On-Farm Composting of Hop Plant Green Waste—Chemical and Biological Value of Compost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Setting—Compost Pile Formation, Temperature Monitoring and Weather Conditions
2.2. Sampling
2.3. The Chemistry of Composts
2.4. Germination Test
2.5. Growth Test
2.6. Respiratory Test
2.7. Number of Bacteria and Fungi
2.8. Statistics
3. Results and Discussion
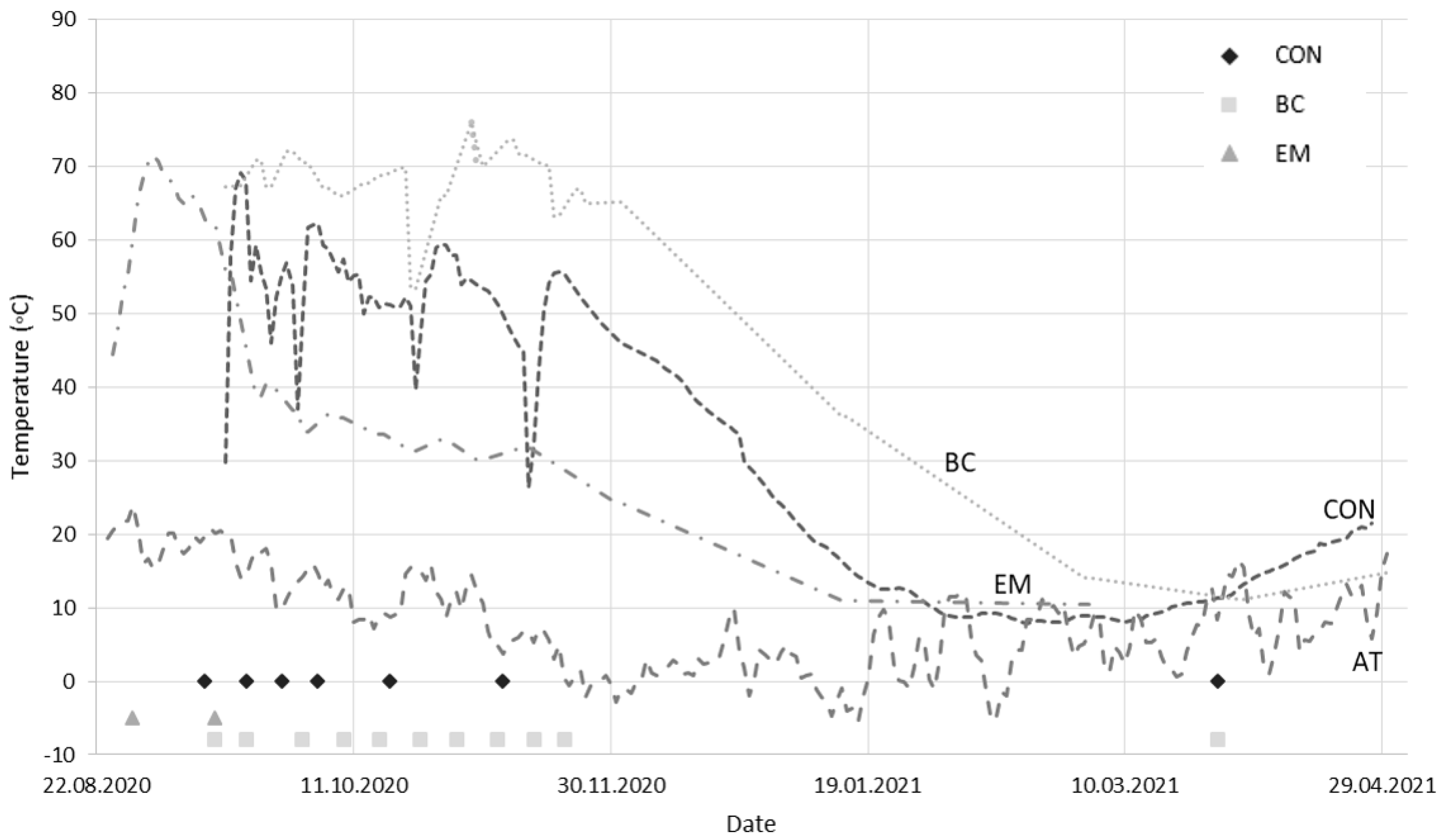
3.1. Temperature
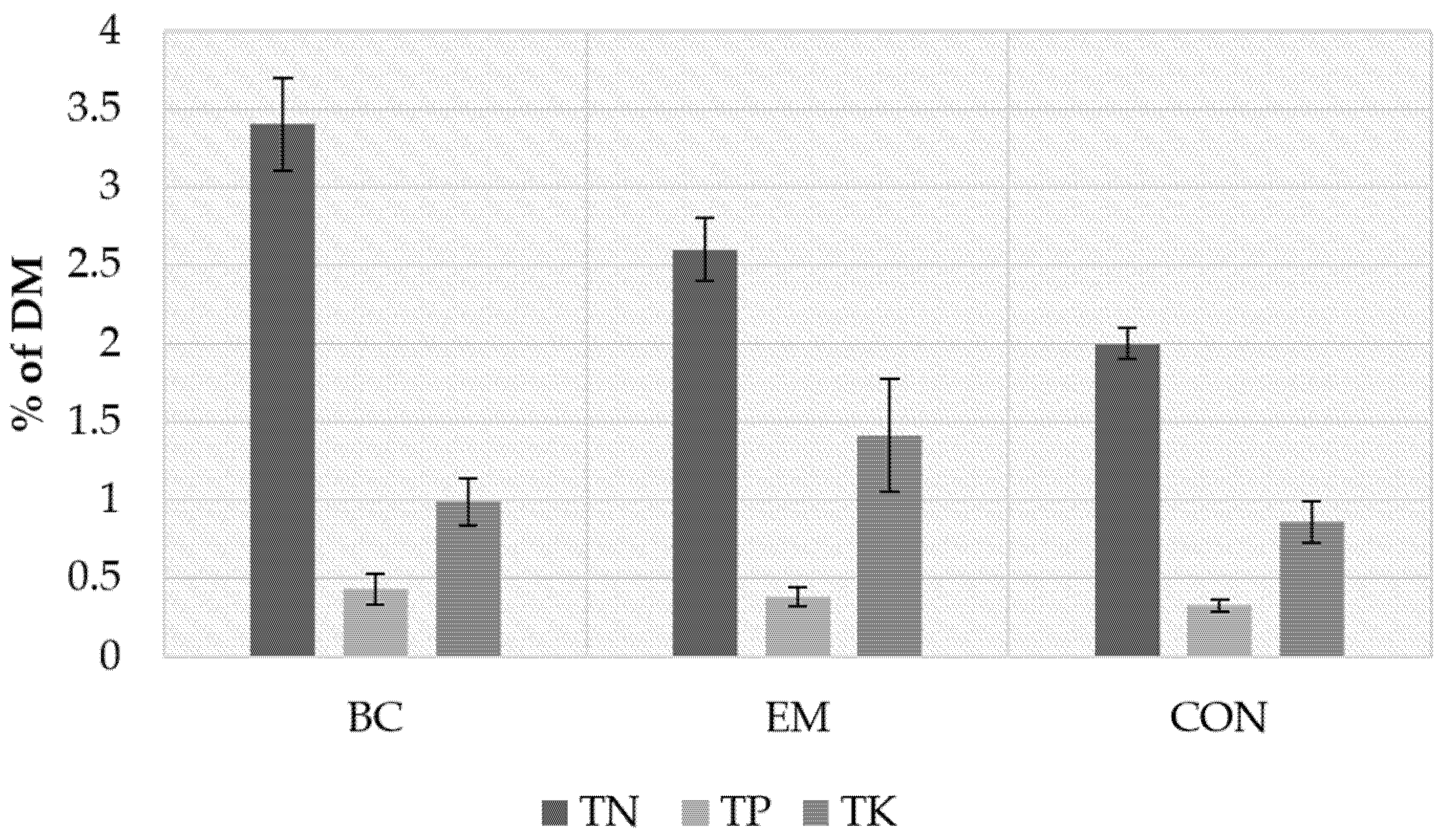
3.2. Chemical Characterization
3.3. Biological Value of Compost
3.3.1. Germination Test
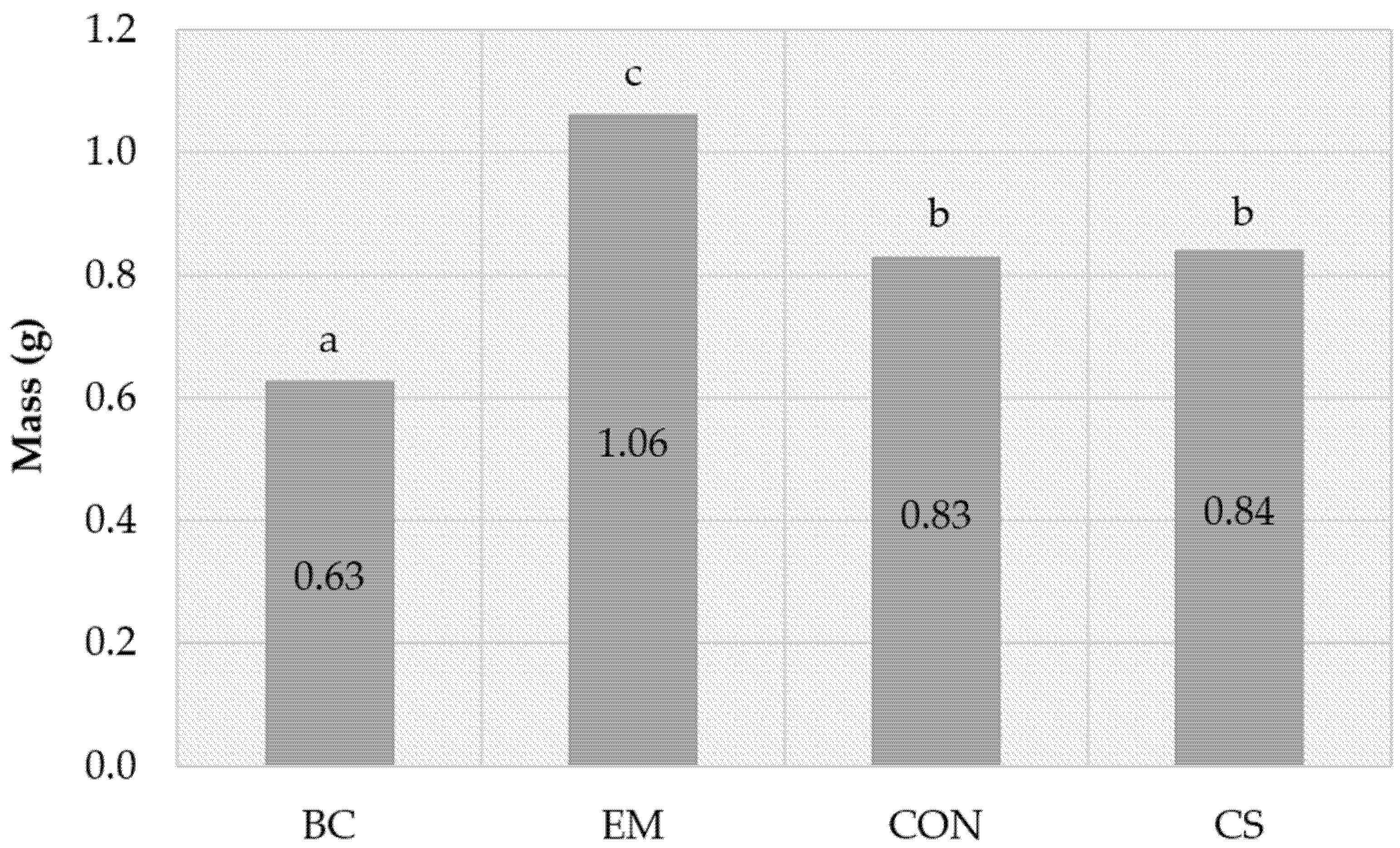
3.3.2. Growth Test
3.3.3. Microbial Respiration
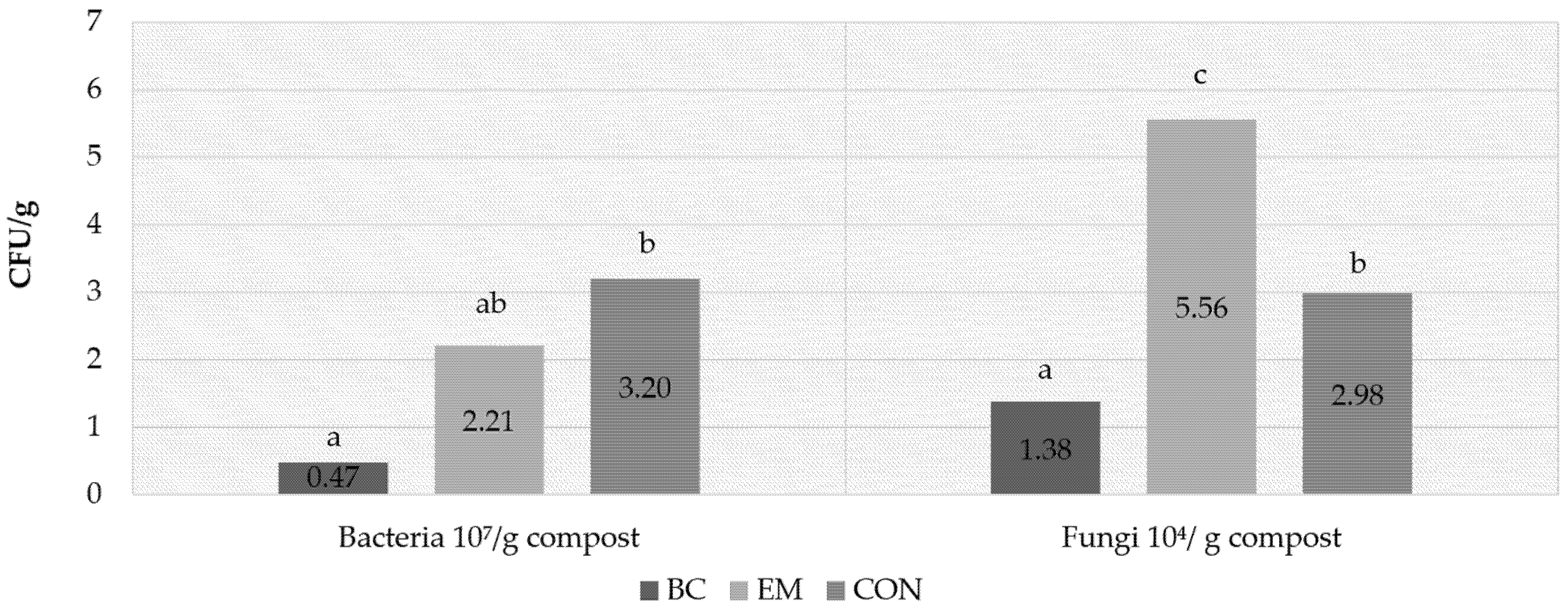
3.3.4. Number of Bacteria and Fungi in Composts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neve, R.A. Hops; Chapman and Hall: London, UK, 1991; 266p. [Google Scholar]
- International Hop Growers’ Convention–IHGC. 2021. Economic Commission-Summary Reports. Available online: http://www.hmelj-giz.si/ihgc/doc/2021_NOV_IHGC_ECReport_public.pdf (accessed on 14 December 2021).
- Čeh, B.; Luskar, L.; Čremožnik, B. Hop biomass after harvest as input material for composting. Hop Bull. 2019, 26, 81–90. [Google Scholar]
- Van Calcar, J.; Eblagon, F. Sustainable hop crop support using compostable PLA twines. Hop Bull. 2019, 26, 69–80. [Google Scholar]
- Life BioTHOP Project. BioTWINE Hop Waste Transformation into Novel Product Assortments for Packaging and Horticulture Sector. 2019. Available online: https://www.life-biothop.eu/ (accessed on 13 December 2021).
- Barrena, R.; Font, X.; Gabarrell, X.; Sánchez, A. Home composting versus industrial composting: Influence of composting system on compost quality with focus on compost stability. Waste Manag. 2014, 34, 1109–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Blanco, J.; Colón, J.; Gabarrell, X.; Font, X.; Sánchez, A.; Artola, A.; Rieradevall, J. The use of life cycle assessment for the comparison of biowaste composting at home and full scale. Waste Manag. 2010, 30, 983–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hait, S.; Tare, V. Transformation and availability of nutrients and heavy metals during integrated composting–vermicomposting of sewage sludges. Ecotoxicol. Environ. Saf. 2012, 79, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tang, J.; Liao, H.; Liu, X.; Zhou, P.; Chen, Z.; Rensing, C.; Zhou, S. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant. Bioresour. Technol. 2018, 265, 146–154. [Google Scholar] [CrossRef]
- Warman, P.R. Evaluation of seed germination and growth tests for assessing compost maturity. Compos. Sci. Util. 1999, 7, 33–37. [Google Scholar] [CrossRef]
- Sundberg, C.; Jönsson, H. Higher pH and faster decomposition in biowaste composting by increased aeration. Waste Manag. 2008, 28, 518–526. [Google Scholar] [CrossRef]
- Ajmal, M.; Aiping, S.; Awais, M.; Ullah, S.M.; Saeed, R.; Uddin, S.; Ahmad, I.; Zhou, B.; Zihao, X. Optimization of pilot-scale in-vessel composting process for various agricultural wastes on elevated temperature by using Taguchi technique and compost quality assessment. Process Saf. Environ. Prot. 2020, 140, 34–45. [Google Scholar] [CrossRef]
- Oazana, S.; Naor, M.; Grinshpun, J.; Halachmi, I.; Raviv, M.; Saadi, I.; Avidov, R.; Varma, V.S.; Rosenfeld, L.; Gross, A.; et al. A flexible control system designed for lab-scale simulations and optimization of composting processes. Waste Manag. 2018, 72, 150–160. [Google Scholar] [CrossRef]
- Liang, C.; Das, K.C.; Mcclendon, R.W. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Xiao, Y.; Zeng, G.M.; Yang, Z.H.; Shi, W.J.; Huang, C.; Fan, C.Z.; Xu, Z.Y. Continuous thermophilic composting (CTC) for rapid biodegradation and maturation of organic municipal solid waste. Bioresour. Technol. 2009, 100, 4807–4813. [Google Scholar] [CrossRef] [PubMed]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.C.; Hafidi, M. Chemical and spectroscopic analysis of organic matter transformation during composting of sewage sludge and green plant waste. Int. Biodeterior. Biodegrad. 2005, 56, 101–108. [Google Scholar] [CrossRef]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.L.; Marschner, P. Compost effects on microbial biomass and soil P pools as affected by particle size and soil properties. J. Soil Sci. Plant Nutr. 2013, 13, 313–328. [Google Scholar]
- Yang, Y.; Awasthi, M.K.; Du, W.; Ren, X.; Lei, T.; Lv, J. Compost supplementation with nitrogen loss and greenhouse gas emissions during pig manure composting. Bioresour. Technol. 2020, 297, 122435. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Zhang, X.; Gao, L.; Tian, Y. Biochar combined with gypsum reduces both nitrogen and carbon losses during agricultural waste composting and enhances overall compost quality by regulating microbial activities and functions. Bioresour. Technol. 2020, 314, 123781. [Google Scholar] [CrossRef]
- Gabhane, J.; William, S.P.; Bidyadhar, R.; Bhilawe, P.; Anand, D.; Vaidya, A.N.; Wate, S.R. Additives aided composting of green waste: Effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour. Technol. 2012, 114, 382–388. [Google Scholar] [CrossRef]
- Tamás, A.; Vrânceanu, N.; Duşa, M.; Stan, V. Steps in organic fraction of municipal solid waste composting and compost quality evaluation. Sci. Pap. Ser. A Agron. 2019, 2, 144–153. [Google Scholar]
- Iannottil, D.A.; Pang, T.; Tothl, B.L.; Elwell, D.L.; Keener, H.M.; Hoitinkl, H.A.J. A quantitative respirometric method for monitoring compost stability. Compos. Sci. Util. 1993, 1, 52–65. [Google Scholar] [CrossRef]
- Lohr, D.; Görl, J.; Meinken, E. Nitrogen dynamics of chopped hop bines-effect of leaf: Stem ratio. Acta Hortic. 2021, 1328, 127–134. [Google Scholar] [CrossRef]
- Cui, E.; Wu, Y.; Zuo, Y.; Chen, H. Effect of different biochars on antibiotic resistance genes and bacterial community during chicken manure composting. Bioresour. Technol. 2016, 203, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Loggers, G.D. Tinytag Plus 2 Internal Temperature (−40 to +85 C) TGP-4017 Data Sheet; Issue 9: 17th October 2014 (E&OE). 2014. Available online: http://gemini2.assets.d3r.com/pdfs/original/1584-tgp-4017.pdf (accessed on 29 December 2021).
- Zucconi, F. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef] [PubMed]
- Milinković, M.; Lalević, B.; Jovičić-Petrović, J.; Golubović-Ćurguz, V.; Kljujev, I.; Raičević, V. Biopotential of compost and compost products derived from horticultural waste—effect on plant growth and plant pathogens’ suppression. Process Saf. Environ. Prot. 2019, 121, 299–306. [Google Scholar] [CrossRef]
- Amlinger, F.; Peyr, S.; Müsken, J. State of the art of Composting. Austrian Minist. Agric. For. Environ. Water Manag. 2009, 115. Available online: https://www.bmk.gv.at/dam/jcr:69c43c71-1844-4c52-8d0c-20e0d9ced59f/Richtlinie_Kompost_en.pdf (accessed on 23 December 2021).
- Van der Wurff, A.W.G.; Fuchs, J.G.; Raviv, M.; Termorshuizen, A.J. Handbook for Composting and Compost Use in Organic Horticulture; BioGreenhouse, 2016; Available online: https://edepot.wur.nl/375218 (accessed on 29 December 2021).
- Theeba, M.; Husni, M.A.; Samsuri, A.W.; Robert, T.B.; Illani, Z.H. Nutrient retention capacity of rice husk biocharcoal in co-composted poultry manure. J. Trop. Agric. Food Sci. 2016, 44, 197–209. [Google Scholar]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
- Hachicha, S.; Sellami, F.; Cegarra, J.; Hachicha, R.; Drira, N.; Medhioub, K.; Ammar, E. Biological activity during co-composting of sludge issued from the OMW evaporation ponds with poultry manured, Physico-chemical characterization of the processed organic matter. J. Hazard. Mater. 2009, 162, 402–409. [Google Scholar] [CrossRef]
- Rynk, R.; Van de Kamp, M.; Willson, G.B.; Singley, M.E.; Richard, T.L.; Kolega, J.J.; Hoitink, H.A. On-Farm Composting Handbook; Northeast Regional Agricultural Engineering Service: Ithaca, NY, USA, 1992. [Google Scholar]
- Tumuhairwe, J.B.; Tenywa, J.S.; Otabbong, E.; Ledin, S. Comparison of four low-technology composting methods for market crop wastes. Waste Manag. 2009, 29, 2274–2281. [Google Scholar] [CrossRef]
- Li, Z.; Deng, H.; Yang, L.; Zhang, G.; Li, Y.; Ren, Y. Influence of potassium hydroxide activation on characteristics and environmental risk of heavy metals in chars derived from municipal sewage sludge. Bioresour. Technol. 2018, 256, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Čeh, B.; Štraus, S.; Hladnik, A.; Oset Luskar, M.; Čremožnik, B. Response of Camelina (Camelina sativa (L.) Crantz) to the lowland agricultural areas. In Proceedings of the 3rd Conference with International Participation Conference VIVUS—On Agriculture, Environmentalism, Horticulture and Floristics, Food Production and Processing and Nutrition, Naklo, Slovenia, 14–15 November 2014. [Google Scholar]
- McFarland, M.J. Biosolids Engineering; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Raza, S.T.; Tang, J.L.; Ali, Z.; Yao, Z.; Bah, H.; Iqbal, H.; Ren, X. Ammonia Volatilization and Greenhouse Gases Emissions during Vermicomposting with Animal Manures and Biochar to Enhance Sustainability. Int. J. Environ. Res. Public Health 2021, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Saarela, T.; Lafdani, E.K.; Laurén, A.; Pumpanen, J.; Palviainen, M. Biochar as adsorbent in purification of clear-cut forest runoff water: Adsorption rate and adsorption capacity. Biochar 2020, 2, 227–237. [Google Scholar] [CrossRef]
- Mihelič, R.; Sušin, J.; Jagodic, A.; Leskošek, M. Slovene guidelines for expert based fertilization in a light of cross compliance rules. Acta Agric. Slov. 2006, 87, 109–119. [Google Scholar]
- Mona, B.; Newbridg, C. Compost Testing and Analysis Service–Interpretation of, Results; Newbridg, Co.: Kildare, Ireland, 2003. [Google Scholar]
- Adebayo, O.S.; Kabbashi, N.A.; Alam, M.; Mirghani, M.S. Recycling of organic wastes using locally isolated lignocellulolytic strains and sustainable technology. J. Mater. Cycles Waste Manag. 2014, 17, 769–780. [Google Scholar] [CrossRef]
- Barrington, S.; Choinière, D.; Trigui, M.; Knight, W. Effect of carbon source on compost nitrogen and carbon losses. Bioresour. Technol. 2002, 83, 189–194. [Google Scholar] [CrossRef]
- Regulation on the Recovery of Biodegradable Waste and the Use of Compost or Digestate; In Slovene: Uredba o Predelavi Biološko Razgradljivih Odpadkov in Uporabi Komposta ali Digestata. 2013. Uradni List Republike Slovenije, št. 99/13. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED6281 (accessed on 14 December 2021).
- Chefetz, B.; Chen, Y.; Hadar, Y. Water-extractable components released during composting of municipal solid waste. In Proceedings of the International Symposium on Composting & Use of Composted Material in Horticulture, Scotland, UK, 5–11 April 1997; Volume 469, pp. 111–118. [Google Scholar]
- Riffaldi, R.; Levi-Minzi, R.; Pera, A.; De Bertoldi, M. Evaluation of compost maturity by means of chemical and microbial analyses. Waste Manag. Res. 1986, 4, 387–396. [Google Scholar] [CrossRef]
- Amery, F.; Vandaele, E.; Körner, I.; Loades, K.; Viaene, J.; Vandecasteele, B.; Willekens, K. Compost Quality Indicators; SOILCOM Report Number 5.1.; 2020; 23p. Available online: https://northsearegion.eu/media/18076/soilcom-report-1-compost-quality-indicators.pdf (accessed on 21 December 2021).
- Zucconi, F.; De Bertoldi, M. Compost specifications for the production and characterization of compost from municipal solid waste. In Compost: Production, Quality and Use; De Bertoldi, M., Ferranti, M.P., L’Hermite, P., Zucconi, F., Eds.; Elsevier Applied Science: London, UK, 1987; pp. 30–50. [Google Scholar]
- Hua, L.; Wu, W.; Liu, Y.; McBride, M.B.; Chen, Y. Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ. Sci. Pollut. Res. 2009, 16, 1–9. [Google Scholar] [CrossRef]
- Raza, S.T.; Zhu, B.; Tang, J.L.; Ali, Z.; Anjum, R.; Bah, H.; Iqbal, H.; Ren, X.; Ahmad, R. Nutrients Recovery during Vermicomposting of Cow Dung, Pig Manure, and Biochar for Agricultural Sustainability with Gases Emissions. Appl. Sci. 2020, 10, 8956. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Tiquia, S.M. Evaluating phytotoxicity of pig manure from the pig-on-litter system. In Proceedings of the International Composting Symposium, Dartmouth/Halifax, NS, Canada, 20 September 1999; Warman, P.R., Taylor, B.R., Eds.; CBA Press Inc.: Truro, NS, Canada, 1999; pp. 625–647. [Google Scholar]
- Emino, E.R.; Warman, P.R. Biological Assay for Compost Quality. Compos. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Xavier, M.C.G.; Santos, C.A.; Costa, E.S.P.; Carmo, M.G.F. Cabbage yield as a function of bokashi doses. Rev. Agric. Neotrop. 2019, 6, 17–22. [Google Scholar] [CrossRef]
- Gómez, R.B.; Lima, F.V.; Ferrer, A.S. The use of respiration indices in the composting process: A review. Waste Manag. Res. 2006, 24, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Biological Treatment of Biowaste. Working Document. 2001. Available online: http://www.cre.ie/docs/EU_BiowasteDirective_workingdocument_2nddraft.pdf (accessed on 7 December 2021).
- Insam, H.; De Bertoldi, M. Microbiology of the composting process. Waste Manag. Ser. 2007, 8, 25–48. [Google Scholar]
- Vizhimalar, A.S.; Vasanthy, M.; Thamaraiselvi, C.; Biruntha, M.; Arockia, J.A.J.; Thirupathi, A.; Chang, S.W.; Xu, Z.; Al-Rashed, S.; Munuswamy-Ramanujam, G.; et al. Greener production of compost from agricultural biomass residues amended with mule dung for agronomic application. Chemosphere 2021, 288, 132561. [Google Scholar] [CrossRef] [PubMed]
- Strom, P.F. Effect of temperature on bacterial species diversity in thermophilic solid-waste composting. Appl. Environ. Microbiol. 1985, 50, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Robledo-Mahón, T.; Martín, M.A.; Gutiérrez, M.C.; Toledo, M.; González, I.; Aranda, E.; Chica, A.F.; Calvo, C. Sewage sludge composting under semi-permeable film at full-scale: Evaluation of odour emissions and relationships between microbiological activities and physico-chemical variables. Environ. Res. 2019, 177, 108624. [Google Scholar] [CrossRef]
- Hori, Y.; Fujita, H.; Hiruma, K.; Narisawa, K.; Toju, H. Synergistic and Offset Effects of Fungal Species Combinations on Plant Performance. Front. Microbiol. 2021, 12, 713180. [Google Scholar] [CrossRef]


| Compost Nr. | Turning | Cover | Particle Size of Hop Biomass (cm) | Additive at Compost Pile Preparation |
|---|---|---|---|---|
| CON | 7-times | / | 2–10 | / |
| BC | 11-times | / | 2–5 | Biochar (11 kg/tonne) |
| EM | 2-times (both before covering the pile) | Black foil cover after 1 month | 1–5 | Effective microorganisms (2 L/tonne) |
| Type of Analysis | Sampling Method |
|---|---|
| Chemistry | 3 samples/pile, each from 12 different spots |
| Microbiology, bacteria and fungi count | 1 sample/pile, each from 4 different spots |
| Respiratory test | 3 samples/pile, each from 4 different spots |
| Germination test | 3 samples/pile, each from 4 different spots |
| Growth test | 1 sample/pile from 12 different spots |
| BC | EM | CON | Average | |
|---|---|---|---|---|
| pH | 6.8 b ** | 6.1 a | - | 6.5 |
| DM (%) | 28.5 b | 25.6 a | 29.4 b | 27.8 |
| TP (%) 1 | 0.26 a | 0.28 a | 0.3 a | 0.28 |
| TK (%) 1 | 1.7 b | 1.17 a | 2.11 c | 1.67 |
| TC (%) 1 | 49.5 b | 50.8 b | 44.0 a | 48.1 |
| TN (%) 1 | 2.8 b | 1.9 a | 3.0 b | 2.6 |
| NO3-N (mg/kg) 2 | 0.5 a | 1.0 a | 1.0 a | 0.8 |
| NH4-N (mg/kg) 2 | 170.3 b | 81.3 a | 257.3 b | 169.6 |
| BC | EM | CON | Average | |
|---|---|---|---|---|
| pH | 7.6 a ** | 8.1 c | 7.8 b | 7.8 |
| DM (%) | 28.6 a | 30.4 a | 34.8 b | 31.2 |
| TP (%) 1 | 0.43 a | 0.38 a | 0.33 a | 0.38 |
| TK (%) 1 | 0.99 ab | 1.41 b | 0.86 a | 1.08 |
| TC (%) 1 | 22.0 ab | 29.8 b | 16.5 a | 22.8 |
| TN (%) 1 | 3.4 c | 2.6 b | 2.0 a | 2.7 |
| NO3-N (mg/kg) 2 | 628.8 c | 84.8 a | 414.2 b | 375.9 |
| NH4-N (mg/kg) 2 | 446.4 a | 380.9 a | 384.0 a | 403.8 |
| Germination Test | Growth Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compost | Garden Cress (Lepidium sativum) | Radish (Raphanus sativus) | Chinese Cabbage (Brassica rapa L. ssp. Pekinensis) | ||||||
| Mean Root Elongation (mm) | Number of Germinated Seeds | GI (%) | Mean Root Elongation (mm) | Number of Germinated Seeds | GI (%) | Germination (%) | Mean Green Mass per Pot (g) | Mass of One Shoot (g) | |
| CON | 21.2 b ** | 9.6 | 203 b | 31.7 a | 9.8 | 88 a | 100 | 8.66 | 0.83 b |
| BC | 22.2 b | 9.8 | 222 b | 32.8 a | 9.7 | 89 a | 95 | 6.07 | 0.63 a |
| EM | 16.4 a | 9.8 | 164 a | 30.8 a | 10 | 89 a | 90 | 9.48 | 1.06 c |
| Control | 9.5 | 10 | 100 | 34.5 | 10 | 100 | 92.5 | 7.78 | 0.84 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luskar, L.; Polanšek, J.; Hladnik, A.; Čeh, B. On-Farm Composting of Hop Plant Green Waste—Chemical and Biological Value of Compost. Appl. Sci. 2022, 12, 4190. https://doi.org/10.3390/app12094190
Luskar L, Polanšek J, Hladnik A, Čeh B. On-Farm Composting of Hop Plant Green Waste—Chemical and Biological Value of Compost. Applied Sciences. 2022; 12(9):4190. https://doi.org/10.3390/app12094190
Chicago/Turabian StyleLuskar, Lucija, Julija Polanšek, Aleš Hladnik, and Barbara Čeh. 2022. "On-Farm Composting of Hop Plant Green Waste—Chemical and Biological Value of Compost" Applied Sciences 12, no. 9: 4190. https://doi.org/10.3390/app12094190
APA StyleLuskar, L., Polanšek, J., Hladnik, A., & Čeh, B. (2022). On-Farm Composting of Hop Plant Green Waste—Chemical and Biological Value of Compost. Applied Sciences, 12(9), 4190. https://doi.org/10.3390/app12094190






