rough filtered through Whatman 54 followed by filtration through Whatman 50 (Cytiva Co., Marlborough, MA, USA). Methanol was removed from the filtrates by rotary evaporation (Buchi Co., New Castle, DE, USA), and the concentrated extracts were redissolved in 150 mL of distilled water. Samples were frozen and lyophilized (Labconco Co., Kansas City, MO, USA), obtaining methanol extracts weighing 40.5 g, 22.2 g, and 13.9 g, respectively, for T. camphoratus, A. blazei, and G. lucidum.
Following methanol extraction, the mushroom powder residues were extracted with 2 L of water using a sonic generator (see above), followed by centrifugation at 1000 rpm for 15 min and filtration of the supernatant through a Whatman Multigrade 150 glass microfiber filter (Cytiva Co., Marlborough, MA, USA). The filtrates were frozen and lyophilized, obtaining water extracts of 62.0 g, 45.8 g, and 48.2 g for T. camphoratus, A. blazei, and G. lucidum, respectively
2.3. Antifungal Test: Agar Plate Assay
Zone of inhibition assays were initially performed to assess the antifungal activity of the mushroom extracts against C. albicans ATCC 10231 and A. fumigatus AF293 as described previously [40]. Methanol or water extracts from mushroom samples were each tested at 0.625, 1.25, 2.5, 5.0, or 10.0% (w/v) on PDA Petri plates (100 mm × 15 mm; Corning Inc.-Life Sciences, Tewksbury, MA, USA). Mushroom extracts were dissolved in a vehicle (DMSO:water (50%:50%)) before spotting (5 μL) each concentration onto the fungus-streaked (lawn) PDA. Control contained the DMSO vehicle only (5 μL) at levels equivalent to that of cohorts receiving the mushroom extracts, within the same set of experiments. The level of zone of inhibition was monitored for three to five days at 35 °C.
The level of antifungal activity of T. camphoratus methanol extract (TcM) was investigated further by monitoring the radial growth of seven fungal pathogens/contaminants (N. fischeri, A. flavus, A. parasiticus, P. expansum W1, W2, FR2, FR3) on PDA containing 0.0%, 0.8%, or 1.6% (w/v) of TcM in triplicate wells of 6-well plates (6 × 16.8 mL; Corning Inc.-Life Sciences, Tewksbury, MA, USA) according to the modified methods described previously [35]. This assay was also performed with juice agar (JA) containing apple or grape juice (70%, final concentration) w/1.5% select agar base. Organically produced white grape (pH 3.08) and honey crisp apple (pH 3.48) juices were purchased from local grocery stores (Berkeley, CA, USA). Fungal inoculum (1 × 103 CFU in 20 μL) was applied to a blank BD Taxo disc (6 mm; BD Life Sciences Franklin Lakes, NJ, USA) at the center of each agar well. Fungal growth (% radial growth) was monitored for five to seven days at 35 °C, except for P. expansum (28 °C).
2.4. Antibacterial Test: Disc Diffusion Assay
L. reuteri (ATCC 23272), L. acidophilus (ATCC 43560), and L. rhamnosus (ATCC 53103) were grown in Lactobacilli MRS (De Man, Rogosa and Sharpe) agar at 37 °C under anaerobic conditions. Strains grown aerobically at 37 °C were: E. coli K-12 MG 1655 (USDA) in Luria Broth, S. enterica pGFP (USDA) in Luria Broth, and L. monocytogenes RM2194 (USDA) in Brain Heart Infusion, and S. aureus (ATCC 6538) in Tryptic Soy. Empty BDL-sensi-discs (6 mm) (BD Life Sciences Franklin Lakes, NJ, USA) were saturated with either vehicle control (DMSO:water (50%:50%)) or mushroom extracts dissolved in the vehicle for 30 min at room temperature [see [40]]. Discs containing vehicle control, mushroom extracts, or various antibiotic discs (levofloxacin (5 μg), gentamicin (10 μg), and gentamicin (120 μg)) were placed onto the bacterial streaked (lawn) agar Petri plates (100 mm × 15 mm; Corning Inc.-Life Sciences, Tewksbury, MA, USA) and incubated overnight at 37 °C (18–24 h). Sensitivity to antibiotics or mushroom extracts was determined via the measurement of zones of inhibition around each disc in mm.
2.5. Risk Assessments: Resistance Testing of Mitogen-Activated Protein Kinase (MAPK) Mutants of A. fumigatus and P. expansum to Salicylaldehyde (SA)
The tolerance of mitogen-activated protein kinase (MAPK) mutants of A. fumigatus (sakAΔ, mpkCΔ) or P. expansum (FR2) to SA was examined in PDA according to the modified method described previously [41]. SA (0.09 M for A. fumigatus; 0.12 M for P. expansum) was applied to membrane filters (1.5 cm diameter; GE Healthcare, Chicago, IL, USA), then either SA- or DMSO (control)-saturated membrane filters were placed onto the lower half of each PDA Petri plate (60 mm × 15 mm; Corning Inc.-Life Sciences, Tewksbury, MA, USA) w/ or w/o FLU (50 μM). SA was delivered to the target fungi as a fumigant. Fungal spores (1 × 103 CFU in 20 μL) were spotted onto the upper half of each PDA, and the inoculated plates were incubated at 35 °C or 28 °C for A. fumigatus or P. expansum, respectively, to determine the development of fungal (MAPK mutants) tolerance to SA. SA was dissolved in DMSO (absolute DMSO amount: <2%, final concentration), and control plates contained DMSO only at levels equivalent to that of cohorts receiving SA, within the same set of experiments. Fungal growth (radial growth) was monitored for three to five days.
2.6. Statistical Analysis
Statistical analysis (Student’s t-test) was performed based on “Statistics to use” [42]; p < 0.05 was considered significant.
3. Results and Discussion
3.1. Antifungal Activity of T. camphoratus Methanol Extract (TcM)
The antimicrobial or health effects of “methanol” extracts from different mushrooms have been investigated elsewhere [13,24,43]. To determine the efficacy of antifungal compounds, it is preferred to perform standard broth microdilution bioassays in microtiter plates according to the protocols outlined by the Clinical Laboratory Standards Institute (CLSI) M38-A [44] or by the European Committee on Antimicrobial Susceptibility Testing (EUCAST); definitive document EDef 7.2 [45] for filamentous fungal or yeast pathogens. In these cases, minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) of compounds can be determined including compound interactions [41,46,47]. However, the standard microdilution bioassays could not be used in our investigation with mushroom extracts since: (a) the final density of the mushroom extracts prepared in the delivery vehicles was high, thus hampering the serial dilution of the extracts in microtiter liquid medium; (b) the turbidity of the food matrices (fruit juices; see Section 3.2) was also high, so that monitoring the level of visible fungal growth for the determination of MICs was obscured; and (c) one of the test (risk assessment) compounds, SA, is volatile so it needs to be remotely applied from the SA-saturated membrane filter placed on solid agar plates (see Section 3.3). Therefore, we pursued the determination of antimicrobial activity of mushroom extracts on a solid agar medium by monitoring zones of inhibition or fungal radial growth.
In Figure 1, a zone of inhibition bioassay showed that only the methanol extract of T. camphoratus (TcM) possessed potent antifungal activity when tested against A. fumigatus AF293 and C. albicans ATCC 10231, the causative agents of human aspergillosis and candidiasis, respectively. Since invasive aspergillosis and candidiasis can also be acquired via contaminated foods, beverages, or dietary supplements, there exists a need to find novel antifungal therapies to combat these threats to our food and environmental safety [1]. In A. fumigatus, the antifungal activity of TcM increased with the concentration of TcM (Figure 1). The level of antifungal activity was steady in C. albicans up to 10% of TcM. The results indicate that a “compound–strain relationship” exists for the differential antifungal activity of TcM, where the filamentous fungal pathogen A. fumigatus was more susceptible to TcM compared to the yeast pathogen C. albicans. The results also showed that the water extract of T. camphoratus (TcW) did not exhibit any antifungal activity against A. fumigatus or C. albicans. Moreover, neither of the A. blazei and G. lucidum extracts exhibited any antifungal activity against test fungi (data not shown).
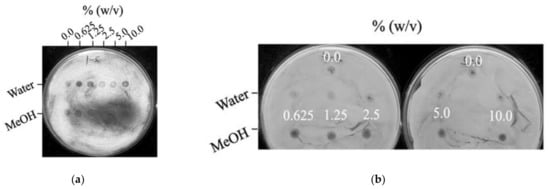
Figure 1.
Antifungal activity of methanol extracts of T. camphoratus (TcM) (0.625% to 10.0%). (a) Incremental increase of TcM activity against A. fumigatus AF293, proportional to the TcM concentrations. (b) A steady antifungal activity of TcM against C. albicans ATCC 10231. Note that no antifungal activity was detected with water extracts of T. camphoratus (TcW).
Differential antifungal susceptibility between yeast and filamentous fungal pathogens has been previously observed. For instance, the benzoic derivative 3,5-dimethoxybenzaldehyde (3,5-DMBA) exhibited potent antifungal activity (average MIC: 1.17 mM) against filamentous fungi such as Aspergillus and Penicillium spp. [41]. In contrast, 3,5-DMBA did not show any antifungal activity against any of the yeast strains tested including C. albicans, C. krusei, C. tropicalis, or Cryptococcus neoformans at up to 6.4 mM [46]. It can be postulated that the tested yeast pathogens possess an intrinsic capability to detoxify 3,5-DMBA. The mechanism of detoxification could be exclusion of toxic compounds via transporters/efflux pumps, vacuolar sequestration, enzymatic degradation/transformation, and other possibilities. A similar detoxification mechanism may also govern the steady response of C. albicans against TcM.
3.2. Antifungal Activity of TcM against Foodborne Pathogens/Contaminants on Potato Dextrose Agar (PDA) or Food Matrices (Apple, Grape Juice Agar): Fungal Antioxidant Mutants as Risk Assessment Tools
3.2.1. Antifungal Activity Test of TcM on PDA (Defined Medium)
We also determined the antifungal activity of TcM against foodborne fungal pathogens/contaminants by monitoring their radial growth on PDA (defined medium) containing 0.8 or 1.6% (w/v) of TcM. As determined in A. fumigatus (Section 3.1), there were incremental increases in antifungal activity with concentrations as shown by a reduction in the radial growth (Table 2). The only exceptions were N. fischeri and P. expansum W2, which showed a similar level of susceptibility to either 0.8% or 1.6% of TcM.

Table 2.
Antifungal activity of TcM on PDA (% radial growth compared to no treatment control).
Of note, two antioxidant mutants of P. expansum, FR2 and FR3, showed a 34% to 46% higher growth rate (tolerance) to TcM when compared to the respective wild type strains, W1 and W2, except for FR3 at 1.6% of TcM, which showed TcM susceptibility similar to that of W2. P. expansum FR2 and FR3 have a mutation in their antioxidant system, possibly in the mitogen-activated protein kinase (MAPK) pathway involved in oxidative stress signaling [38,48]. As determined in the A. fumigatus MAPK mutants [41], FR2 and FR3 mutants were tolerant to the phenylpyrrole fungicide, FLU [38,48]. The antifungal action of certain antifungal agents such as FLU, fenpiclonil, aromatic hydrocarbons, etc. is mediated through the normal antioxidant signaling system, especially the intact MAPK pathway [49,50]. Therefore, it is speculated that the antifungal activity of TcM could also be mediated through the intact antioxidant signaling system in fungi, while fungi with mutations in the system escape the toxicity triggered by the mushroom extracts (Table 2) (see also Section 3.3 below).
3.2.2. Antifungal Activity Test of TcM on Apple or Grape JA (Complex Medium)
The antifungal activity of TcM was determined further against foodborne fungal pathogens/contaminants by monitoring their radial growth on food matrices, apple, or grape JA containing 0.8 or 1.6% (w/v) of TcM. As determined in PDA (see Section 3.2.1), there were incremental increases in TcM antifungal efficacy (reduction in a fungal radial growth) in either apple or grape JA as the TcM concentration increased (Table 3), except with A. parasiticus on grape JA, with a similar level of susceptibility to either 0.8% or 1.6% of TcM (Table 3).

Table 3.
Antifungal activity of TcM on apple or grape JA (% radial growth compared to no treatment control).
The results show that the antifungal efficacy of TcM was relatively higher in apple JA than in grape JA; average % radial growth in apple JA was 65 ± 13% or 41 ± 16% at 0.8% or 1.6% of TcM, respectively, while that in grape JA was 75 ± 18% or 64 ± 15% at the same concentrations of TcM. Hence, the order of antifungal efficacy was high to low: (a) PDA > apple JA > grape JA at 0.8%, and (b) PDA, apple JA > grape JA at 1.6%.
It seems that the ingredients in the JA (complex medium) might negatively affect the antifungal efficacy of TcM when compared to PDA (defined medium). Of note, interference of the efficacy of antifungal agents by the ingredients of food matrices has been previously documented. For instance, the polyene drug natamycin (NAT) is a redox-active antifungal molecule commercially applied for control of fungi infecting various crops or contaminating processed foods [51,52,53,54,55]. We previously observed that while the antifungal efficacy of NAT (tested against filamentous fungi) was generally enhanced with increasing doses of NAT in JA, the level of NAT efficacy varied depending on the types of JA tested [35]. Therefore, it can be concluded that the ingredients in food matrices could interfere with the antifungal efficacy of sanitary compounds. Changes in NAT efficacy in response to food ingredients such as organic acids, salts, etc., have been documented further in different food matrices [35 and references therein].
Furthermore, as observed in PDA (see Section 3.2.1), two P. expansum antioxidant mutants (FR2, FR3) showed 8% to 100% of higher growth rate (namely, tolerance) to TcM on JA at both 0.8% and 1.6% of TcM, when compared to their respective wild type strains W1 and W2 (Table 3).
3.3. Resistance of Mitogen-Activated Protein Kinase (MAPK) Mutants of A. fumigatus and P. expansum to SA: Proof-of-Concept
As mentioned previously, the fungicidal effect of the commercial fungicide FLU is exerted via the “intact” oxidative (osmotic) stress signaling system in fungi, namely the MAPK pathway [49]. FLU disrupts fungal growth by triggering unusual, excessive stimulation of the oxidative (osmotic) stress signaling MAPK system, thus causing cellular energy drain [49]. This MAPK pathway is responsive to oxidative (osmotic) cues, and hence protects the wild type fungal cells from environmental oxidative (osmotic) stressors. However, fungi with mutations in this MAPK system are not affected by the toxicity of FLU, which results in the development of FLU tolerance in the environment [49].
As a proof of concept, we examined the susceptibility of the wild type and MAPK mutants of A. fumigatus (sakAΔ and mpkCΔ) and of P. expansum (FR2) (Table 1) [36,37,38] to a benzoic compound SA, a natural aromatic constituent found in many plant species [56]. In a prior study, aromatic hydrocarbons such as pentachloronitro-benzene also exerted its antifungal activity via an action similar to FLU [50]. SA is a redox-active benzoic derivative, possessing both antioxidant and prooxidant potential [57,58,59]. Therefore, we reasoned that SA exerts its antifungal activity by interfering with fungal antioxidant systems such as the MAPK pathway, whereby the wild type fungus will be sensitive to the treatment while the antioxidant MAPK mutants, as shown in this study, will be tolerant or less susceptible. According to its vapor pressure value (5.93 × 10−1 mm Hg, 25 °C), SA exists mainly as a vapor in the environment [60]. To target fungi, SA was remotely applied from the SA-saturated membrane filter placed on each plate.
As shown in Figure 2, the growth of the wild type of A. fumigatus (AF293) or P. expansum (W1) was completely inhibited by FLU (50 μM), while MAPK mutants (sakAΔ, mpkCΔ, FR2) of both species exhibited tolerance to FLU.
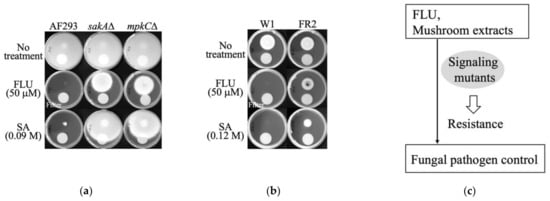
Figure 2.
Resistance of antioxidant signaling mutants of fungi to FLU and SA. (a) Response of A. fumigatus wild type (AF293) and MAPK mutants (sakAΔ, mpkCΔ). (b) Response of P. expansum wild type (W1) and antioxidant mutant (FR2). (c) Diagram describing the tolerance of fungal signaling mutants to FLU or the mushroom extract TcM. The round circle at the lower half of each plate indicates a membrane filter where SA or DMSO (no treatment control) was applied. Fungal radial growth is shown at the upper half of each plate; note that A. fumigatus fully covered the control (no treatment) plates, reflecting rapid growth.
Fungal MAPK mutants were also discovered to be tolerant to the natural benzoic SA (Figure 2). For example, A. fumigatus sakAΔ and mpkCΔ mutants showed higher tolerance (higher radial growth) to FLU or SA compared to the wild type AF293, which showed almost no growth under the same treatment. Similar trends were also observed in the P. expansum FR2 mutant when compared to the wild type W1. Results indicate that, similar to FLU, SA also acts as an inducer of “oxidative (osmotic) imbalance” in fungal cells, thus indicating that both SA and FLU might share a common mechanism of antifungal action.
Antioxidant MAPK pathway mutants have also demonstrated tolerance under treatment of cell wall-disrupting agents. Efficacy of cell wall disrupting drugs required both the “cell wall integrity” and “antioxidant” MAPK systems to be intact, while mutations in the MAPK system resulted in drug resistance [61,62,63]. For instance, antioxidant MAPK system mutants of the baker’s yeast Saccharomyces cerevisiae, hog1 (MAPK) or pbs2 (MAPK kinase; MAPKK) exhibited tolerance to drugs targeting the cell wall [61,62,63]. Of note, mutants of the upstream sensors including transmembrane (sho1) or histidine kinase (sln1) osmo-sensors in the antioxidant MAPK signaling system were also partially tolerant to the cell wall-inhibitory agent, calcofluor white [61,62,63].
It is also worth noting that, among the benzo analogs examined thus far, the development of fungal tolerance seemed specific to SA. In our previous study, application of other benzo derivatives such as cinnamaldehyde, 2-hydroxy-5-methoxybenzaldehyde, 2,3- or 2,5-dihydroxybenzoic acid, 2-acetoxybenzoic acid, etc., did not result in tolerance by fungal MAPK mutants to the treatments [41,64]. In fact, MAPK mutants, rather, exhibited increased susceptibility to other benzo derivatives when compared to the wild type. We initially speculated that the sensitivity was due to the high redox-activity of benzo derivatives that make the antioxidant MAPK mutants more susceptible to the treatments [57,58,59].
However, the mechanism of differential antifungal activity (“tolerant” versus “sensitive” response) between SA and other benzo derivatives during the targeting of fungal MAPK mutants remains to be elucidated. A previous study with methanol and water extracts from Shiitake mushroom (Lentinula edodes (Berk.) Pegler) revealed that the water extract from L. edodes possessed the most potent radical scavenging activity [43]. Hence, it is thought that TcW mostly possesses radical scavenging activity and low antifungal activity, while TcM possesses molecules exerting potent antifungal activity.
Types of bioactive components of T. camphoratus and their bioactivities have recently been reviewed [20] (see also Table 4).

Table 4.
Exemplary chemical compounds identified in T. camphoratus.
The chemical components of T. camphoratus mainly include: (a) Polysaccharides such as sulfated polysaccharides (SPS) exhibiting anticancer activity via the downregulation of epidermal growth factor receptor (EGFR) signaling, galactomannan (ACP) possessing immunostimulatory effect, which degrades transforming growth factor β (TGFβ) and TGFβ receptors (TGFR), etc.; (b) terpenoids such as triterpenes inhibiting diabetes or increasing wound healing, antcin-H exhibiting anticancer activity via the inhibition of ERK1/2-AP-1/c-Fos and C/EBP-β signaling system, antcin-K inducing apoptosis through the increased generation of reactive oxygen species (ROS) and reduction in ATP levels (thus, suited for liver cancer therapy); (c) maleic and succinic acid derivatives such as antrodin C lowering high glucose-triggered cellular toxicity/senescence while it upregulates antioxidant genes; and (d) other compounds such as antrodan, antrolone, or 2,3,5-trimethoxy-4-cresol exhibiting anti-prostate hyperplasia, anti-inflammatory, or anticancer activity, respectively [20]. However, the antifungal activity and/or fungal tolerance potentiated by these chemical components have not been investigated thus far.
In this investigation, it was speculated that, similar to FLU, SA could also trigger unusual, excessive stimulation of the oxidative (osmotic) stress signaling MAPK system, thus causing energy exhaustion and cell death. However, MAPK mutants escape TcM-triggered toxicity. Although a recent study identified several benzenoid and benzoquinone derivatives in T. camphoratus possessing high redox free radical scavenging activities [67], precise determination of the causative molecules triggering tolerance as well as elucidation of the tolerance mechanism of MAPK mutants to TcM warrants future in-depth investigation. Conversely, the antioxidant MAPK mutants of fungi could serve as effective risk assessment tools for the industry to screen safe antifungal molecules/ingredients to be used in food matrices, thus avoiding compounds that trigger fungal tolerance.
3.4. Antibacterial Activities of T. camphoratus Methanol (TcM) or Water (TcW) Extracts
Antibacterial activity of three mushroom extracts were explored further against the wild type foodborne bacteria. We found that, as is the case in fungi described above, only TcM exhibited potent antibacterial activity against S. aureus and Lactobacillus spp., while TcW possessed limited antibacterial activity against L. monocytogenes (Table 5) (note that the lack of any antibacterial activity by the A. blazei and G. lucidum extracts are not shown.)

Table 5.
Antibacterial activity (% zone of inhibition compared to the Gentamicin control) of T. camphoratus extracts 1.
The following were determined: (a) In the species of Lactobacillus, the level of antibacterial activity of TcM increased with the concentrations of TcM. L. acidophilus showed the highest susceptibility to TcM (67% growth inhibition at 0.625% TcM), followed by L. monocytogenes (30% growth inhibition at 0.625% TcM) and L. reuteri (40% growth inhibition at 1.25% TcM; no inhibition at 0.625% TcM); (b) TcW possessed a limited antibacterial efficacy; only L. monocytogenes showed around 35% growth inhibition at the highest concentration tested (10% TcW); (c) S. aureus exhibited 41% of growth inhibition at 10% of TcM, while E. coli and S. enterica did not exhibit any susceptibility to TcM or TcW; and (d) as determined in fungi, it seems that “compound–strain specificity” also exists for the bacterial responses to TcM. A similar type of differential antibacterial activity was observed when extracts from tomato (leaves, stems, fruit) were applied as antibacterial agents against bacterial pathogens [40]. As stated above for the fungal study, elucidation of the comprehensive mechanism of “compound—strain specificity” as well as the functional response(s) of antioxidant signaling mutants to mushroom extracts (namely, tolerant vs. sensitive responses) in the bacterial study also warrants future in-depth investigation.
4. Conclusions
In summary, antimicrobial activities of three medicinal mushrooms were investigated against fungal and bacterial pathogens/contaminants in a defined (PDA) or complex (food matrices) medium. It was found that mainly the methanol extract of T. camphoratus (TcM) exhibited a promising antifungal or antibacterial activity and that the ingredients of commercial food matrices (JA) had a negative impact on TcM efficacy. Compound–strain specificity was observed in both fungal and bacterial testing. As a proof-of-concept, application of a redox-active natural molecule, SA, resulted in the tolerance of P. expansum and A. fumigatus antioxidant signaling mutants to SA, thus suggesting that certain natural ingredients in mushroom extracts such as redox-active molecules can negatively affect the antifungal efficacy of TcM when antioxidant mutants are targeted.
Collectively, the data suggest that: (1) TcM has the potential to be developed as an antimicrobial food ingredient; (2) the determination of parameters affecting TcM efficacy in different foods as well as the elucidation of the resistance mechanisms need further study; and (3) alternatively, antioxidant signaling mutants such as MAPK mutants can be adopted as risk assessment tools in compound screening processes, whereby selected molecules do not disrupt food integrity by triggering the resistance of environmental mutants contaminating the food matrices. This approach will ultimately promote sustainable food production/processing, thus ensuring food safety and public health.
Author Contributions
Conceptualization, J.H.K. and K.M.L.; Methodology, J.H.K., C.C.T., K.L.C., N.M. and K.M.L.; Validation, J.H.K., C.C.T. and K.L.C.; Formal analysis, J.H.K. and C.C.T.; Investigation, J.H.K., C.C.T. and K.M.L.; Resources, K.M.L., N.M., L.W.C. and M.F.; Data curation, J.H.K. and C.C.T.; Writing—original draft preparation, J.H.K.; Writing—review and editing, J.H.K., K.L.C., C.C.T., N.M., K.M.L. and M.F.; Visualization, J.H.K. and K.L.C.; Supervision, L.W.C.; Project administration, L.W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated by this study are available in this paper.
Acknowledgments
This research was conducted under USDA-ARS CRIS Project 2030-42000-054-00-D. We thank Siov Sarreal and DeAngela Ford, Foodborne Toxin Detection and Prevention Research Unit, Western Regional Research Center, USDA-ARS, for their technical assistance. We also graciously thank David Ojcius, University of the Pacific School of Dentistry (San Francisco, CA, USA), for the mushroom samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benedict, K.; Chiller, T.M.; Mody, R.K. Invasive fungal infections acquired from contaminated food or nutritional supplements: A review of the literature. Foodborne Pathog. Dis. 2016, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Luz, L.; Mendonça, M.; Bernardes Fogaça, M.; Kipnis, A.; Bhunia, A.K.; Bührer-Sékula, S. Listeria monocytogenes: Review of pathogenesis and virulence determinants-targeted immunological assays. Crit. Rev. Microbiol. 2021, 47, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, R.J.; Mahoney, N.; Kim, J.H.; Campbell, B.C. Mycotoxins in edible tree nuts. Int. J. Food Microbiol. 2007, 119, 72–78. [Google Scholar] [CrossRef] [PubMed]
- RASFF—The Rapid Alert System for Food and Feed. Food and Food Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff-food-and-feed-safety-alerts_en (accessed on 15 March 2022).
- Alves, M.J.; Ferreira, I.C.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins—New antifungal antibiotics from the basidiomycete Strobilurus tenacellus. J. Antibiot. 1977, 30, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.T.; Lopes, I.; Pardal, M.Â. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: A review. Environ. Int. 2013, 53, 18–28. [Google Scholar] [CrossRef]
- Heick, T.M.; Justesen, A.F.; Jørgensen, L.N. Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic-Baltic region—A status. Eur. J. Plant Pathol. 2017, 149, 669–682. [Google Scholar] [CrossRef]
- Wood, P.M.; Hollomon, D.W. A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Qo site of Complex III. Pest Manag. Sci. 2003, 59, 499–511. [Google Scholar] [CrossRef]
- Shen, H.-S.; Shao, S.; Chen, J.-C.; Zhou, T. Antimicrobials from Mushrooms for Assuring Food Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 316–329. [Google Scholar] [CrossRef] [Green Version]
- United States Food and Drug Administration. GRAS Notice GRN No. 740. Glycolipids from Dacryopinax spathularia. Available online: https://www.fda.gov/media/113331/download (accessed on 23 March 2022).
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The Antibacterial Activity of Coriolus versicolor Methanol Extract and Its Effect on Ultrastructural Changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.J.; Lai, K.C.; Lee, A.S.; Chang, C.H.; Liu, C.L.; Chung, C.H. Novel Antrodia cinnamomea extract reduced cancer stem-like phenotype changes and resensitized KRAS-mutant colorectal cancer via a microRNA-27a pathway. Cancers 2019, 11, 1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-T.; Ruan, J.-W.; Chang, C.-S.; Ko, M.-L.; Chou, H.-C.; Lin, C.-C.; Lin, C.-M.; Huang, C.-T.; Wei, Y.-S.; Liao, E.-C.; et al. Antrodia cinnamomea confers obesity resistance and restores intestinal barrier integrity in leptin-deficient obese mice. Nutrients 2020, 12, 726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.; Cheng, S.-C.; Tsou, D.; Kong, Z.-L. Attenuation of reproductive dysfunction in diabetic male rats with timber cultured Antrodia cinnamomea ethanol extract. Biomed. Pharmacother. 2019, 112, 108684. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Yadav, V.K.; Srivastava, P.; Wu, A.T.; Huynh, T.-T.; Wei, P.-L.; Huang, C.-Y.F.; Huang, T.-H. Antrodia cinnamomea enhances chemo-sensitivity of 5-FU and suppresses colon tumorigenesis and cancer stemness via up-regulation of tumor suppressor miR-142-3p. Biomolecules 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-T.; Lan, Y.-W.; Chen, C.-M.; Ko, Y.-F.; Ojcius, D.M.; Martel, J.; Young, J.D.; Chong, K.-Y. Antrodia cinnamomea induces anti-tumor activity by inhibiting the STAT3 signaling pathway in lung cancer cells. Sci. Rep. 2019, 9, 5145. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef]
- da Silva de Souza, C.A.; Correa, G.V.; de Almeida Goncalves, G.; Soares, A.A.; Bracht, A.; Peralta, M.R. Agaricus blazei bioactive compounds and their effects on human health: Benefits and controversies. Curr. Pharm. Des. 2017, 23, 2807–2834. [Google Scholar] [CrossRef]
- Wei, Q.; Huang, L.; Li, J.; Chen, B.; Xie, B.; Teng, H.; Chen, L.; Jiang, Y. The beneficial effects of Agaricus blazei Murrill on hepatic antioxidant enzymes and the pancreatic tissue recovery in streptozotocin-induced diabetic rats. J. Food Biochem. 2020, 44, e13170. [Google Scholar] [CrossRef]
- Živković, L.; Borozan, S.; Čabarkapa, A.; Topalović, D.; Ciptasari, U.; Bajić, V.; Spremo-Potparević, B. Antigenotoxic properties of Agaricus blazei against hydrogen peroxide in human peripheral blood cells. Oxid. Med. Cell Longev. 2017, 2017, 8759764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh Gobi, V.; Rajasankar, S.; Ramkumar, M.; Dhanalakshmi, C.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M.; Chidambaram, R.; Kalandar, A. Agaricus blazei extract abrogates rotenone-induced dopamine depletion and motor deficits by its anti-oxidative and anti-inflammatory properties in Parkinsonic mice. Nutr. Neurosci. 2018, 21, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Furutani, Y.; Matsuoka, R.; Furukawa, T. Hot water extract of Agaricus blazei Murrill specifically inhibits growth and induces apoptosis in human pancreatic cancer cells. BMC Complement. Altern. Med. 2018, 18, 319. [Google Scholar] [CrossRef]
- Lin, M.-H.; Lee, K.-M.; Hsu, C.-Y.; Peng, S.-Y.; Lin, C.-N.; Chen, C.-C.; Fan, C.-K.; Cheng, P.-C. Immunopathological effects of Agaricus blazei Murill polysaccharides against Schistosoma mansoni infection by Th1 and NK1 cells differentiation. Int. Immunopharmacol. 2019, 73, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Qiu, M. Meroterpenoids from Ganoderma species: A review of last five years. Nat. Prod. Bioprospecting 2018, 8, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.K.; Gaikwad, S.; Nagaonkar, D.; dos Santos, C.A. Current advances in the antimicrobial potential of species of genus Ganoderma (higher Basidiomycetes) against human pathogenic microorganisms (Review). Int. J. Med. Mushrooms 2015, 17, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Gabryelska, K.; Grabarczyk, M. Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules 2019, 24, 4075. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2019, 121, 1160–1178. [Google Scholar] [CrossRef]
- Li, L.-D.; Mao, P.-W.; Shao, K.-D.; Bai, X.-H.; Zhou, X.-W. Ganoderma proteins and their potential applications in cosmetics. Appl. Microbiol. Biotechnol. 2019, 103, 9239–9250. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, X.; Liu, S.; Huang, L.; Gu, J. Ganoderma: A cancer immunotherapy review. Front. Pharmacol. 2018, 9, 1217. [Google Scholar] [CrossRef] [Green Version]
- Bienemann, B.; Ruschel, N.S.; Campos, M.L.; Negreiros, M.A.; Mograbi, D.C. Self-reported negative outcomes of psilocybin users: A quantitative textual analysis. PLoS ONE 2020, 15, e0229067. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Che, T.; Yin, Y.; Yu, G.; Yang, Q.; Liu, W.; Ye, X.; Yu, W.; Alok, S.; Chen, Y.; et al. Lethal protein in mass consumption edible mushroom Agrocybe aegerita linked to strong hepatic toxicity. Toxicon 2014, 90, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Tam, C.C.; Chan, K.L.; Cheng, L.W.; Land, K.M.; Friedman, M.; Chang, P.-K. Antifungal efficacy of redox-active natamycin against some foodborne fungi—Comparison with Aspergillus fumigatus. Foods 2021, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Nguyen, C.K.; Romans, A.; May, G.S. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot. Cell 2004, 3, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Reyes, G.; Romans, A.; Nguyen, C.K.; May, G.S. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell 2006, 5, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Xiao, C.L. Characterization of fludioxonil-resistant and pyrimethanil-resistant phenotypes of Penicillium expansum from apple. Phytopathology 2008, 98, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-Z.; Liang, Y.-C.; Lin, S.-Y.; Lin, Y.-S.; Wu, W.-C.; Hou, W.-C.; Su, C.-H. Antihypertensive Activities of a Solid-State Culture of Taiwanofungus camphoratus (Chang-Chih) in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2007, 71, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Tam, C.C.; Nguyen, K.; Nguyen, D.; Hamada, S.; Kwon, O.; Kuang, I.; Gong, S.; Escobar, S.; Liu, M.; Kim, J.; et al. Antimicrobial properties of tomato leaves, stems, and fruit and their relationship to chemical composition. BMC Complement. Med. Ther. 2021, 21, 229. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.L.; Mahoney, N.; Campbell, B.C. Antifungal activity of redox-active benzaldehydes that target cellular antioxidation. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, T.W. Statistics to Use. Available online: http://www.physics.csbsju.edu/stats/ (accessed on 17 March 2022).
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard–Second Edition. CLSI Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Faria, N.; Martins, M.; Chan, K.; Campbell, B. Enhancement of antimycotic activity of amphotericin B by targeting the oxidative stress response of Candida and Cryptococcus with natural dihydroxybenzaldehydes. Front. Microbiol. 2012, 3, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Xiao, C.L. Use of chemosensitization to overcome fludioxonil resistance in Penicillium expansum. Lett. Appl. Microbiol. 2010, 51, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Takano, Y.; Yoshimi, A.; Tanaka, C.; Kikuchi, T.; Okuno, T. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 2004, 53, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.T.; Klein, B.S. Uncertainty surrounding the mechanism and safety of the post-harvest fungicide fludioxonil. Food Chem. Toxicol. 2019, 123, 561–565. [Google Scholar] [CrossRef]
- Chen, D.; Forster, H.; Adaskaveg, J. Baseline sensitivities of major citrus, pome, and stone fruits postharvest pathogens to natamycin and estimation of the resistance potential in Penicillium digitatum. Plant Dis. 2021, 105, 2114–2121. [Google Scholar] [CrossRef]
- Haack, S.E.; Ivors, K.L.; Holmes, G.J.; Förster, H.; Adaskaveg, J.E. Natamycin, a new biofungicide for managing crown rot of strawberry caused by QoI-resistant Colletotrichum acutatum. Plant Dis. 2018, 102, 1687–1695. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhao, H.; Lyu, L.; Huang, Z.; Fan, S.; Wu, W.; Li, W. Synergistic effect of natural antifungal agents for postharvest diseases of blackberry fruits. J. Sci. Food Agric. 2019, 99, 3343–3349. [Google Scholar] [CrossRef]
- Medina, A.; Jiménez, M.; Mateo, R.; Magan, N. Efficacy of natamycin for control of growth and ochratoxin A production by Aspergillus carbonarius strains under different environmental conditions. J. Appl. Microbiol. 2007, 103, 2234–2239. [Google Scholar] [CrossRef]
- Saito, S.; Wang, F.; Xiao, C.L. Efficacy of natamycin against gray mold of stored mandarin fruit caused by isolates of Botrytis cinerea with multiple fungicide resistance. Plant Dis. 2020, 104, 787–792. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service. Dr. Duke’s Phytochemical and Ethnobotanical Databases. Salicylaldehyde. Available online: https://phytochem.nal.usda.gov/phytochem/chemicals/show/15858?qlookup=salicylaldehyde&offset=0&max=20&et= (accessed on 17 March 2022).
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Guillén, F.; Evans, C.S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 1994, 60, 2811–2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6998, Salicylaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Salicylaldehyde (accessed on 16 March 2022).
- Lai, M.H.; Silverman, S.J.; Gaughran, J.P.; Kirsch, D.R. Multiple copies of PBS2, MHP1 or LRE1 produce glucanase resistance and other cell wall effects in Saccharomyces cerevisiae. Yeast 1997, 13, 199–213. [Google Scholar] [CrossRef]
- Jiang, B.; Ram, A.F.J.; Sheraton, J.; Klis, F.M.; Bussey, H. Regulation of cell wall β-glucan assembly: PTC1 negatively affects PBS2 action in a pathway that includes modulation of EXG1 transcription. Mol. Gen. Genet. 1995, 248, 260–269. [Google Scholar] [CrossRef] [Green Version]
- García-Rodriguez, L.J.; Durán, A.; Roncero, C. Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: Evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 2000, 182, 2428–2437. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; May, G.S. Enhancement of fludioxonil fungicidal activity by disrupting cellular glutathione homeostasis with 2,5-dihydroxybenzoic acid. FEMS Microbiol. Lett. 2007, 270, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.-B.; Guan, Y.-Y.; Hu, P.-F.; Chen, L.; Xu, G.-R.; Liu, L.; Cheung, P.C.K. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Hu, P.-F.; Huang, J.; Hu, Y.-D.; Chen, L.; Xu, G.-R. Current Advances on the Structure, Bioactivity, Synthesis, and Metabolic Regulation of Novel Ubiquinone Derivatives in the Edible and Medicinal Mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405. [Google Scholar] [CrossRef]
- Wu, M.-D.; Cheng, M.-J.; Wang, W.-Y.; Huang, H.-C.; Yuan, G.-F.; Chen, J.-J.; Chen, I.-S.; Wang, B.-C. Antioxidant activities of extracts and metabolites isolated from the fungus Antrodia cinnamomea. Nat. Prod. Res. 2011, 25, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).