Abstract
In recent years, the usefulness of infrared thermography (IRT) as a valuable supplementary imaging method in medical diagnostics, as well as for assessing the effects of the treatment of musculoskeletal injuries, has been increasingly confirmed. At the same time, great importance is attached to the standards of thermographic research, the fulfillment of which determines the correct methodology and interpretation of the results. This article discusses the medical applications of infrared thermography in musculoskeletal system diseases, with particular emphasis on its usefulness in assessing the therapeutic effects of physical treatments used in rehabilitation. The literature from the last decade that is available in the Medline and Web of Science databases has been reviewed. Among the physiotherapeutic methods used, the following were selected that directly affect the musculoskeletal system: cryotherapy, laser therapy, electrotherapy, diathermy, and massage. The article summarizes all the guidelines and recommendations for IR imaging in medicine and rehabilitation.
1. Introduction
Recently, there has been radical progress in diagnostic technologies; on the other hand, applications based on already known technologies are constantly emerging in the world of medicine and rehabilitation. There are various diagnostic imaging methods used for the assessment of muscosceletal disorders (MSDs), both for diagnosis and monitoring, namely plain radiographs (X-rays, XR), dual-energy X-ray absorptiometry (DXA), ultrasonography, arthroscopy, computed tomography (CT), or magnetic resonance imaging (MRI), all of which image the morphological structures. In recent years, more and more efforts have been made to investigate the causes of MSDs and take preventive action. Considerable support is provided by supplementary tests assessing the neuromuscular function, not only the structure, including electromyography [1] and infrared thermography [2]. Imaging may be used to confirm a clinical diagnosis, or its verification when there is clinical doubt [3].
The musculoskeletal system is structurally diverse, comprising bones, muscles, joints, cartilage, ligaments, tendons, and bursae. Each of these structures can be temporarily or permanently damaged or affected by many disorders. Musculoskeletal diseases and injuries are among the most disabling diseases affecting humans and have become a major public health problem worldwide, while being a leading cause of work disability, sickness absenteeism from work, and loss of productivity [4]. According to the World Health Organization (WHO), about 1.71 billion people worldwide suffer from musculoskeletal disorders that result in severe and prolonged pain [5,6].
Musculoskeletal conditions include acute and chronic diseases that affect joints (osteoarthritis, chronic polyarthritis, rheumatoid arthritis, psoriatic arthritis, gout, and ankylosing spondylitis), bones (osteoporosis, osteopenia, and osteomalacia osteonecrosis), muscles (dystrophy, myasthenia gravis, and sarcopenia, tendonitis), and the spine (low back and neck pain), as well as multiple body areas or systems with symptoms (regional and widespread pain disorders, inflammatory diseases, connective tissue diseases, and vasculitis) affecting the musculoskeletal system.
In patients with MSDs, an accurate differential diagnosis is a key to selecting appropriate treatments; moreover, monitoring of treatment effects, apart from subjective patient assessment, should also be based on objective diagnostic methods that are affordable and do not burden the patient. Research conducted in recent years has shown that infrared thermography (IRT) is useful in medical diagnostics, and also in the case of MSDs [7]. One of the arguments in favor of conducting research on the use of IRT to diagnose MSDs may be the possibility of lowering the costs of diagnosis and treatment, and minimizing radiation harmful to patients. Thermography can be useful in early diagnosis and for monitoring the effectiveness of rehabilitation because of its high sensitivity in several situations: to assess pain during muscle injuries, inflammation in injuries and in degenerative and rheumatic changes, occupational disorders, ischemic areas, excessive friction in dentures, joint overload, asymmetry of muscle tone and activity, and various motor diseases.
In this article, the authors discuss the medical applications of IRT for musculoskeletal system diseases, with particular emphasis on its usefulness in assessing the therapeutic effects of physical procedures used in rehabilitation. The authors provide guidelines and recommendations for those interested.
2. Technical Fundamentals of IRT
Infrared thermography is a touch-free imaging technique that has the ability to map the isotherms of the surface of a selected area or an entire object, owing to the detection and registration of infrared emissions [8]. Importantly, in contrast to other imaging techniques in medicine, IRT is a completely passive (non-invasive and non-radiating) technique. The possibility of creating an image on the screen plane of the temperature distribution of a body with a temperature higher than absolute zero, as observed from a certain distance, results from several laws of physics, which are the basis for the operation of thermal imaging cameras: Stefan-Boltzmann’s law; Wien’s law; Planck’s law; and Kirchhoff’s law. Those interested in these processes are referred to theoretical studies [9,10,11] for the details.
All living objects consist of matter in random motion, containing heat from kinetic energy and having surface temperatures above absolute zero (0 K; −273.15 °C). They emit electromagnetic radiation characterized by two features: its wavelength (λ) and intensity (Q). Both the intensity and the wavelength at which the radiation is most intense depend on the surface temperature of the emitting body. Thus, objects with a certain temperature emit radiation of different wavelengths.
Though the spectral range of visibility changes from person to person, the human eye is sensitive to a fraction of the electromagnetic spectrum, ranging approximately from 400 to 760 nm [12], but it cannot detect the IR radiation emitted by human skin. The infrared emissions from human skin at 27 °C lie within the wavelength range of 2–20 μm, and they peak around 10 μm. Based on Plank’s law, roughly 90% of the infrared radiation emitted by humans is of a longer wavelength (8–15 µm) [13]. For medical applications, a very narrow wavelength band corresponding to the range 8–12 μm, termed as body infrared rays, is in general use [14].
The human body’s surface temperature can be calculated based on the total radiant power per surface area M (Wm−2). The principle of the operation of thermal imaging cameras is to measure and convert the energy of the radiation emitted by the body into a temperature value.
The total amount of energy consists of the object’s emissions, the emissions reflected from the source of the environment, and the emissions from the atmosphere. The detectors of an IR camera convert the incoming power of the electromagnetic rays emitted by the body into electronic video signals that are sequentially amplified and transmitted to monitors. Thermal images generated by IR cameras are called thermograms, where each image pixel corresponds to a digital value proportional to the amount of received energy. By reason of the achieved time resolutions of 30 frames per second (fps), it is possible to analyze dynamic changes in temperature. To calculate the surface temperature correctly based on the emitted radiation, it is necessary to know the emissivity of the object [15]. Regarding the spectral region, human skin is a black-body radiator with an emissivity factor of 0.97–0.99 [16] and is therefore a perfect emitter of infrared radiation at room temperature.
The skin microcirculatory flow determines infrared irradiation from the human surface and, in that way, also skin temperature (Tsk). There is a complex relationship between skin, the metabolism of internal tissues, and the functions of local blood vessels. The skin and skeletal muscles have a relatively low resting metabolism, therefore postganglionic neurons of the sympathetic nervous system (SNS) extend to the target organs, and also to the blood vessels and the sweat glands with the peripheral nerves or blood vessels playing a dominant role in regulating the flow in its microcirculation. For this reason, SNS is the most important way by which human body temperature is regulated through thermoregulatory mechanisms of heat exchange between organs, tissues, and skin [17]. The skin is an effectively controlled “heat radiator” system, and 60% of the total heat loss of the naked human being occurs as infrared radiation [18]. Tsk of different areas of the body is related to the area of anatomical variation in the area of subcutaneous tissue and skin, as well as innervation and blood supply.
3. Standardization in Thermographic Research
Continuous progress in the field of thermovision based on establishing measurement standards for thermography, the development of computer technologies, and the design of sensitive detectors makes the analysis of thermal temperature distributions on selected surfaces of various objects applicable in more and more different fields of science and technology, including medicine and rehabilitation. Due to the very different technical capabilities of cameras, the individual differences in temperatures of selected areas, and the diversity of research objectives, it is necessary to standardize the technique of thermal imaging in medicine. General, basic guidelines for thermographic research were introduced in 2002 and then, in 2015–2016, detailed guidelines for research in the area of neuromuscular-skeletal, dental, and systemic diseases were defined [19]. In 2017, a consensus was reached and TISEM (Thermographic Imaging in Sports and Exercise and Medicine checklist) was developed, based on Moreira et al. [20].
The temperature distribution of the human body surface is influenced by many factors, which translates directly into the results of thermographic tests; therefore, the impact of any stimuli interfering with the measurements should be minimized and the rules for the proper conduct of the experiment should be followed and described in detail in the methodology of thermal imaging studies. There are many conditions for the technical and interpretative correctness of thermal imaging studies (environmental, individual, and technical). The most important are the research room’s conditions, the patient preparation and pre-examination acclimatization period, the adjustment equipment, and the subject of the size and position of the regions of interest (ROI).
The detailed conditions for carrying out thermographic measurements for medical purposes are determined by the standards that have been developed by thermographic associations such as the American Academy of Thermology (AAT), the European Thermological Society, and the Polish Society of Thermographic Diagnostics in Medicine.
Based on the guidelines of these societies and the experiences of researchers, the requirements for thermo-imaging standards in medicine have been compiled below, taking into account factors influencing the recorded results:
- Concerning the examination room where the thermovision measurements are carried out: it should allow for the convenient and correct placement of the measuring devices and the visualization of the entire examined area and it should not be smaller than 6 m2 and it should be without unnecessary equipment; it should have a stable temperature and a relative humidity system; air-conditioning equipment should be located so that draughts are not directed at the patient and that overall air speed is kept as low as possible; airflow should be inferior to 2 m/s and the illumination should have protections to avoid incident lightning over the subject; laboratory windows have to be shut (to prevent solar radiation).
- Concerning the environmental conditions: a range of temperatures from 18 °C to 25 °C should be adjustable; the selection of the ambient temperature in which the research is conducted depends on the type and purpose of the tests (recommendation for the examination of the extremities: a warmer ambient temperature of 22–24 °C; large body surfaces examinations within 25–27 °C; lower temperatures enable better diagnosis of inflammatory changes (20 °C)); for medical examinations, it is recommended to keep the room humidity at the level of 45–55%.
- Concerning the patients: the intrinsic factors that should be taken into account during the implementation and interpretation of the research results are sex, age, anthropometric measurements, body composition, circadian and infradian rhythms, hair density, skin emissivity, medical history, metabolic rate, skin blood flow, genetics, and emotions; those that should be avoided are factors that alter metabolism (smoking, drinking sparkling water and/or hot coffee and tea; a heavy meal a minimum of two hours prior to the investigation; alcohol or drug consumption; and physiotherapy or sports on the image-collection day); also on the day of the examination, the patient must not apply any cosmetics to the tested body surface; the patient should report any infections and any medications taken; the subject’s acclimatization or equilibration period should be 15–20 min, while during this process the body surface that will be recorded must be uncovered; to minimize thermal reflections, the person under testing should be as far as possible from all equipment and walls; physical activity during all testing procedures should be kept to a minimum; the analyzed area of the body must not be touched; the examined person should not have any jewelry and should avoid crossing legs or holding arms close to or on the body; seating should be abstained from, to prevent marks that can result in skin temperature changes.
- Concerning the technical determinants of measurements: in medicine, cameras with a tunable focal length (zoom) are most often used, with a minimum focusing distance of approx. 0.5 m, a row’s field of view 25° × 19°, and an angle of divergence equal to or less than 1 mrad; an optimized temperature range (approximately 20–50 °C) will maximize the sensitivity of the sensor; a larger number of pixels (resolution) means more thermal information; it is important to control the calibration; the distance between the camera and the analyzed object should not be less than 1–1.5 m; in the selection of the ROI, the body region must be perpendicular to the lens of the infrared camera; when setting an ROI manually, including random pixels from the background or from the borders to the ROI in the analysis should be avoided [11,19,21,22,23].
It should be remembered that for the correct execution and the thermogram, it is required to enter a number of settings into the thermal imaging camera related to the external conditions of thermal imaging.
The standardization protocols for thermovision testing are constantly improving, and also in the field of medical research. The latter’s guidelines for measuring devices and the methodology of the measurements themselves were included in the report of the International Organization for Standardization in 2017, ISO 18251-1:2017 [24], entitled Medical electrical equipment—deployment, implementation and operational guidelines for identifying febrile humans using a screening thermograph, which is considered to be one of the best conductors for estimating the temperature of the tested object [25]. The American Society for Testing and Materials (ASTM) also made a significant contribution to the development and standardization of thermographic research, developing standards for testing systems and thermographic devices, and proposing methods for determining the basic parameters of thermal imaging cameras [26,27,28,29,30,31].
Thermography is used in sports, medicine, and rehabilitation, both in the terms of research on adults and children, and in physiological and pathological conditions. IRT is increasingly applicable in medicine, as well as in rehabilitation, especially in fields related to musculoskeletal dysfunctions. Thermal detectors for medical applications need to be safe, easy to use, and inexpensive. It is fast, and simultaneous large-area monitoring is possible. In medical applications, thermal analysis mainly refers to the local skin temperatures, measured at fixed points/areas on the surface of the body, as well as to the mean skin temperature over the entire body surface, which can be estimated by calculating a weighted average of a series of local skin temperatures related to the surfaces that characterize it [32].
Thermovision is used in a dozen or more fields of medicine. Those most frequently described in the literature are vascular disorders, surgeries, rheumatology, neurological disorders, gynecology, traumatology, dermatology, ophthalmology, laryngology, endocrinal disorders, and musculoskeletal disorders, including, in particular, the assessment of skin temperature changes for the diagnosis, localization, and assessment of the extent and intensity of inflammation in the examined tissue, based on the phenomenon of high thermo-emission as a result of local increased blood supply and tissue metabolism. Thermal imaging can detect joint inflammation in patients with arthritis. Arthritis has been diagnosed for centuries with the underlying symptoms of inflammation (pain, swelling, redness, and loss of function). An increase in temperature around the affected joint indicates exacerbation of inflammation. It is now known that the results of thermographic analysis strongly correlate with both the severity of the inflammation in the joints and the results of scintigraphy, which was confirmed both in human studies and in an animal model. It was found that infrared imaging can be used as a complementary method, especially in the early stages of the disease, as it can provide information on the dynamics of the pathophysiological processes in the joints [33,34]. The correlation between thermographic images and the severity of the degenerative changes has also been documented [35].
The diagnostic potential of thermography is also indicated in spine pain of various origins including root pain [36], complex regional pain syndrome [37,38], and fibromyalgia [39]. In non-specific MSD, a relationship between the Tsk changes and pain intensity was found [40].
Schmitt and Guillot assessed the usefulness of thermography in muscle injuries in athletes. They found that thermal images are helpful in assessing the severity of an injury, especially in the acute phase, in monitoring its evolution, and in prognosticating healing and recovery [41].
Correlation was confirmed between the clinical, intraoperative, and thermal images in the case of supraspinatus and Achilles tendon [42]. It has been proved that in the case of scoliosis there is thermal asymmetry, mainly in the areas of the upper back, thigh, and back shank, and the high positive correlations of the angle of trunk rotation with the size of the thermal asymmetry occurs [43].
A clear and relatively wide area of interest of researchers related to rehabilitation and the use of objective tools for the assessment of therapeutic effects is the use of IRT in the assessment of the effectiveness of physiotherapeutic treatments.
4. Materials and Methods
4.1. Study Design
Among the physiotherapeutic methods used, the following were selected that directly affect the musculoskeletal system: cryotherapy, laser therapy, electrotherapy, diathermy, and massage.
4.2. Search Strategies
In this review, relevant articles were searched for in electronic databases, including the National Library of Medicine (MEDLINE) and the Web of Science. The search process used the Boolean operator AND/OR in the combinations of the following keywords: thermovision or thermography and 1. cryotherapy, 2. laser therapy, laser treatment, 3. electrotherapy, 4. diathermy, and 5. massage.
4.3. Article Protocol Selection
Articles were included for review if they met the following inclusion criteria: (1) published in English with publication date from 2012 to February 2022, (2) original studies about selected physiotherapeutic methods monitored by thermal imaging, (3) fully accessed articles, of which a copy could be obtained by the authors, and (4) studies involving humans. Articles were excluded if the main results were not in line with the purpose of this literature review. The complete search process is illustrated in the PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow chart (Figure 1). The search results are presented in the individual subchapters of the manuscript. The summary is provided in Table 1.
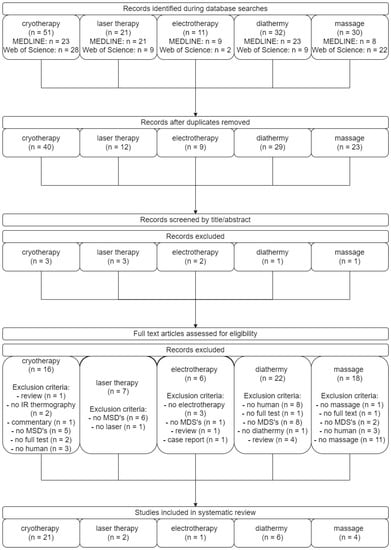
Figure 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow chart.

Table 1.
Summary of found articles by adopted criteria.
Data synthesis and analysis were performed based on a narrative summary of the results of the included studies.
5. The Use of Thermovision to Assess the Effects of Chosen Physiotherapy Treatments
5.1. Cryotherapy
Of the 51 papers found (PubMed n = 23, Web of Science n = 28), a total of 21 works were analyzed after applying the inclusion and exclusion criteria and the exclusion of copies. A total of 963 people (including 379 women, 566 men, and 18 non-identified subjects) took part in the study. The larger sample was composed of 480 subjects [49]; the smallest sample was 10 [53,57,62]. Most studies used local cryotherapy (LC) (n = 12) [44,45,47,50,53,54,59,60,61,62,63,64]; less often, whole body cryotherapy was used (n = 7) [46,48,49,51,52,55,58]. One study used both WBC and LC (using liquid nitrogen vapor) [56], and one used WBC and PBC [57].
In the case of local cryotherapy, thermovision was used primarily to compare the effects of various cooling factors on the change in the temperature of the facial skin [44], the surface of the hand [45], quadriceps surface [47], and the front of the thigh [61] and knee joint [64]. In one of the studies found, thermography was used to assess the effect of exposure to one coolant (gel pack) depending on the duration of the procedure (5 min vs. 10 min) [53].
In the Adamczyk et al. paper [54], thermovision was used to compare the assessment of the effectiveness of ice massage and immersion in cold water in the context of using it as a method of supporting post-workout regeneration. Thermovision has also made it possible to describe the optimal cooling protocols of the anterior thigh in the rugby player population [60]. On the other hand, Muñoz-Alcamí [62], using thermography, assessed changes in skin temperature in the anterior part of the thigh in response to a cold stress test after fatigue protocols during physical exercise.
Vargas et al. [59] examined the influence of cold and thermotherapy on pain tolerance, and Keramidas et al. [50] the influence of normobaric hypoxia on hand temperature reactions after a cold-water immersion test. One study demonstrated the reproducibility of the skin temperature of the lower extremities after the cold-endurance test [63].
In the case of whole body cryotherapy (WBC), thermography was used to analyze the distribution and dynamics of the temperature changes on the surface of both the whole body [46,48,55] and the individual regions of the body [49,51,52,58]. Four studies focused on analyzing the body’s response to WBC exposure [46,48,51,52], and one assessed changes in the temperature distribution of the body surface under the influence of WBC, classic massage, and hot stone massage [58]. Thermovision made it possible to determine the safety conditions of the WBC procedure [49]. One study used thermography to validate the new WBC technology [55].
Polidori et al. [57] used thermography to compare whole-body temperature distribution between WBC and PBC, while Cholewka et al. [56], focusing on the spinal region of Th5/Th6-L5/S1, verified whether PBC improves thermal diagnostics in spinal diseases in a manner comparable to WBC.
Cryotherapy (cryostimulation) is a physical procedure that exposes the body to low or extremely low temperatures. Treatments are divided into whole-body cryotherapy (WBC), partial-body cryotherapy (PBC), and local cryotherapy (LC) [77]. In WBC and PBC, patients are exposed to extremely low temperatures (−110 °C to −160 °C) for a maximum of 3 min in a specially designed chamber (cryo-chamber) or cabin (cryo-cabin). In the PBC chamber (also called a cryosauna) the patient’s head and neck are not exposed to the cold [78].
Cryotherapy is used in medicine, health, and sports, and its type depends on the desired effect. Exposure to cold causes strong fluctuations in skin temperature and leads to the stimulation of skin thermoreceptors, and thus to the stimulation of the thermoregulation center in the hypothalamus [79]. In local cryotherapy, a small area of the body is exposed to cold [64]. Cooling factors include liquid nitrogen vapors, ice bags, cold air [64], frozen gel bags [59], wetted ice, crushed ice [60], watered ice [61], cold-water immersion [50], ethyl chloride spraying, ice-block rubbing [44], or special systems [60,63].
5.2. Laser Therapy
Of the 31 papers found (PubMed n = 12, Web of Science n = 19), a total of two works were analyzed after applying the inclusion and exclusion criteria and the exclusion of copies. A total of 55 people (including 17 women and 38 men) took part in the study. Both studies used low-level laser therapy (LLLT).
Bilska et al. [65] demonstrated the usefulness of thermovision as a prognostic tool during treatment of stage III and IV pressure ulcers. Stamborowski et al. [66], based on thermograms, investigated the effect of photomodulation from low-level laser therapy on muscle fatigue and the temperature of the brachial biceps.
Low-level laser therapy (LLLT) energy is so low that it does not exhibit photobiological effects, but it is sufficient to elicit a stimulatory response from body tissue. The wavelength used is in the range 390–10,600 nm and the output power is up to 500 mW. LLLT does not produce thermal or ablative effects (rather a photochemical effect); therefore, it is called “soft” laser therapy or cold laser therapy [80]. LLLT has found application in promoting tissue regeneration, relieving pain, and reducing inflammation [81,82].
5.3. Electrotherapy
Of the 11 papers found (PubMed n = 9, Web of Science n = 2), a total of one work was analyzed after applying the inclusion and exclusion criteria and the exclusion of copies.
Benito-Martínez et al. [67] examined 45 people (25 women and 20 men) and used thermography to investigate the effects of applying symmetrical biphasic square currents on skin temperature.
Electrotherapy is one of the basic methods used in physiotherapy, which consists of introducing a certain amount of physical energy into the biological system. The result is physiological changes that are used for therapeutic purposes [83]. Depending on the expected effect, direct current, pulsed low-frequency, and an alternating current of medium and high frequencies are used [84]. Applied by Benito-Martínez et al. [67], neuromuscular electrical stimulation (NMES) is used both for selective muscle overtraining and swelling control, and for maintaining muscle mass and strength during longer periods of immobilization.
5.4. Diathermy
Of the 32 papers found (PubMed n = 23, Web of Science n = 9), a total of six works were analyzed after applying the inclusion and exclusion criteria and the exclusion of copies. A total of 186 people (including 96 women and 90 men) took part in the study. The larger sample was composed of 60 subjects [68], and the smallest sample was 10 [70]. In the found works, the diathermy methods included extracorporeal shock waves (ESW) [68], short- [69,72,73] and microwave diathermy [69], and Tecar therapy (TT) [70,71].
Dymarek et al. [68] used thermography to detect thermal conditions in the region of the carpal flexor muscles to assess the effects of the radial extracorporeal shock wave stimulation. In case of short- and microwave diathermy, thermography was used to evaluate the temperature behavior and arterial blood flow after lower limb administration [69] and to evaluate local and general temperature variations in the area of the right knee joint [72]. Furthermore, Benincá [73], using the three capacitive techniques of shortwave diathermy treatment (contra-, coplanar, and longitudinal arrangement), compared the effectiveness in changing skin temperature. Clijse [70], using thermography, verified whether TT administered in two modes (resistive and capacitive) floats on intramuscular blood flow, perfusion of skin microcirculation, and skin temperature. In turn, Yeste-Fabregat [71], in their research among professional basketball players, analyzed the effect of TT on latent myofascial trigger points on skin temperature, range of ankle motion, and pain.
Diathermy is a thermotherapeutic method that uses high-frequency electromagnetic currents to induce heat [85]. Diathermy includes ultrasound, shortwave, and microwave [86]. Both shortwave diathermy (SWD) and microwave diathermy (MWD) have been used in the treatment of musculoskeletal conditions [69]. In SWD, heat is generated at a deep tissue level by generating electromagnetic vibrations, which in turn lead to ion movement, deformation of molecules, and the formation of electric vortices. The treatment causes vasodilation, increases metabolism in the body, and normalizes muscle tension [72]. In addition, they can reduce pain and promote wound healing [87].
5.5. Massage
Of the 30 papers found (PubMed n = 8, Web of Science n = 22), a total of four works were analyzed after applying the inclusion and exclusion criteria and the exclusion of copies. A total of 127 people (including 85 women and 42 men) took part in the study. The larger sample was composed of 40 subjects [58,76], and the smallest sample was 12 [74]. The studies using thermography included rhythmical [74], classic [58,75,76], isometric [76], and with hot stone [58] massage.
Wälchli et al. [74], using thermography, analyzed the heat distribution after the treatment sessions. In the case of classic massage, two works by the same authors were found [75,76]. In the first one, the authors focused on determining the relationship between the classic sports massage of the hands and forearms and the temperature of the surface of the muscles of the upper limbs [75]. In the second, they focused on the relationship between isometric and classic massage and selected parameters of the quadriceps muscle of the thigh [76]. Classic massage was also the subject of the study of Gruszka et al. [58], who assessed the changes in the temperature distribution of the body surface under the influence of WBC, classic massage, and hot stone massage using thermography.
According to the Ottawa Panel definition, massage is “soft tissue and joint manipulation using the hands or a handheld device” [88]. Massage therapy improves blood and lymph flow, reduces muscle tension, lowers blood pressure, and provides relief in many diseases of the musculoskeletal system [89].
6. Conclusions
As the review shows, thermovision is an undeniably important diagnostic tool in the physiotherapeutic methods used in musculoskeletal dysfunctions. Most of the studies found concern cold-therapy treatments. Nevertheless, thermovision is becoming more and more popular among other therapeutic methods (e.g., diathermy or massage). However, there is a significant gap regarding the influence of laser therapy and electrotherapy on the distribution of temperature in the treatment area.
Thermovision makes it possible to evaluate the effectiveness of a given procedure, and it provides information on the temperature distribution of the whole body and individual areas. As a non-contact and non-invasive method, it does not affect the results of the procedure; therefore, the obtained results are reliable and authoritative.
Authors recommend the use of thermography to assess the effectiveness of physiotherapeutic procedures, in accordance with the test standards and taking into account the factors affecting the recorded results.
Author Contributions
Conceptualization, A.L. and W.P.; methodology, A.L. and W.P.; software, A.L. and W.P.; validation, A.L. and W.P.; formal analysis, A.L.; investigation, A.L. and W.P.; resources, A.L.; data curation, W.P.; writing—original draft preparation, A.L. and W.P.; writing—review and editing, A.L. and W.P.; visualization, W.P.; supervision, A.L.; project administration, A.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zawawi, T.N.S.T.; Abdullah, T.; Sudriman, R.; Saad, N.M.; Too, J.; Shair, E.F. Classification of EMG signal for health screening task for musculoskeletal disorder. Int. J. Eng. Technol. 2019, 8, 219–226. [Google Scholar] [CrossRef]
- Albuquerque, N.F.; Lopes, B.S. Musculoskeletal applications of infrared thermography on back and neck syndromes: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 386–396. [Google Scholar] [CrossRef]
- Wenham, C.Y.J.; Grainger, A.J.; Conaghan, P.G. The role of imaging modalities in the diagnosis, differential diagnosis and clinical assessment of peripheral joint osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1692–1702. [Google Scholar] [CrossRef]
- Bevan, S. Economic impact of musculoskeletal disorders (MSDs) on work in Europe. Best Pract. Res. Clin. Rheumatol. 2015, 29, 356–373. [Google Scholar] [CrossRef]
- Musculoskeletal Conditions. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 27 February 2022).
- Mock, C.; Cherian, M.N. The global burden of musculoskeletal injuries: Challenges and solutions. Clin. Orthop. Relat. Res. 2008, 466, 2306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Sun, H.; Jiang, Y.; Lou, J.; He, X.; Fang, J. Infrared thermography in the diagnosis of musculoskeletal injuries: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e23529. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Zeilberger, K.; Francis, E.; Ring, J.; Raschner, C. The application of medical infrared thermography in sports medicine. In An International Perspective on Topics in Sports Medicine and Sports Injury; Zaslav, K.R., Ed.; Intech Open: London, UK, 2012. [Google Scholar] [CrossRef]
- Minkina, W. Theoretical basics of radiant heat transfer—Practical examples of calculation for the infrared (IR) used in infrared thermography measurements. Quant. Infrared Thermogr. J. 2021, 18, 269–282. [Google Scholar] [CrossRef]
- Speakman, J.R.; Ward, S. Infrared thermography: Principles and applications. Zoology 1998, 101, 224–232. [Google Scholar]
- Vardasca, R.; Vaz, L.; Mendes, J. Classification and decision making of medical infrared thermal images. In Lecture Notes in Computational Vision and Biomechanics; Springer: Berlin/Heidelberg, Germany, 2018; Volume 26, pp. 79–104. [Google Scholar]
- Batista-Leyva, A.J. Radiometry and photometry: Two visions of one phenomenon. Rev. Cub. Fis. 2019, 36, 66–72. [Google Scholar]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An overview of recent application of medical infrared thermography in sports medicine in Austria. Sensors 2010, 10, 4700. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Diakides, N.A. Infrared Imaging in Medicine; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849390272. [Google Scholar]
- Pereira, C.B.; Yu, X.; Dahlmanns, S.; Blazek, V.; Leonhardt, S.; Teichmann, D. Infrared thermography. In Multi-Modality Imaging; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–30. ISBN 9783319989747. [Google Scholar]
- Steketee, J. Spectral emissivity of skin and pericardium. Phys. Med. Biol. 1973, 18, 686–694. [Google Scholar] [CrossRef]
- Merla, A.; Romani, G.L. Functional infrared imaging in medicine: A quantitative diagnostic approach. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 224–227. [Google Scholar]
- Guyton, A.; Hall, J. Textbook of Medical Physiology, 11th ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2006; ISBN 9780323597128. [Google Scholar]
- Shterenshis, M. Challenges to global implementation of infrared thermography technology: Current perspective. Cent. Asian J. Glob. Health 2017, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.E.; Costa, C.M.A.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fujimasa, I. Standardization of techniques for thermal imaging testing: The current situation. Part 1. Basic information. Biomed. Thermol. J. Jpn. Soc. Thermorogy 1995, 15, 63–68. [Google Scholar]
- Al-Nakhli, H.H.; Petrofsky, J.S.; Laymon, M.S.; Berk, L.S. The use of thermal infra-red imaging to detect delayed onset muscle soreness. J. Vis. Exp. 2012, 59, e3551. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuevas, I.; Bouzas Marins, J.C.; Arnáiz Lastras, J.; Gómez Carmona, P.M.; Piñonosa Cano, S.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- ISO TR13154; Medical Electrical Equipment—Deployment, Implementation and Operational Guidelines for Identifying Febrile Humans Using a Screening Thermograph. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33. [Google Scholar] [CrossRef] [PubMed]
- ASTM E 1213; Standard Practice for Minimum Resolvable Temperature Difference for Thermal Imaging Systems. ASTM International: West Conshohocken, PA, USA, 2009.
- ASTM E 1256; Standard Test Methods for Radiation Thermometers (Single Waveband Type). ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM E 1311; Standard Test Method for Minimum Detectable Temperature Difference for Thermal Imaging Systems. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM E 1543; Standard Test Method for Noise Equivalent Temperature Difference of Thermal Imaging Systems. ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM E 1862; Standard Test Methods for Measuring and Compensating for Reflected Temperature Using Infrared Imaging Radiometers. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM E 1933; Standard Test Methods for Measuring and Compensating for Emissivity Using Infrared Imaging Radiometers. ASTM International: West Conshohocken, PA, USA, 2005.
- Jasti, N.; Bista, S.; Bhargav, H.; Sinha, S.; Gupta, S.; Chaturvedi, S.K.; Gangadhar, B.N. Medical applications of infrared thermography: A narrative review. In Stem Cells in Disease Pathogenesis; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 415–453. ISBN 9781536196580. [Google Scholar]
- Brenner, M.; Braun, C.; Oster, M.; Gulko, P.S. Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity. Ann. Rheum. Dis. 2006, 65, 306. [Google Scholar] [CrossRef]
- Lasanen, R.; Piippo-Savolainen, E.; Remes-Pakarinen, T.; Kröger, L.; Heikkilä, A.; Julkunen, P.; Karhu, J.; Töyrös, J. Thermal imaging in screening of joint inflammation and rheumatoid arthritis in children. Physiol. Meas. 2015, 36, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Denoble, A.E.; Hall, N.; Pieper, C.F.; Kraus, V.B. Patellar skin surface temperature by thermography reflects knee osteoarthritis severity. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, 69–75. [Google Scholar] [CrossRef]
- Zuzda, J.G.; Topczewska, M.; Borkowski, P.; Latosiewicz, R. The influence of rotational training on muscle activity of young adults in thermographic imaging. Stud. Logic Gramm. Rhetor. 2018, 56, 91–105. [Google Scholar] [CrossRef]
- Choi, E.; Lee, P.B.; Nahm, F.S. Interexaminer reliability of infrared thermography for the diagnosis of complex regional pain syndrome. Skin Res. Technol. 2013, 19, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Conwell, T.D.; Hobbins, W.B.; Giordano, J. Sensitivity, specificity and predictive value of infrared cold water autonomic functional stress testing as compared with modified IASP criteria for CRPS. Thermol. Int. 2010, 20, 60–68. [Google Scholar]
- Mitani, Y.; Fukunaga, M.; Kanbara, K.; Takebayashi, N.; Ishino, S.; Nakai, Y. Evaluation of psychophysiological asymmetry in patients with fibromyalgia syndrome. Appl. Psychophysiol. Biofeedback 2006, 31, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zaproudina, N. Methodological Aspects of Use of Infrared Thermography in Healthy Individuals and Patients with Non-Specific Musculoskeletal Disorders. Doctoral Dissertation, University of Eastern Finland, Kuopio, Finland, 2011. [Google Scholar]
- Schmitt, M.; Guillot, Y. Thermography and muscular injuries in sports medicine. In Recent Advances in Medical Thermology; Springer: Boston, MA, USA, 1984; pp. 439–445. [Google Scholar] [CrossRef]
- Park, J.Y.; Hyun, J.K.; Seo, J.B. The effectiveness of digital infrared thermographic imaging in patients with shoulder impingement syndrome. J. Shoulder Elb. Surg. 2007, 16, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Gajewska, E. Temperature distribution of selected body surfaces in scoliosis based on static infrared thermography. Int. J. Environ. Res. Public Health 2020, 17, 8913. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.G.; Park, H.J.; Chae, H.Y.; Kim, B.G.; Lim, H.S.; Park, J.I.; Kim, J.H. Comparison of changes in facial skin temperature caused by ethyl chloride spraying, ice block rubbing and cold gel packing in healthy subjects. J. Oral Rehabil. 2012, 39, 931–940. [Google Scholar] [CrossRef]
- Korman, P.; Straburzyńska-Lupa, A.; Romanowski, W.; Trafarski, A. Temperature changes in rheumatoid hand treated with nitrogen vapors and cold air. Rheumatol. Int. 2012, 32, 2987–2992. [Google Scholar] [CrossRef][Green Version]
- Cholewka, A.; Stanek, A.; Sieroń, A.; Drzazga, Z. Thermography study of skin response due to whole-body cryotherapy. Ski. Res. Technol. 2012, 18, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo de Carvalho, A.; Lazzeri de Medeiros, D.; Tibes de Souza, F.; Francine de Paula, G.; Mantovani Barbosa, P.; Olegini Vasconcellos, P.R.; Rosângela Buzanello, M.; Flor Bertolini, G.R. Temperature variation of the quadriceps femoris muscle exposed to two forms of cryotherapy by means of thermography. Rev. Bras. Med. Esporte 2012, 18, 109–111. [Google Scholar]
- Zalewski, P.; Klawe, J.J.; Pawlak, J.; Tafil-Klawe, M.; Newton, J. Thermal and hemodynamic response to whole-body cryostimulation in healthy subjects. Cryobiology 2013, 66, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Dȩbiec-Ba̧k, A.; Skrzek, A.; Podbielska, H. Application of thermovision for estimation of the optimal and safe parameters of the whole body cryotherapy. J. Therm. Anal. Calorim. 2013, 111, 1853–1859. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Kölegård, R.; Mekjavic, I.B.; Eiken, O. Acute effects of normobaric hypoxia on hand-temperature responses during and after local cold stress. High Alt. Med. Biol. 2014, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Chudecka, M.; Zaborski, D.; Lubkowska, A.; Grzesiak, W.; Klimek, A.; Modrzejewski, A. Temperature changes in selected areas of body surface induced by systemic cryostimulation. Aviat. Space. Environ. Med. 2014, 85, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Dębiec-Bąk, A.; Pawik, Ł.; Skrzek, A. Thermoregulation of football players after cryotherapy in thermography. J. Therm. Anal. Calorim. 2016, 126, 1633–1644. [Google Scholar] [CrossRef]
- Vellard, M.; Arfaoui, A. Detection by infrared thermography of the effect of local cryotherapy exposure on thermal spreadin skin. J. Imaging 2016, 2, 20. [Google Scholar] [CrossRef]
- Adamczyk, J.G.; Krasowska, I.; Boguszewski, D.; Reaburn, P. The use of thermal imaging to assess the effectiveness of ice massage and cold-water immersion as methods for supporting post-exercise recovery. J. Therm. Biol. 2016, 60, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Arfaoui, A.; Grappe, F.; Ravier, G.; Jarlot, B.; Dugue, B. Validation of a new whole-body cryotherapy chamber based on forced convection. J. Therm. Biol. 2017, 65, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cholewka, A.; Stanek, A.; Wójcik, M.; Sieroń-Stołtny, K.; Drzazga, Z. Does local cryotherapy improve thermal diagnosis similar to whole-body cryotherapy in spinal diseases? J. Therm. Anal. Calorim. 2017, 127, 1155–1162. [Google Scholar] [CrossRef]
- Polidori, G.; Taiar, R.; Legrand, F.; Beaumont, F.; Murer, S.; Bogard, F.; Boyer, F.C. Infrared thermography for assessing skin temperature differences between partial body cryotherapy and whole body cryotherapy devices at −140 °C. Infrared Phys. Technol. 2018, 93, 158–161. [Google Scholar] [CrossRef]
- Gruszka, K.; Szczuka, E.; Całkosiński, I.; Sobiech, K.A.; Chwałczyńska, A. Thermovision analysis of surface body temperature changes after thermal stimulation treatments in healthy men. Acta Bioeng. Biomech. 2018, 20, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Vargas e Silva, N.C.O.; Rubio, A.L.; Alfieri, F.M. Pain tolerance: The influence of cold or heat therapy. J. Chiropr. Med. 2019, 18, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Rhodes, D.D.; Birdsall, D.; Selfe, P.J. Comparison of cryotherapy modality application over the anterior thigh across rugby union positions; a crossover randomized controlled trial. Int. J. Sports Phys. Ther. 2020, 15, 210–220. [Google Scholar] [CrossRef] [PubMed]
- De Estéfani, D.; Ruschel, C.; Benincá, I.L.; dos Santos Haupenthal, D.P.; de Avelar, N.C.P.; Haupenthal, A. Volume of water added to crushed ice affects the efficacy of cryotherapy: A randomised, single-blind, crossover trial. Physiotherapy 2020, 107, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Alcamí, M.; Priego-Quesada, J.I.; Gimeno Raga, M.; Durán Lozano, Á.; Gil-Calvo, M. Effect of fatigue strength exercise on anterior thigh skin temperature rewarming after cold stress test. J. Therm. Biol. 2021, 101, 103098. [Google Scholar] [CrossRef] [PubMed]
- Priego-Quesada, J.I.; Gandia-Soriano, A.; Pellicer-Chenoll, M.T.; Catalá-Vilaplana, I.; Bermejo-Ruiz, J.L.; Encarnación-Martínez, A.; Salvador-Palmer, R.; Cibrián Ortiz de Anda, R. Reproducibility of skin temperature response after cold stress test using the game ready system: Preliminary study. Int. J. Environ. Res. Public Health 2021, 18, 8295. [Google Scholar] [CrossRef] [PubMed]
- Radecka, A.; Pluta, W.; Lubkowska, A. Assessment of the dynamics of temperature changes in the knee joint area in response to selected cooling agents in thermographic tests. Int. J. Environ. Res. Public Health 2021, 18, 5326. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Stangret, A.; Pyzlak, M.; Wojdasiewicz, P.; Szukiewicz, D. Skin surface infrared thermography in pressure ulcer outcome prognosis. J. Wound Care 2020, 29, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Stamborowski, S.F.; de Oliveira Spinelli, B.M.; Lima, F.P.S.; Costa, D.R.; de Silveira Souza, G.A.; Lima, M.O.; Lopes Martins, R.A.B. The influence of photobiomodulation on the temperature of the brachial biceps during muscle fatigue protocol. Lasers Med. Sci. 2021, 36, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martínez, E.; Senovilla-Herguedas, D.; de la Torre-Montero, J.C.; Martínez-Beltrán, M.J.; Reguera-García, M.M.; Alonso-Cortés, B. Local and contralateral effects after the application of neuromuscular electrostimulation in lower limbs. Int. J. Environ. Res. Public Health 2020, 17, 9028. [Google Scholar] [CrossRef]
- Dymarek, R.; Taradaj, J.; Rosińczuk, J. The effect of radial extracorporeal shock wave stimulation on upper limb spasticity in chronic stroke patients: A single-blind, randomized, placebo-controlled study. Ultrasound Med. Biol. 2016, 42, 1862–1875. [Google Scholar] [CrossRef]
- De Sousa, N.T.A.; Guirro, E.C.D.O.; Calió, J.G.; de Queluz, M.C.; Guirro, R.R.D.J. Application of shortwave diathermy to lower limb increases arterial blood flow velocity and skin temperature in women: A randomized controlled trial. Braz. J. Phys. Ther. 2017, 21, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the application of tecar therapy affect temperature and perfusion of skin and muscle microcirculation? A pilot feasibility study on healthy subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar] [CrossRef]
- Yeste-Fabregat, M.; Baraja-Vegas, L.; Vicente-Mampel, J.; Pérez-Bermejo, M.; Bautista González, I.J.; Barrios, C. Acute effects of tecar therapy on skin temperature, ankle mobility and hyperalgesia in myofascial pain syndrome in professional basketball players: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 8756. [Google Scholar] [CrossRef]
- Kaźmierska, B.; Sobiech, K.A.; Demczuk-Włodarczyk, E.; Chwałczyńska, A. Thermovision assessment of temperature changes in selected body areas after short-wave diathermy treatment. J. Therm. Anal. Calorim. 2021, 1–8. [Google Scholar] [CrossRef]
- Benincá, I.L.; de Estéfani, D.; Pereira de Avelar, N.C.; Pacheco dos Santos Haupenthal, D.; Lock Silveira, P.C.; Haupenthal, A. Coplanar arrangement of shortwave diathermy is the most effective in skin temperature change: A randomized crossover trial. J. Bodyw. Mov. Ther. 2021, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wälchli, C.; Saltzwedel, G.; Krüerke, D.; Kaufmann, C.; Schnorr, B.; Rist, L.; Eberhard, J.; Decker, M.; Simões-Wüst, A.P. Physiologic effects of rhythmical massage: A prospective exploratory cohort study. J. Altern. Complement. Med. 2014, 20, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, D.; Adamczyk, J.G.; Urbańska, N.; Mrozek, N.; Piejko, K.; Janicka, M.; Białoszewski, D. Using thermal imaging to assess the effect of classical massage on selected physiological parameters of upper limbs. Biomed. Hum. Kinet. 2014, 60, 20–25. [Google Scholar] [CrossRef]
- Boguszewski, D.; Adamczyk, J.G.; Hadamus, A.; Mosiołek, A.; Ochal, A.; Białoszewski, D. Evaluation of the effect of isometric and classic massage on selected physiological and biomechanical parameters of the lower extremities. Acta Kinesiol. 2020, 14, 109–114. [Google Scholar]
- Roszkowska, K.; Witkowska-Pilaszewicz, O.; Przewozny, M.; Cywinska, A. Whole body and partial body cryotherapies—Lessons from human practice and possible application for horses. BMC Vet. Res. 2018, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Fonda, B.; Sarabon, N. Actual temperature during and thermal response after whole-body cryotherapy in cryo-cabin. J. Therm. Biol. 2013, 38, 186–191. [Google Scholar] [CrossRef]
- Hirvonen, H.; Kautiainen, H.; Moilanen, E.; Mikkelsson, M.; Leirisalo-Repo, M. The effect of cryotherapy on total antioxidative capacity in patients with active seropositive rheumatoid arthritis. Rheumatol. Int. 2017, 37, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Baroni, B.M.; Rodrigues, R.; Freire, B.B.; de Azevedo Franke, R.; Geremia, J.M.; Vaz, M.A. Effect of low-level laser therapy on muscle adaptation to knee extensor eccentric training. Eur. J. Appl. Physiol. 2015, 115, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, J. Basic concepts in electricity and electrotherapy. In Electrical Stimulation for Pelvic Floor Disorders; Springer: Cham, Switzerland, 2015; pp. 61–74. ISBN 9783319069470. [Google Scholar]
- Putowski, M.; Piróg, M.; Podgórniak, M.; Padała, O.; Sadowska, M.; Bazylevycz, A.; Wdowiak, A. The use of electromagnetic radiation in the physiotherapy. Eur. J. Med. Technol. 2016, 2, 53–58. [Google Scholar]
- Benincá, I.L.; de Estéfani, D.; Pereira de Souza, S.; Weisshahn, N.K.; Haupenthal, A. Tissue heating in different short wave diathermy methods: A systematic review and narrative synthesis. J. Bodyw. Mov. Ther. 2021, 28, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Lineaweaver, W.C.; Zhang, F.; Zhang, J. Role of shortwave and microwave diathermy in peripheral neuropathy. J. Int. Med. Res. 2019, 47, 3569–3579. [Google Scholar] [CrossRef]
- Farahat, A.E.; Kahil, H.M.; Hussein, F.A. Microwave diathermy for deep heating therapy of knee joint. Prog. Electromagn. Res. C 2020, 99, 15–33. [Google Scholar] [CrossRef]
- Brosseau, L.; Wells, G.A.; Poitras, S.; Tugwell, P.; Casimiro, L.; Novikov, M.; Loew, L.; Sredic, D.; Clément, S.; Gravelle, A.; et al. Ottawa panel evidence-based clinical practice guidelines on therapeutic massage for low back pain. J. Bodyw. Mov. Ther. 2012, 16, 424–455. [Google Scholar] [CrossRef]
- Ernst, E. Massage therapy for cancer palliation and supportive care: A systematic review of randomised clinical trials. Support. Care Cancer 2009, 17, 333–337. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).