Abstract
Black garlic is produced as a result of the so-called “fermentation processes” of whole heads or cloves kept under controlled conditions of temperature and humidity for several weeks. During this long-term heat treatment, garlic undergoes enzymatic and non-enzymatic browning reactions, which greatly change its taste, aroma, physicochemical, organoleptic and bioactive properties. Black garlic is most often produced in the form of cloves, and recently also in the form of paste and powder. This work focused on the comparison of functional properties of black garlic, such as volatile compounds, taste profile, total polyphenols content, antioxidant activity, color (CIE L*, a*, b*), water activity (aw), pH, soluble solids content (°Brix) and moisture content, depending on the form of its occurrence: cloves, spreading paste and powder. After long-term heat treatment, garlic was characterized by a higher content of dry matter and water-soluble solids, respectively at about 22% and 24% for spreading paste and 166% and 44% for powder. The conducted research showed significant differences in the bioactive properties of the tested garlic samples, with the lowest content of polyphenols and antioxidant properties in fresh, unprocessed garlic (6.05 ± 0.07 mg GAE/1 g d.m. and 232.95 ± 4.06 µM TEAC/1 g d.m., respectively), while in garlic subjected to long-term heat treatment, the total polyphenols content and antioxidant potential were two times higher than in the unprocessed garlic. The polyphenol content and antioxidant properties were the highest in the spread garlic (respectively, 15.16 ± 0.08 mg GAE/1 g d.m. and 638.46 ± 3.37 µM TEAC/1 g d.m.) and the lowest in the powdered samples (respectively, 11.02 ± 0.51 mg GAE/1 g d.m. and 541.71 ± 5.22 µM TEAC/1 g d.m.). Obtained black garlic samples gain completely different sensory characteristics determined using instrumental methods. In black garlic and its preparations, the intensity of unpleasant taste and aroma is reduced as a result of the appearance of metabolites during the long-term heat treatment, which in turn determined the specific, delicate sweet–sour taste and pleasant aroma, completely unrelated to the aroma of the unprocessed product. Taking into account the obtained results, it can be stated that black garlic, in the form of cloves, paste and powder, exhibits completely different properties than white garlic.
1. Introduction
Garlic (Allium sativum L.) is a plant with a high content of various bioactive compounds (including sulfur compounds—alliin, polyphenolic compounds, minerals—manganese, selenium, calcium, B vitamins and vitamin C), and therefore for centuries it has been considered a medicine for many diseases [1]. Garlic, due to its diaphoretic, expectorant, antispasmodic, bacteriostatic and antiviral properties, is commonly used in the treatment of bronchial and upper respiratory tract inflammation, abdominal pain, recurrent infections and influenza [2]. Its antibacterial, antiviral, antidiabetic, anticancer, hypotensive, hypolipidemic, antiatherosclerotic, cardioprotective and antioxidant properties are also widely documented and widely known to consumers [3,4,5,6,7,8].
The main compounds responsible for the health-promoting properties of garlic are sulfur-containing compounds, such as S-allyl-l-cysteine sulfoxide (alliin) and γ-glutamyl-S-allyl-l-cysteine, which under the influence of the allinase enzyme are hydrolyzed to sulfides, disulfides and trisulfides, diallyl and ajoenes and other organosulfur metabolites such as S-allylcysteine [1,3,4,9,10]. These compounds, apart from showing health-promoting properties, are also responsible for the characteristic, distinctive, sharp taste and smell of garlic, which are unacceptable to some consumers [11,12]. Therefore, technologists and food producers are looking for solutions to meet the expectations of consumers related to the supply of garlic with high bioactive properties, which is also characterized by a neutral taste and smell. A product with such properties is the so-called “black garlic” formed during the prolonged heating of garlic under certain temperature and humidity conditions [13,14,15].
Black garlic is produced as a result of the so-called “fermentation processes” of whole heads or cloves kept under controlled conditions of temperature (60–90 °C) and humidity (70–90%) for a period of several weeks (usually 30–90 days). This process takes place without the participation of microorganisms (bacteria and fungi) and without the addition of preservatives, but only under the influence of enzymes naturally occurring in the raw material, as a result of natural reactions and biochemical transformations of compounds present in the raw material [16,17,18]. During this long-term heat treatment, garlic undergoes enzymatic and non-enzymatic browning reactions, such as oxidation, caramelization and Maillard reactions, which greatly change the physicochemical, organoleptic and bioactive properties of garlic [19,20,21]. Moreover, as a result of fermentation processes, unstable organosulfur compounds present in raw garlic (mainly allicin responsible for the sharp and unpleasant taste and smell of fresh garlic) are transformed into stable, water-soluble, odorless compounds, the most important of which are S-allylcysteine, S-allyl-mercapto cysteine and other organosulfur ingredients, 5-hydroxymethylfurfural, alkaloids and polyphenols (including phenolic acids and flavonoids), which show very high antioxidant activity [21,22,23,24,25,26]. For this reason, black garlic has many times higher antioxidant potential than raw garlic; thus, fermentation improves the bioactive profile of raw garlic [27,28,29,30]. In addition, the content of reducing sugars and organic acids significantly increases during the ageing process of black garlic, which also changes the taste of black garlic to sweet and sour, which is much more acceptable to consumers [23,31,32]. All these mentioned compounds possess a strong correlation with biological and pharmacological properties such as antioxidant, anti-inflammatory, anti-cancer, anti-allergic, anti-allergic, hypolipidemic, neuroprotective and hepatoprotective activities [13,26,33,34]. The functional properties of “black garlic”, especially the content of bioactive compounds such as polyphenols, and the antioxidant activity are widely described in the recent literature [26,35,36]. Most of the available studies compare the functional properties of “black garlic” with unprocessed “white” cloves due to the fact that black garlic is most often produced in the form of cloves [14,15,16,17,18,19,20,28,29,30,31,32,33,34,35,36].
Recently, many products obtained from black garlic have appeared on the food market, including black garlic in the form of a paste or powder. Black garlic produced in these forms greatly facilitates its use by consumers, both in terms of the possibility of its longer storage (paste heat-fixed by pasteurization, powder with a very low aw), as well as its use during the preparation of meals.
The research carried out by our team [31,32] confirmed the high bioactive properties of black garlic, which were found to be much higher compared to traditional white garlic. Previous manuscripts have focused on comparing the main physicochemical parameters of black garlic with white garlic, such as moisture, extract, sugar, pH and bioactive properties, i.e., polyphenol content and antioxidant potential. This work is a continuation of the above-mentioned research; however, in this work, the research was conducted on black garlic paste and powder, which have recently been introduced to the market.
The first objective of this research was to determine dry matter, moisture content, pH, soluble solids content (°Brix), total polyphenols content and antioxidant activity. The second objective was to determine volatile compounds, taste profile and color measurement depending on the form of the occurrence of garlic: cloves, spreading paste and powder.
2. Materials and Methods
2.1. Materials
The research was carried out in 2021 on garlic (Allium sativum L.), cultivar “Harnaś”, grown in an ecological cultivation system (farm located in the Warmińsko-Mazurskie Voivodeship, Lubawa, Poland, 53°38′39.2″ N 19°50′48.6″ E).
The fresh material was cleaned and stored in a refrigerator at 10 °C and 85% relative air humidity for 24 h. One part of the fresh, unprocessed white (WG) garlic was used for physicochemical tests, while the rest was used for the production of black garlic (BG). Black garlic was produced by the thermal treatment of whole garlic bulbs in climate chamber (Humidity Chamber HCP70, Memmert GmbH & Co., KG, Schwabach, Germany) at 70 °C temperature and with 80% relative humidity for 45 days, as previously described [31,32].
The flow diagram of the study is presented in Figure 1.
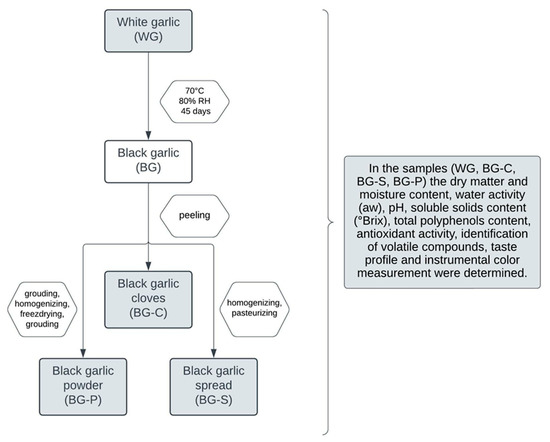
Figure 1.
The flow diagram showing the methodology followed in the study.
The black garlic was peeled, the cloves were separated, and all material obtained in this way was divided into three parts (BG-C, BG-P, BG-S). Garlic in the form of cloves (BG-C) was stored in a glass vessel under refrigeration conditions (10 °C and 85% relative air humidity) until physicochemical tests were carried out. To prepare the black garlic powder (BG-P), the cloves were ground, homogenized for 1 min (10,000 rpm), (IKA T18 Basic ULTRA-TURRAX®, IKA® Poland Ltd., Warsaw, Poland), placed in in plastic containers, frozen in liquid nitrogen and freeze-dried (Alpha 1-4 LSC, Martin Christ GmbH, Osterodeam Harz, Germany) at a chamber pressure of 10 Pa, a drying chamber temperature of −50 °C and a shelf of 21 °C for 72 h. The freeze-dried black garlic macerate was ground in a laboratory knife mill (Grindomix GM 200, Retsch GmbH, Haan, Germany), packed into sterile plastic containers and stored in a −20 °C freezer until analysis. The black garlic spread (BG-S) was prepared by the fragmentation and homogenization for 1 min (5000 rpm) (IKA T18 Basic ULTRA-TURRAX®, IKA® Poland Ltd., Warsaw, Poland) of black garlic cloves. The macerate prepared in this way was placed in a dark glass jar and then pasteurized (at 70 ± 2 °C temperature for 15 ± 2 min).
In the research material obtained in this way, i.e., in cloves of fresh, white (WG) and black (BG-C) garlic, in powder (BG-P) and spread (BG-S) of black garlic (Figure 2), the dry matter and moisture content, water activity (aw), pH, soluble solids content (°Brix), total polyphenols content, antioxidant activity, identification of volatile compounds, taste profile and instrumental color measurement were determined.
Figure 2.
Plant material used in the research: (a) WG—white garlic, (b) BG-C—black garlic cloves, (c) BG-S—black garlic spread and (d) BG-S—black garlic spread.
2.2. Methods
2.2.1. Dry Matter and Moisture Content, Water Activity, pH, Soluble Solids Content (°Brix)
To determine the dry matter content in the samples of white and black garlic, the gravimetric method was used in accordance with the AOAC method [37]. Weighed weighing vessels were filled with garlic samples, reweighed again and dried in a laboratory drier (SUP 200W, Wamed, Warsaw, Poland) at 105 °C. After 72 h, the samples were cooled to 21 °C and weighed. The dry matter and moisture content was calculated from the difference in weight, and the results were expressed as a percentage (%). Water activity (aw) was measured using an AquaLab Water Activity Meter with a temperature stabilizer (Decagon Devices. Inc., Pullman, WA, USA). In all garlic samples, in a 1:5 homogenate/powder mixture with deionized water, the pH was measured potentiometrically using a pH meter (Elmetron CP-511, Elmetron G.P., Zabrze, Poland) at room temperature (20 °C). The content of soluble solids (°Brix) was measured with an Abbe refractometer (ORT-1, Kern & Sohn GmbH, Balingren-Frommern, Germany) at room temperature (20 °C) and expressed as a percentage (%). Dry matter and moisture content, water activity (aw), pH and soluble solids content (°Brix) were determined in three independent replications for each garlic samples.
2.2.2. Total Polyphenols Content and Antioxidant Activity
Total polyphenols content and antioxidant activity were determined in 80% methanol extracts (Sigma-Aldrich, Poznań, Poland). To sterile 50 mL Falcone tubes, on an analytical balance (AS 220/X, Radwag, Radom, Poland) 1.0 g of the tested samples was weighed (with an accuracy of 0.001 g), 30 mL of 80% methanol solution was added and vortexed (Wizard Advanced IR Vortex Mixer, VELP Scientifica Srl, Usmate, Italy) for 60 s (2000 rpm) and incubated in a shaking incubator (IKA KS 4000i Control, IKA® Poland Ltd., Warsaw, Poland) for 60 min (40 °C, 200 rpm). After incubation, the samples were vortexed again (60 s, 2000 rpm) and centrifuged (MPW-380 R, MPW Med. Instruments, Poland, Warsaw) for 15 min (3 °C, 10,000 rpm). In the obtained clear supernatants, the total content of polyphenols and antioxidant activity were determined.
The total polyphenols content was determined by the colorimetric spectrophotometric method using Folin–Ciocalteu reagent (Sig-Ma-Aldrich, Poznań, Poland) according to the modified method of Singleton and Rossi (1965) [38]. The amounts of extracts (determined in a predetermined dilution scheme) were transferred to 50 mL volumetric flasks; then, 2.5 mL of Folin–Ciocalteu reagent and 5.0 mL of 20% sodium carbonate (Sigma-Aldrich, Poznań, Poland) were added, made up to the mark with distilled water and incubated for 60 min at room temperature (20 °C) protected from the light. After incubation, the absorbance was measured at a wavelength λ = 750 nm with a spectrophotometer (UV/Vis UV-6100A, Metash Instruments Co., Ltd., Shanghai, China). After taking into account the applied dilution schemes, based on the calibration curve (y = 2.231 + 0.1315, R2 = 0.9995) for the standard substance, i.e., gallic acid (Sigma-Aldrich, Poznań, Poland), the results of total polyphenolic content were calculated and expressed as mg GAE (Gallic Acid Equivalents)—i.e., mg gallic acid per 1 g of dry matter (mg GAE/g d.m.).
Antioxidant activity was determined by the colorimetric spectrophotometric method using ABTS+• (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic) acid) (Sigma-Aldrich, Poznań, Poland) radical cation assay according to modified method of Re et al. (1999) [39]. The amounts (0.5–1.5 mL) of extracts (determined in a predetermined dilution scheme) were transferred into 10 mL glass test tubes; then, 3.0 mL of ABTS+• radical cations in PBS solution (Phosphate Buffer Solution, Sigma-Aldrich, Poznań, Poland) was added and incubated for 6 min at room temperature (20 °C). After incubation, the absorbance was measured at a wavelength λ = 734 nm with a spectrophotometer (UV/Vis UV-6100A, Metash Instruments Co., Ltd., Shanghai, China). After taking into account the applied dilution schemes, based on the calibration curve (y = –5.7012 + 0.7039, R2 = 0.9998) for the standard substance, i.e., Trolox (Sigma-Aldrich, Poznań, Poland), the results of antioxidant activity were calculated and expressed as a µmols TEAC (Trolox Equivalent Antioxidant Capacity)—i.e., μM TEAC per 1 g of dry matter (μM TEAC/1 g d.m.).
The determinations of total polyphenolic content ant antioxidant activity were performed in six independent replications for each garlic sample.
2.2.3. Volatile Compounds
Volatile compounds were identified using the Heracles Neo Ultrafast Gas Chromatograph (HS-GC) (Alpha M.O.S., Toulouse, France). The instrument consists of a robotic autosampler system and two capillary chromatographic columns of various polarities—MXT-5 (non-polar; 10 m × 18 µm, Restek) and MXT-1701 (slightly polar; 10 m × 18 µm, Restek)—connected to two flame ionization detectors (FIDs). One gram of sample (with an accuracy up to 0.001 g) was placed in a 20 mL headspace vial and capped with a teflon faced silicon rubber cap. Each sample was incubated for 20 min at 40 °C, while the agitation speed was maintained at the level of 500 rpm. After incubation, the headspace was collected in a syringe; the injection volume on the GC was 1.0 mL, the injection speed was 125 mL/s, and the injector temperature was 200 °C. The temperatures of the injector and the detector (FID1 and FID2) were, respectively, 200 and 260 °C. The injection on e-nose was carried out on three replicates. The method was calibrated using an alkane solution (C6 to C16) in order to convert the retention time in Kovats indices and to identify the volatile compounds using the AroChemBase database. The AlphaSoft v16.0 software was used to process the data.
2.2.4. Taste Profile
The taste profiles of the fresh and processed garlic were measured using an Astree electronic tongue (Alpha MOS, Toulouse, France). The e-tongue is composed of an automatic sampler, seven potentiometric chemical sensors (sensor set #7: AHS, SCS, ANS, CPS, NMS, CTS, PKS), a reference electrode of Ag/AgCl and a data acquisition system. The potentiometric difference between each sensitive electrode and the Ag/AgCl reference electrode in the equilibrium state was recorded as a response signal. The electronic tongue sensor was preconditioned and calibrated using a 0.01 mol/L hydrochloric acid solution. The diagnostic started when the calibration passed. For the diagnostic, a 0.01 mol/L hydrochloric acid solution, MSG and sodium chloride were used to judge the sensitivity and discernment of the electronic tongue sensors. The sourness, saltiness and umami of the samples were measured using 0.1 M HCl, 0.1 M NaCl and 0.1 M MSG as reference materials for the electronic tongue sensor, respectively. The samples (0.5 g) were dissolved in 50 mL deionized water. Then, the samples were incubated in a shaking incubator (IKA KS 4000i Control, IKA® Poland Ltd., Warsaw, Poland) for 60 min (20 °C, 200 rpm). After that, the samples were centrifuged in a centrifuge (MPW-380 R, MPW Med. Instruments, Warsaw, Poland) for 15 min (2 °C, 10,000 rpm). The obtained supernatant was transferred into the electronic tongue sample beaker. Ten replicate measurements were conducted for each sample, and five points after stabilization were used for further data processing. The signal of each electrode was recorded each second, and the detection time was set to 120 s to ensure the sensors acquired enough taste information for each sample. Afterward, the sensors were rinsed in ultrapure water for 10 s to reach a stable state and prepared for the detection of the next sample. The detection sensors used in this analysis included CTS (for salty taste), NMS (for umami), AHS (for sourness), ANS (for sweetness), SCS (for bitterness), as well as PKS and CPS (both for general purpose) [40].
2.2.5. Instrumental Color Measurement
The colors of white (WG) and black garlic (BG) were instrumentally measured by computer image analysis using a Visual Analyzer 400 color analyzer (Alpha M.O.S., Toulouse, France). For this purpose, the tested garlic (homogenate/powder) samples were placed in pots in the measuring chamber, under controlled upper and lower lighting conditions (in order to avoid the shadow effect). Photos of the samples were taken with a Fujifilm lens (Fujifilm, Tokyo, Japan) with a diameter of 16 mm and a focal length of 0.1 m. Measurements were made in CIE Lab systems (L-brightness, + a-red, − a-green, + b-yellow, − b-blue), and the values of the L*, a* and b* parameters were calculated as weighted averages, taking into account the frequency of occurrence of individual color codes. Measurements and photos were taken in three independent replications for each garlic sample.
2.2.6. Statistical Analysis
The data are presented as the mean ± standard deviation (SD) of three to six independent replications and analyzed by one-way ANOVA using Duncan’s multiple range test and the statistical program Statistica 13.0 (Tibco Software Inc., Palo Alto, California, USA). Differences were considered statistically significant at p < 0.05 for all assays.
Principal component analysis (PCA) was used to assess the similarities and differences between the tested parameters of the garlic samples that were evaluated according to a covariance matrix. The AlphaSoft v16.0 software was used to process the data and obtain a PCA chart.
3. Results and Discussion
The physicochemical characterizations of fresh, white and black garlic are shown in Table 1. Concerning the results from the conducted research, all garlic samples significantly (p < 0.05) differed in terms of dry matter and moisture content, water activity and pH. Significant differences were also found in the content of soluble solids (°Brix). The lowest dry matter content (37.1 ± 0.13%) with the highest moisture content (62.86 ± 0.13%) was found in fresh, white, unprocessed garlic (WG) samples, which was confirmed by other authors’ research showing the water content in fresh garlic to be at the level of 57–65% [13,17,18,31,32,41]. The average dry matter content in the tested samples of black garlic was significantly higher and depended on the treatment of black garlic used during the production of its various products. Among the various black garlic products, the highest dry matter content was found in powder (BG-P), significantly lower in black garlic in the form of cloves (BG-C), and the lowest content was found in black garlic paste (BG-S). The results obtained in this study are in line with the literature data, in which the authors found a varied dry matter content (30–65%) in garlic cloves, depending, e.g., on the conditions of the garlic aging process such as temperature, relative humidity and the time of heat treatment of the garlic [13,17,18,31,32,41]. Tahir et al. (2022) [36] showed a significant reduction in moisture content in black garlic samples, similar to Sunanta et al. (2021) [25], showing a decrease in the moisture content of fresh garlic samples from about 72% to about 56% in black garlic, which is consistent with our research. The available literature lacks information on the comparison of these parameters in clove, powder and black garlic spreading paste. Black garlic’s moisture content is an important factor affecting its texture and consistency properties. A water content at the level of 35–40% results in a reduction of flexibility, and a value lower than 35% leads to excessively high hardness and dryness of the product. According to the literature, a product containing about 40–50% water is characterized by adequate flexibility, softness and firmness [13,17].

Table 1.
Physicochemical characterization of white garlic and black garlic products.
Fresh garlic’s (WG) water activity averaged 0.98, which is consistent with the results obtained by other authors (0.97–0.98) [16,31,32,41]. Long-term heat treatment leading to the production of black garlic (BG-C) decreased this parameter (0.94). Other authors also found water activity (aw) at a level similar (0.91 to 0.98) to the results of the research in this study [31,32,42]. The water activity of black garlic, remaining at a fairly high level, is a result of the specific conditions under which black garlic is produced. The high humidity maintained during the black garlic production process makes it difficult to drain water, but it is necessary for the proper course of this process [29,41]. With a high moisture content, a high aw cannot be a determinant of the microbiological safety of the final product, and in order to improve it, it is recommended to dry black garlic to an aw below 0.85 [42]. The produced black garlic was subjected to further processing. Black garlic powder (BG-P) was characterized by several times lower water activity (0.20), while garlic spreading paste (BG-S) was additionally pasteurized, which probably improved its microbiological properties. According to the literature, pasteurization, as a fairly mild method of heat treatment of food (below 100 °C), leads to the reduction or elimination of both pathogenic and non-pathogenic microorganisms in food, especially that with a high water content [43].
During the production of black garlic, the pH of the product decreased significantly (Table 1). According to the conducted research, the pH of fresh, unprocessed garlic (WG) was 6.37 ± 0.04, which is in line with the results of other authors showing a pH in the range of 5.99–6.52 [18,25,26]. Long-term heat treatment, resulting in the formation of black garlic, significantly (p < 0.05) decreased this parameter (on average to 3.80 ± 0.27 for the tested black garlic products). Other authors also showed this tendency [18,19,20,31,32]. Sunanta et al. (2021) [25] obtained results similar to ours, showing a significant decrease in pH for fresh unprocessed garlic (6.46) to about 3.79–4.44 in black garlic (depending on the time of heat treatment). In addition, Tahir et al. (2022) [36] found a significant decrease in pH from about 6.01 for fresh to 4.09 for black garlic during the aging process. The lowering of the pH during the aging process of black garlic depends on the conditions of its course. Lower pH is obtained in a product fermented over a longer period of time [42] or at a higher temperature [41,42]. According to the literature, the low pH in black garlic is a result of the Maillard reactions occurring during the aging process, leading to an increase in the synthesis of organic acids such as formic, succinic, 3-hydroxypropionic or pyroglutamic acid due to the oxidation of aldehyde groups in aldoses [20,44], or the degradation of hexose increasing the acetic acid content [45].
In the garlic samples tested in the study, the content of soluble solids (°Brix) was significantly (p < 0.05) the lowest in unprocessed, fresh garlic (WG) (35.88 ± 1.35%), and the applied process of long-term heat treatment resulted in a significant increase of this parameter for all black garlic products, averaging at a value of 47.78 ± 3.15%. The obtained results are consistent with the studies of other authors [18,28,31,42]. Among the tested black garlic products, the highest value for °Brix was found in BG-P powder (51.74 ± 0.48%). As reported in the literature, the increase in the content of soluble solids depends on the time and temperature of the garlic aging process, as well as on the way the material is fermented (in the form of head/cloves) [28] and finally on the variety of garlic and its condition as a crop (conventional/ecological cultivation) [31,32,42]. According to the literature, the increase in soluble solids content in black garlic is mainly attributed to the processes of hydrolytic degradation of polysaccharides to oligosaccharides and monosaccharides [27,46], accompanied by an approximately 80% increase in the content of reducing sugars determining the characteristic sweet taste of black garlic [46,47,48].
Chemical processes occurring during the long-term heat treatment of garlic, leading to the production of its black counterpart, are primarily Maillard reactions [19,20], chemical oxidation of phenols [49] or changes in the metabolism of organosulfur compounds in garlic [50], leading to the production of compounds originally absent from white garlic, as well as an increase in the content of bioactive components present in fresh, unprocessed garlic, such as polyphenolic compounds. Therefore, in this study, the total polyphenols content and the antioxidant activity of white (fresh) and black garlic as well as products of its processing (powder, spreading paste) were investigated, and the obtained results are presented in Figure 3.
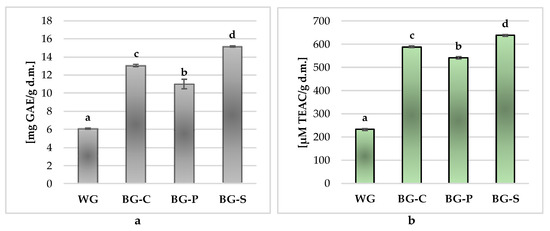
Figure 3.
Total polyphenols content (a) and antioxidant activity (b) in white garlic and black garlic products. Values are means ± standard deviation (n = 6). Different letters in bars (a–d) are significantly different (p < 0.05). WG—white garlic; BG-C—black garlic cloves; BG-P—black garlic powder; BG-S—black garlic spread.
The conducted research showed significant differences in the content of total polyphenolic compounds in the tested garlic samples, with the significantly lowest (p < 0.05) content of these components being found in fresh, unprocessed white garlic (WG), where it was 6.05 ± 0.07 mg GAE/1 g d.m., while in garlic subjected to long-term (45 days) heat treatment (70 °C) (BG), the total value of total polyphenols was on average 13.09 ± 1.81 mg GAE/1 g d.m. and was two times higher than in the WG. The obtained results are confirmed by the studies of other authors, who found a two-tenth increase in the content of these components as a result of garlic fermentation processes [14,15,18,31,32,38,42,51,52]. According to Sunanta et al. (2021) [25], the content of total polyphenols and flavonoids is higher the longer the heat treatment process continues. The authors showed a several-fold increase in the content of total polyphenols and an almost four-fold increase in the content of flavonoids in black garlic compared to fresh garlic. Similarly, Nakagawa et al. (2020) [21] found a several-fold increase in the content of phenolic components, at the same time showing a significant differentiation in the dynamics of the process of their synthesis. While in the initial phase of the aging process (0–7 days), the increase in the content of polyphenols was relatively small, and the synthesis was significantly higher with the lengthening of the aging process, reaching the maximum values on the 28th day of heat treatment. The authors explain this process by the probable polymerization of polyphenols and the decomposition of macromolecules during long-term heat treatment of garlic in high temperature and humidity conditions. According to the literature, the differences in the growth dynamics of the content and profile of polyphenolic compounds result from the conditions used during the aging of garlic. The content of these components varies depending on the temperature [42,51,52], which probably results from their easier release from esterified and glycosylated complex structures under the influence of high temperature [51,53] or time and high relative humidity [42,51,52], which in turn generates an increase in these components as a result of the later phases of the browning reaction [54].
The literature lacks data on the total polyphenolic content in various products obtained from black garlic. According to our research (Figure 3a), black garlic in the form of cloves (BG-C) contained two times more (13.08 ± 0.13 mg GAE/1 g d.m.) polyphenols than WG. The powder produced from it had a significantly lower content (11.02 ± 0.51 mg GAE/1 g d.m.). Interestingly, the spreading paste made from cloves of black garlic had significantly (p < 0.05) the highest content of total polyphenols among all tested black garlic products (15.16 ± 0.08 mgvGAE/1 g d.m.), which was probably due to the pasteurization (70 °C) used during its production and high macerate moisture (54.60 ± 0.99%), creating conditions for the further course of the garlic aging process.
Apart from changes in the content of polyphenolic compounds, their profile also changes during the production of black garlic. According to the literature, the content of phenolic acids may increase even eight-fold, among which derivatives of hydroxycinnamic acid dominate [31,32,51], which is attributed to the high stability of these compounds compared to other phenolic structures [51,55,56,57]. The profile of flavonoid compounds is also changing, the amount of which may increase by about four [51] or five times [52]; however, due to the very large variety of their chemical structure and thermal stability [57], the share of individual flavonoids varies widely at different stages of the garlic aging process, depending on the thermal conditions and the production time of black garlic [51,52,57].
Black garlic is known primarily for its antioxidant properties, which are determined by the increasing content of phenolic components during the aging process, as well as changes in the content and profile of sulfur-organic compounds [13,20,41,52]. Therefore, the study investigated the antioxidant activity of fresh, unprocessed garlic (WG), black garlic (BG-C) obtained after 45 days of heat treatment at 70 °C and 80% relative humidity and the products of its processing—i.e., powder (BG-P) and spreading paste (BG-S), (Figure 3b).
All garlic samples were characterized by a high ability to deactivate synthetic cation radicals ABTS+• (on average 500.50 ± 165.28 µM TEAC/1 g d.m.), and there were statistically significant (p < 0.05) differences in this parameter between the tested samples. White, unprocessed garlic (WG) was characterized by over two times lower antioxidant potential (232.95 ± 4.06 µM TEAC/1 g d.m.) than black garlic (on average, all black garlic products showed activity at the level of approximately 589.68 ± 42.05 µM TEAC/1 g d.m.). The results are similar to these obtained by other authors [18,31,32,41], which show a several-fold (2–9) increase in the antioxidant capacity of garlic during long-term heat treatment, leading to the production of black garlic, depending on the time and thermal conditions of the aging process [16,17,19,30,41,42] or the type of aged garlic (heads/cloves) [41,42]. Some authors show even several dozen times higher antioxidant activity of black garlic compared to white garlic [13,48]. However, the literature lacks comparative data for various black garlic processing products. According to our research, among the analyzed black garlic products, the highest antioxidant activity (similarly to the total content of polyphenols) was found in black garlic spreading paste (BG-S), where the value of this parameter reached an average of 638.46 ± 3.37 µM TEAC/1 g d.m. (Figure 3b).
According to the literature, the key compounds shaping the high antioxidant potential of black garlic include, among others, various polyphenolic or organosulfur compounds, both those that are present in fresh, unprocessed garlic and those that arise during its processing [13,41]. Already during the pre-treatment of garlic and during its grinding (slicing, grinding, biting the clove), odorless sulfur compounds such as alliin (S-allyl-l-cysteine sulfoxide, SAC) and γ-glutamyl-S-allyl-cysteine (GSAC), are metabolized under the influence of the enzyme released then—alliinase—to strongly aromatic, irritating substances that determine the specific sharp taste of garlic, such as allicin (diallyl thiosulfonate) and its diallyl sulfide derivatives, such as DAS (diallyl sulfides), DADS (diallyl disulfides), DTS (diallyl trisulfides) and others [12,58].
According to the literature, during the long-term heat treatment of garlic, during its aging, the content of alliin and allicin metabolites, such as DAS, DADS, DTS or SAC, may increase several times (6–7 times), which increases the antioxidant activity of black garlic [13,48]. The temperature-dependent γ-glutamyl transpeptidase (γ-GTP) from γ-glutamyl-S-allylcysteine (GSAC) in raw unprocessed garlic produces S-allyl-cysteine [11,12,52,59], a substance with strong antioxidant properties. According to the recently published studies by Liu et al. (2022) [50], the thermal stability of γ-glutamyl-S-allylcysteine (GSAC) is much higher than that of S-allyl-l-cysteine (SAC), and its content during the fermentation processes of black garlic (in conditions of high temperature and humidity) can increase by over 20% [50]. In addition, the antioxidant activity of black garlic significantly increases during the aging process as a result of the Maillard reactions taking place at that time (with different intensities depending on temperature and humidity conditions) [18,19,20,27]. In turn, the share, profile and mutual proportions of the Maillard reaction products determine not only the antioxidant properties of black garlic, but also its specific taste and smell [60,61,62].
Therefore, in this study, we focus on the identification of volatile compounds contained in fresh, unprocessed white garlic (WG) (responsible for its specific, pungent taste and aroma) and in black garlic (BG) and its processed products, in which the unpleasant taste and the aroma are lowered and improved as a result of the metabolites appearing during the long-term heat treatment of garlic, which in turn determine the specific, delicate sweet and sour taste and pleasant aroma [60,61].
Table 2 shows the main volatile compounds detected in all garlic samples, while Figure 4 shows the relative areas of peaks of the main volatile compounds indemnified in the headspace of the garlic samples. Based on chromatograms, the 43 compounds were detected in WG, 25 in BG-C, 24 in BG-P and 23 in BG-S. Fresh garlic contain 13 sulfur compounds, while in black garlic 10 compounds were found, and in paste and powder, 8 compounds were detected. Similarly, Ma et al. (2021) [63] showed that the total sulfide content tended to decrease during the aging process, possibly due to the strong volatility of sulfur compounds. The dominant compounds in fresh garlic cloves were sulfur compounds and ketones followed by aldehydes. According to the literature, similar compounds were detected in fresh garlic samples in several studies [63,64,65]. With aging, the number of sulfur compounds decreased; thus, the relative area (%) of detected peaks decreased. Some of the sulfur volatiles in fresh garlic, such as 1-pentanehiol, dimethyl sulfoxide, 3-mercapto-3-methylbutyl formate and 1-decanethiol, were not detected in black garlic.

Table 2.
Characterization of the main volatile compounds identified in the headspace of fresh garlic, black garlic, black garlic powder and paste.
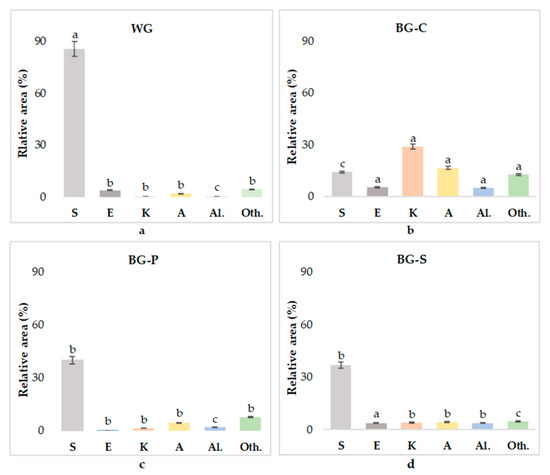
Figure 4.
Relative area of peaks (%) for the MTX-G column of the main volatile compounds identified in the headspace of garlic samples. WG—white garlic (a), BG-C—black garlic cloves (b), BG-P—black garlic powder (c), BG-S—black garlic spread (d). Means (n = 3) with different small letters (a–c) in the same chemistry family group are significantly different (p < 0.05). S—sulfur compounds, E—ester, K—ketone, A—aldehyde, Al.—alcohol, Oth.—other compounds.
However, with the aging process, multiple compounds could be detected in black garlic samples, including carbon disulfide, 2-methyl-2-propanethiol, 2-methylothio-ethanol, 2-furanmethanol, 2-octanone, alfa-pinene, 2,3,-dimethyl-pyrazine, 4-hydroxy-5-methyl-3-furanone and 5-methylfurfural.These substances may contribute to the roast, burnt, caramelized, green, fruity aroma of black garlic. Five-methylfurfural is a furfural derivative, which confirmed that the Maillard reaction took place during the aging of black garlic. Flavor compounds, such as pyrroles, thiophenes, furans and pyrazines, are formed through the generation of various volatile compounds in this process [66]. The fresh garlic cloves contained smaller amounts of green grass, floral and fruity aroma substances, and with the aging process, substances such as methyl acetate, 2-octanone, 1-hydroxy-2-propanone and (E,E) 2,4-decadienal improved the smell of the black garlic. Figure 5 shows the aromatic profile in a PCA plot based on the VOCs in the samples. The electronic taste sensor reactivity values of the tested samples are shown in Figure 5. Additionally, to the best of our knowledge, the taste profile of the garlic and black garlic was for the first time analyzed on an electronic tongue, which is a novelty of the current work.
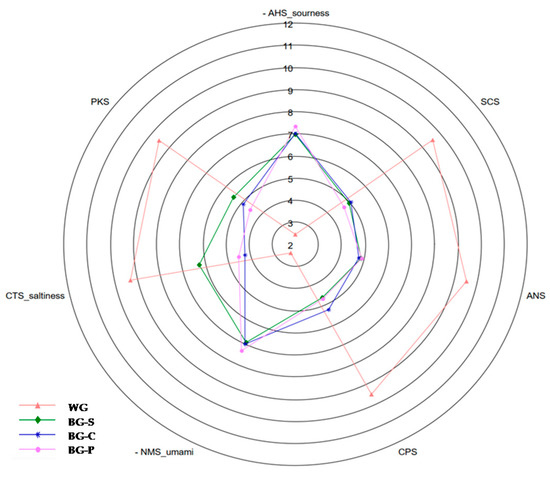
Figure 5.
Radar map of e-tongue data for fresh and black garlic. WG—white garlic, BG-C—black garlic cloves, BG-P—black garlic powder, BG-S—black garlic spreading paste.
Sourness (AHS) increased after the fermentation process (1.90–7.30), and the values between black garlic samples were similar between groups. In the case of umami (NMS), taste was enhanced after thermal process and increased from 1.90 (WG) to 7.00–7.40 in BG and BG-P, respectively. According to the results obtained by Park and Kim (2022) [67], lyophilized chives (Allium), which contain free amino acids, may enhance the umami taste in the product. The saltiness (CTS) decreased after the fermentation process, from 9.30 to 6.80 (BG-S), 5.50 (BG-P) and 5.20 (BG-C). The fresh garlic showed a strong bitter taste (SCS), and with a thermal process, the bitter taste strongly decreased, which may be due to the decrease of the total sulfide compounds, which tended to decrease during the thermal process. As previously reported, BG products in taste are very mild with sweet and sour tones, and fructooligosaccharides contribute to the sweet taste of black garlic [18,27].
The taste profiles (fingerprints) were obtained using a PCA based on the reactivity of all the sensors, which was derived from the e-tongue analysis results of the individual samples. The analysis results are shown in Figure 6. A total contribution rate of over 97% suggested the feasibility of the method. The variance contribution rate of PC1 was 99.50%, and PC2 was 0.26%. The classification of the groups showed that samples WG and BG were classified correctly. According to the PCA chart, the results from the e-tongue were able to completely distinguish the flavor of fresh and processes garlic cloves. Therefore, the black garlic samples (BG-C, BG-S, BG-P) shown on the x-axis had quite similar taste profiles. However, the BG-C and BG-P showed slightly different taste profiles from BG-S. The samples of WG cloves were positive along the y-axis and negative along the x-axis, indicating a greater diversity from BG samples. Therefore, an electronic tongue can also be used to distinguish white garlic and black garlic samples. The results based on the e-tongue measurement were significantly correlated with the human sensory evaluation scores, indicating that the e-tongue can be utilized to characterize the flavor of garlic products or other food types.
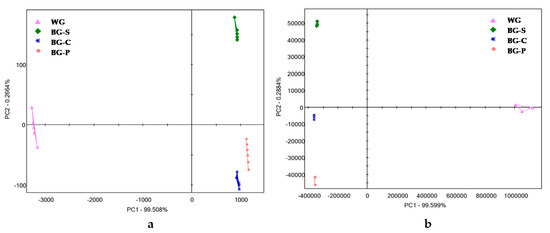
Figure 6.
PCA plot of the similarities and differences in the taste and profile of volatile compounds identified in the garlic samples: (a) e-tongue, (b) e-nose. WG—white garlic, BG-C—black garlic cloves, BG-P—black garlic powder, BG-S—black garlic spread.
According to the results from the research, the chemical processes occurring during the long-term heat treatment of garlic as well as its further processing (production of black garlic powder or spreading paste) significantly changed the physicochemical and bioactive properties (such as the content of polyphenols, antioxidant potential) or the content of the volatile compounds. They changed the aroma and taste profile of the products produced in this way, which gained completely different organoleptic or sensory properties and characteristics. The caramelization, enzymatic and non-enzymatic browning reactions taking place during the aging of garlic and the products formed as a result of the Maillard reaction also significantly changed the color parameters of processed garlic [19,20,68].
Therefore, in this study, an instrumental measurement of the color of unprocessed white garlic (WG) and black garlic (BG) and the products of its further processing, i.e., powder (BG-P) and spreading paste (BG-S), was performed, and the obtained results are presented in Table 3 and Figure 7.

Table 3.
Color parameters in L*a*b* space obtained using the instrumental method (“electronic eye”) in white garlic and black garlic products.
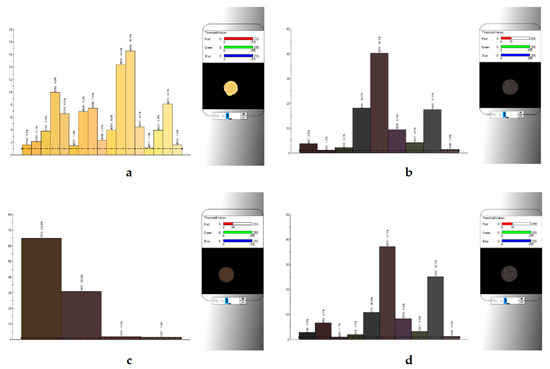
Figure 7.
Detailed color analysis of white garlic and black garlic products using the instrumental method (“electronic eye”). (a) WG—white garlic, (b) BG-C—black garlic cloves, (c) BG-P—black garlic powder, (d) BG-S—black garlic spread.
Significant (p < 0.05) differences were found in all color parameters in the L* a* b* color space between white unprocessed garlic (WG) and various black garlic products (BG) (Table 3). Large differences were noted in the case of the L* parameter, describing the lightness. White garlic (WG) was over 3.3 times brighter (85.94 ± 0.26) than black garlic (average for all black garlic products: 25.44 ± 0.35), which confirms the caramelization or Maillard reactions during the fermentation of black garlic leading to the production of browning products in black garlic, changing the color of the product to dark brown or even black. The obtained results are similar to the results of other authors [13,16,28,31,32,42,52]. Additionally, differences in the lightness of the black garlic products were found. The L* parameter in the garlic clove (BG-C) and spreading paste (BG-S) samples were at a similar level (25.66 ± 0.15), while the garlic powder (BG-P) had the lowest lightness (25.00 ± 0.01) and therefore the darkest color, which would indicate a greater intensity of browning.
Significant differences were also found in the a* color parameter related to the red tone. The value of this parameter in unprocessed white garlic (WG) was on average 3.11 ± 0.11, while as a result of the long-term fermentation processes of garlic, it was significantly reduced to 2.15 ± 0.05 for garlic in the form of cloves (BC-C) and spreading paste (BC-S), which did not differ in this parameter. Interestingly, the black garlic powder (BG-P) had the significantly highest (p < 0.05) value of the a* parameter among all analyzed samples (4.71 ± 0.02), which proves the greatest red shift in the case of this product. For the b* color parameter, describing shades of yellow, the significantly highest value was found in white garlic (WG) (53.77 ± 1.67), while the samples of black garlic showed an almost four times lower intensity of this color (average 13.94 ± 4.13). Furthermore, in the case of shades of yellow, the different products obtained from black garlic showed significant differences.
Black garlic in the form of cloves (BG-C) and black garlic spreading paste (BG-S) did not differ significantly in terms of yellow color saturation (average 11.18 ± 0.03), and similarly to the parameter a*; however, they differed significantly from the black garlic powder (BG-P), which showed the greatest color shift towards the yellow color (19.45 ± 0.08). No comparative data were found in the literature for various black garlic products—i.e., clove, powder and spreading paste. Figure 7 above shows a detailed analysis of the color of unprocessed white (WG) and black (BG) garlic and its processing products by an instrumental method (“electronic eye”).
According to the results from our research, the differences in the color parameters in the L* a* b* system in the tested garlic samples indicate a significant influence of both the long-term heat treatment processes and the further processing of black garlic (powder/spreading paste), resulting in the production of products with very different properties, including organoleptic ones. In the literature, the changes in the color of black garlic are explained by the Maillard reactions occurring during long-term heat treatment of garlic, generating dark-colored substances, such as melanoids, formed from sugars and amino acids at various stages of these chemical reactions [27,69,70]. The conditions of the long-term heat treatment of this product have an impact on the intensity and dynamics of the color change of black garlic. According to the literature, time and temperature [16,42,48] positively correlate with the intensity of browning black garlic, in contrast to relative humidity, where the relationship is opposite, slowing the Maillard reaction rate and thus the intensity of browning garlic [41].
4. Conclusions
In our work, the multidimensional pattern of the chemical processes occurring during long-term heat treatment of garlic as well as its further processing (production of black garlic powder or spreading paste) was analyzed and significantly changed the physicochemical and bioactive properties as well as the content of the volatile compounds and the taste profile of white garlic. After long-term heat treatment, garlic was characterized by a higher content of dry matter and water-soluble solids, and the highest values were found in the powdered garlic. The polyphenol content and antioxidant properties were the highest in the spread garlic and the lowest in the powdered samples. Obtained black garlic samples gained completely different sensory characteristics determined using instrumental methods. In black garlic and its preparations, the intensity of the unpleasant taste and aroma was reduced as a result of the appearance of metabolites during the long-term heat treatment, which in turn determined the specific, delicate sweet–sour taste and pleasant aroma, completely unrelated to the aroma of the unprocessed product. The conducted research showed significant differences in the bioactive properties of the tested garlic samples, with the lowest content of polyphenols and antioxidant properties in fresh, unprocessed garlic, while in garlic subjected to long-term heat treatment, the total polyphenolic content and antioxidant potential was two times higher than in the unprocessed one. The spread samples had a higher content of polyphenols and the highest antioxidant capacity.
Taking the above into account, it can be stated that black garlic, both in the form of cloves, paste and powder, exhibits completely different properties than white garlic. The paste is characterized by the highest content of polyphenols and the highest antioxidant properties, while the powder has the highest level of soluble solid. All the black garlic samples showed a similar flavor and aroma profile, with the clove and powder samples being the most closely related.
Author Contributions
Conceptualization, K.N.; methodology, K.N. and K.K.; validation, K.N. and K.K.; formal analysis, K.N., K.K. and A.S.; investigation, K.N., K.K. and A.S.; resources, K.N. and K.K.; writing—original draft preparation, K.N. and K.K.; writing—review and editing, A.S.; visualization, K.N. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was prepared as part of the statutory activity of the Department of Functional and Ecological Food. The research for this publication was carried out with the use of the equipment purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhosha, S.G.; Prakash, J.; Prabhavathi, S.N. Bioactive components of garlic and their physiological role in health maintenance: A review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najman, K.; Leontowicz, H.; Leontowicz, M. The influence of plants from the Alliaceae family on morphological parameters of the intestine in atherogenic rats. Nutrients 2021, 13, 3876. [Google Scholar] [CrossRef]
- Najman, K.; Sadowska, A.; Buczak, K.; Leontowicz, H.; Leontowicz, M. Effect of heat-treated garlic (Allium sativum L.) on growth parameters, plasma lipid profile and histological changes in the ileum of atherogenic rats. Nutrients 2022, 14, 336. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [Green Version]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude garlic extract inhibits cell proliferation and induces cell cycle arrest and apoptosis of cancer cells in vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef]
- González, R.E.; Soto, V.C.; Sance, M.M.; Camargo, A.B.; Galmarini, C.R. Variability of solids, organosulfur compounds, pungency and health-enhancing traits in garlic (Allium sativum L.) cultivars belonging to different ecophysiological groups. J. Agric. Food Chem. 2009, 57, 10282–10288. [Google Scholar] [CrossRef]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanazawa, A.; Toru, N.; Fujikawa, M.; Nassage, S.; Masamoto, K.; et al. Physical, chemical and biological properties of S-allylcysteine, an amino acid derived from garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive compounds. J. Nutr. 2001, 131, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.J.; Lee, S.J.; Kang, M.J.; Cho, H.S.; Sung, N.J.; Shin, J.H. Physicochemical characteristics of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 465–471. [Google Scholar] [CrossRef]
- Kang, O.J. Physicochemical characteristics of black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2016, 21, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Moreno-Rojas, R. Influence of variety and storage time of fresh garlic on the physicochemical and antioxidant properties of black garlic. Foods 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Bedrníček, J.; Laknerová, I.; Lorenc, F.; de Moraes, P.P.; Jarošová, M.; Samková, E.; Tříska, J.; Vrchotová, N.; Kadlec, J.; Smetana, P. The use of a thermal process to produce black garlic: Differences in the physicochemical and sensory characteristics using seven varieties of fresh garlic. Foods 2021, 10, 2703. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The comparison of the contents of sugar, Amadori, and Heyns compounds in fresh and black garlic. J. Food Sci. 2016, 81, 1662–1668. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef]
- Nakagawa, K.; Maeda, H.; Yamaya, Y.; Tonosaki, Y. Maillard reaction intermediates and related phytochemicals in black garlic determined by EPR and HPLC analyses. Molecules 2020, 25, 4578. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Chen, Y.T.; Chen, H.J.; Hsieh, C.W.; Liao, P.C. Comparative UHPLC-Q-Orbitrap HRMS-based metabolomics unveils biochemical changes of black garlic during aging process. J. Agric. Food Chem. 2020, 68, 14049–14058. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Moreno-Rojas, R.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M. Bioaccessibility of bioactive compounds of ‘fresh garlic’ and ‘black garlic’ through in vitro gastrointestinal digestion. Foods 2020, 9, 1582. [Google Scholar] [CrossRef]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relatioship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Sunanta, P.; Pankasemsuk, T.; Jantanasakulwong, K.; Chaiyaso, T.; Leksawasdi, N.; Phimolsiripol, Y.; Rachtanapun, P.; Seesuriyachan, P.; Sommano, S.R. Does curing moisture content affect black garlic physiochemical quality? Horticulture 2021, 7, 535. [Google Scholar] [CrossRef]
- Ahmed, T.; Wang, C.K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef]
- Qiu, Z.; Zheng, Z.; Zhang, B.; Sun-Waterhouse, D.; Qiao, X. Formation, nutritional value, and enhancement of characteristic components in black garlic: A review for maximizing the goodness to humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 801–834. [Google Scholar] [CrossRef] [Green Version]
- Toledano-Medina, M.A.; Merinas-Amo, T.; Fernández-Bedmar, Z.; Rafael Font, R.; Del Río-Celestino, M.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Alonso-Moraga, Á.; Moreno-Rojas, R. Physicochemical characterization and biological activities of black and white garlic: In vivo and in vitro assays. Foods 2019, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Dai, C.H.; Hou, F.R.; Zhu, P.P.; He, R.H.; Ma, H.L. Study on the ageing method and antioxidant activity of black garlic residues. Czech J. Food Sci. 2018, 36, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Najman, K.; Sadowska, A.; Hallmann, E. Influence of thermal processing on the bioactive, antioxidant, and physicochemical properties of conventional and organic agriculture black garlic (Allium sativum L.). Appl. Sci. 2020, 10, 8638. [Google Scholar] [CrossRef]
- Najman, K.; Sadowska, A.; Hallmann, E. Evaluation of bioactive and physicochemical properties of white and black garlic (Allium sativum L.) from conventional and organic cultivation. Appl. Sci. 2021, 11, 874. [Google Scholar] [CrossRef]
- Yu, J.H.; Shan, Y.; Li, S.; Zhang, L.F. Potential contribution of Amadori compounds to antioxidant and angiotensin I converting enzyme inhibitory activities of raw and black garlic. LWT Food Sci. Technol. 2020, 129, 109553. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Rasheed, R.; Hussain, M.; Aamir, M.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Faqir, M.; Anjum, F.M. Nutritional, biological, and therapeutic properties of black garlic: A critical review. Int. J. Food Prop. 2021, 24, 1387–1402. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Supapvanich, S.; Kaewthong, P.; Takeungwongtrakul, S. Impact of steaming pretreatment process on characteristics and antioxidant activities of black garlic (Allium sativum L.). J. Food Sci Technol. 2021, 58, 1869–1876. [Google Scholar] [CrossRef]
- Tahir, Z.; Saeed, F.; Nosheen, F.; Ahmed, A.; Anjum, F.M. Comparative study of nutritional properties and antioxidant activity of raw and fermented (black) garlic. Int. J. Food Prop. 2022, 25, 116–127. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Weng, Z.; Sun, L.; Wang, F.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Assessment the flavor of soybean meal hydrolyzed with Alcalase enzyme under different hydrolysis conditions by E-nose, E-tongue and HS-SPME-GC-MS. Food Chem. X 2021, 12, 100141. [Google Scholar] [CrossRef]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT Food Sci. Technol. 2014, 55, 397–402. [Google Scholar] [CrossRef]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Wyk, J.V. Antioxidant activity of Maillard reaction products (MRPs) derived from fructose–lysine and ribose–lysine model systems. Food Chem. 2013, 137, 92–98. [Google Scholar] [CrossRef]
- Liang, T.; Wei, F.; Lu, Y.; Kodani, Y.; Nakada, M.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT Food Sci. Technol. 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Li, N.Y.; Lu, X.M.; Pei, H.B.; Qiao, X.G. Effect of freezing pretreatment on the processing time and quality of black garlic. J. Food Process Eng. 2015, 38, 329–335. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, M.; Liu, R.; Gao, Y.; Xu, M.; Zhang, M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J. Food Biochem. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Liu, C.; Lu, L.; Yang, C.; Niu, C.; Wang, J.; Zheng, F.; Li, Q. Effects of thermal treatment on alliin and its related sulfides during black garlic processing. LWT Food Sci. Technol. 2022, 159, 113158. [Google Scholar] [CrossRef]
- Kim, J.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidant processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, S.M.; Park, W.P.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006, 97, 472–479. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Camp, J.V. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Corzo-Martinez, M.; Corso, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Kim, N.Y.; Park, M.H.; Jang, E.Y.; Lee, J. Volatile distribution in garlic (Allium sativum L.) by solid phase microextraction (SPME) with different processing conditions. Food Sci. Biotechnol. 2011, 20, 775–782. [Google Scholar] [CrossRef]
- Molina-Calle, M.; Priego-Capote, F.; de Castro, M.D.L. Headspace−GC–MS volatile profile of black garlic vs. fresh garlic: Evolution along fermentation and behavior under heating. LWT Food Sci. Technol. 2017, 80, 98–105. [Google Scholar] [CrossRef]
- Martínez-Casas, L.; Lage-Yusty, M.; López-Hernández, J. Changes in the aromatic profile, sugars and bioactive compounds when purple garlic is transformed into black garlic. J. Agric. Food Chem. 2017, 65, 10804–10811. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, D.; Wang, Z.; Jiang, J.; Jiang, T.; Cui, F.; Fan, X. Effect of ultrahigh pressure treatment on volatile compounds in garlic. J. Food Process Eng. 2011, 34, 1915–1930. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Volatile compounds of fresh and processed garlic. Exp. Ther. Med. 2020, 19, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.H.; Wu, C.M.; Liou, Y.C. Volatile compounds from garlic. J. Agric. Food Chem. 1989, 37, 725–730. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kovis, T.J.K.; Yi, N.J. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.Y. Effect of lyophilized chive (Allium wakegi Araki) supplementation to the frying batter mixture on quality attributes of fried chicken breast and tenderloin. Food Chem. X 2022, 13, 100216. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biological activities of Maillard reaction products (MRPs) in a sugar-amino acid model system. J. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical characterization and antioxidant properties of coffee melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar] [CrossRef]
- Tressl, R.; Wondrak, G.T.; Garbe, L.A.; Krüger, R.P.; Rewicki, D. Pentoses and hexoses as sources of new melanoidin-like Maillard polymers. J. Agric. Food Chem. 1998, 46, 1765–1776. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).