Sodium-Conducting Ionic Liquid Electrolytes: Electrochemical Stability Investigation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Electrochemical Measurements
3. Results and Discussion
3.1. Electrochemical Stability
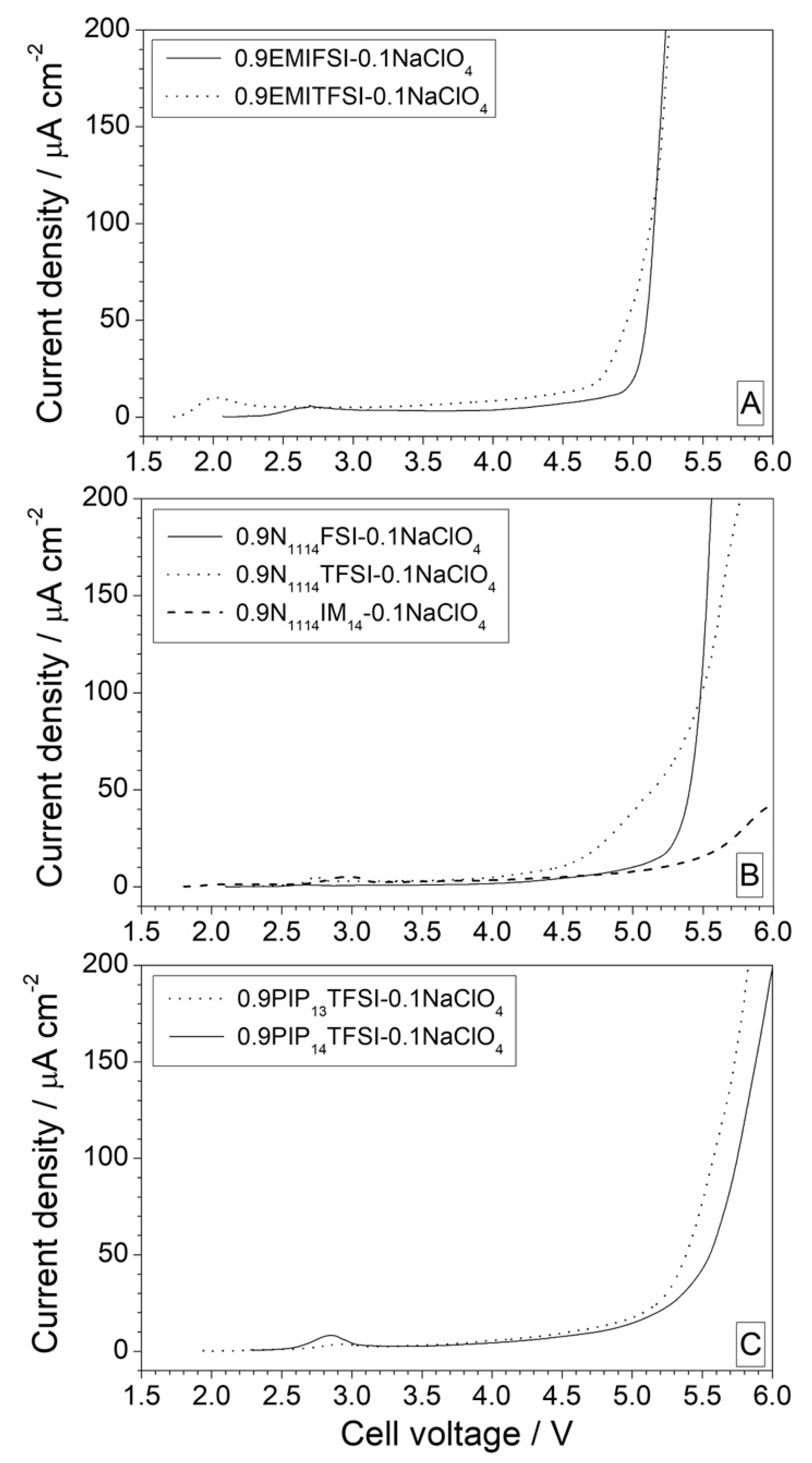
3.1.1. Preliminary LSV Tests
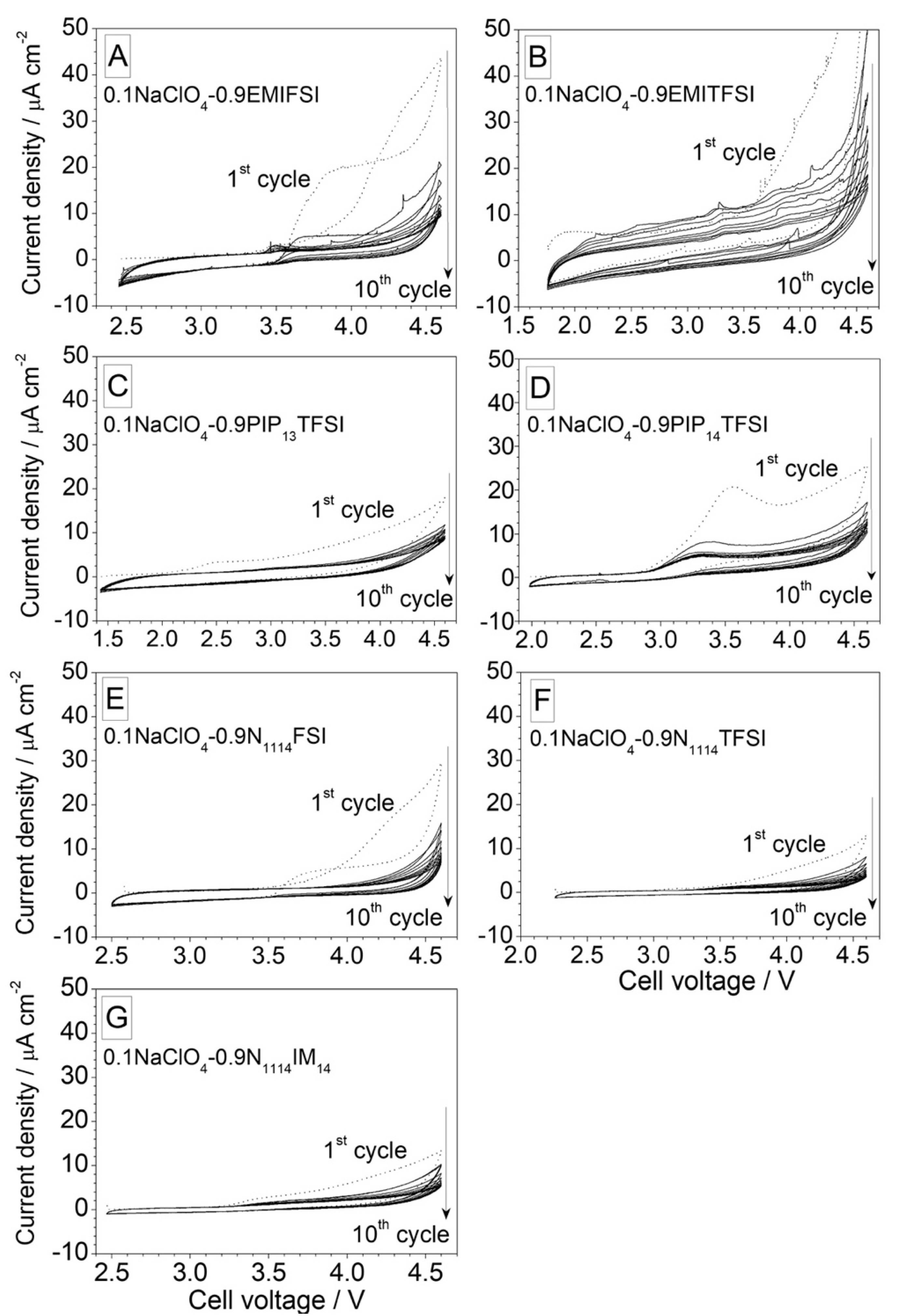
3.1.2. Anodic Stability
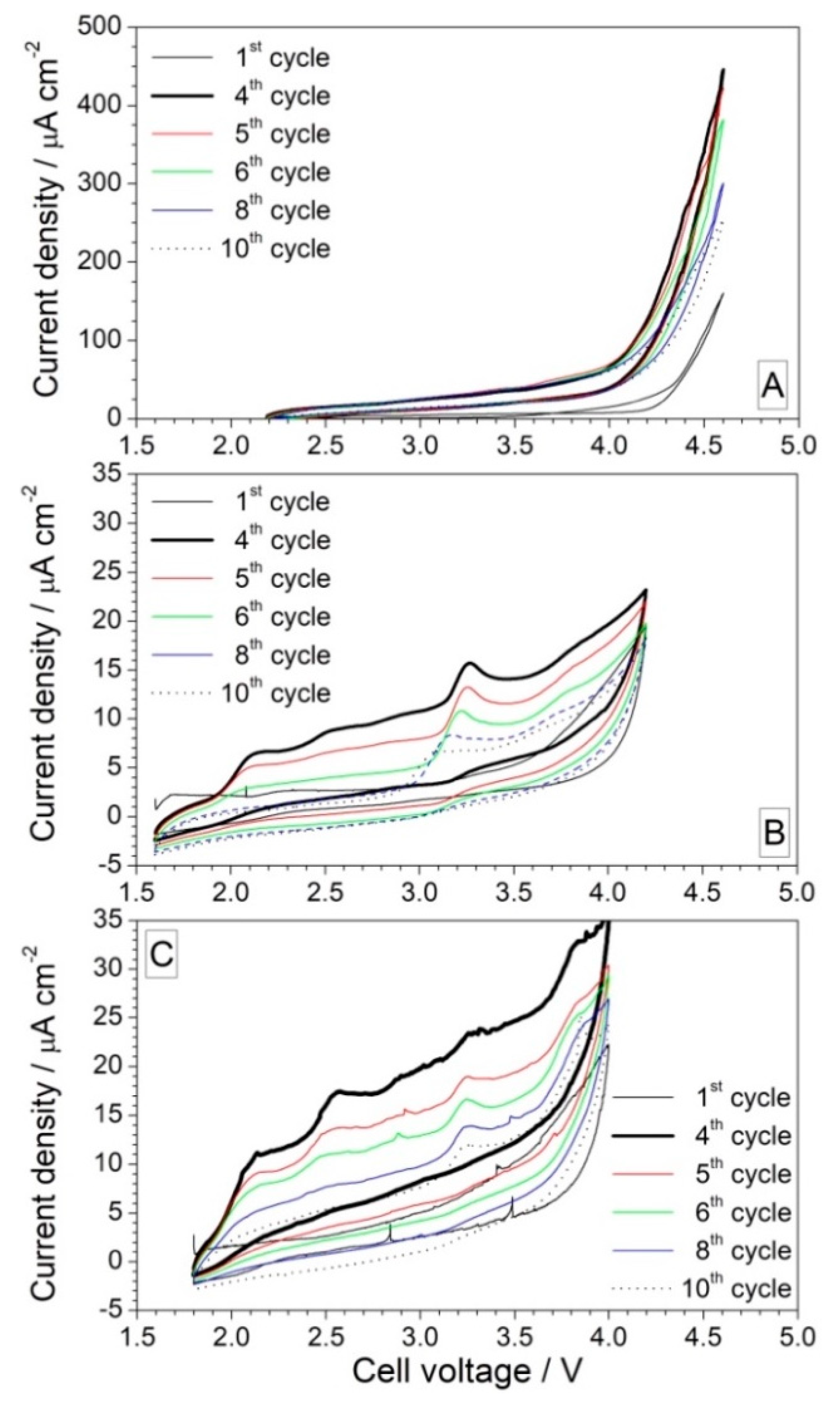
3.1.3. Effect of Contaminants on the Anodic Stability
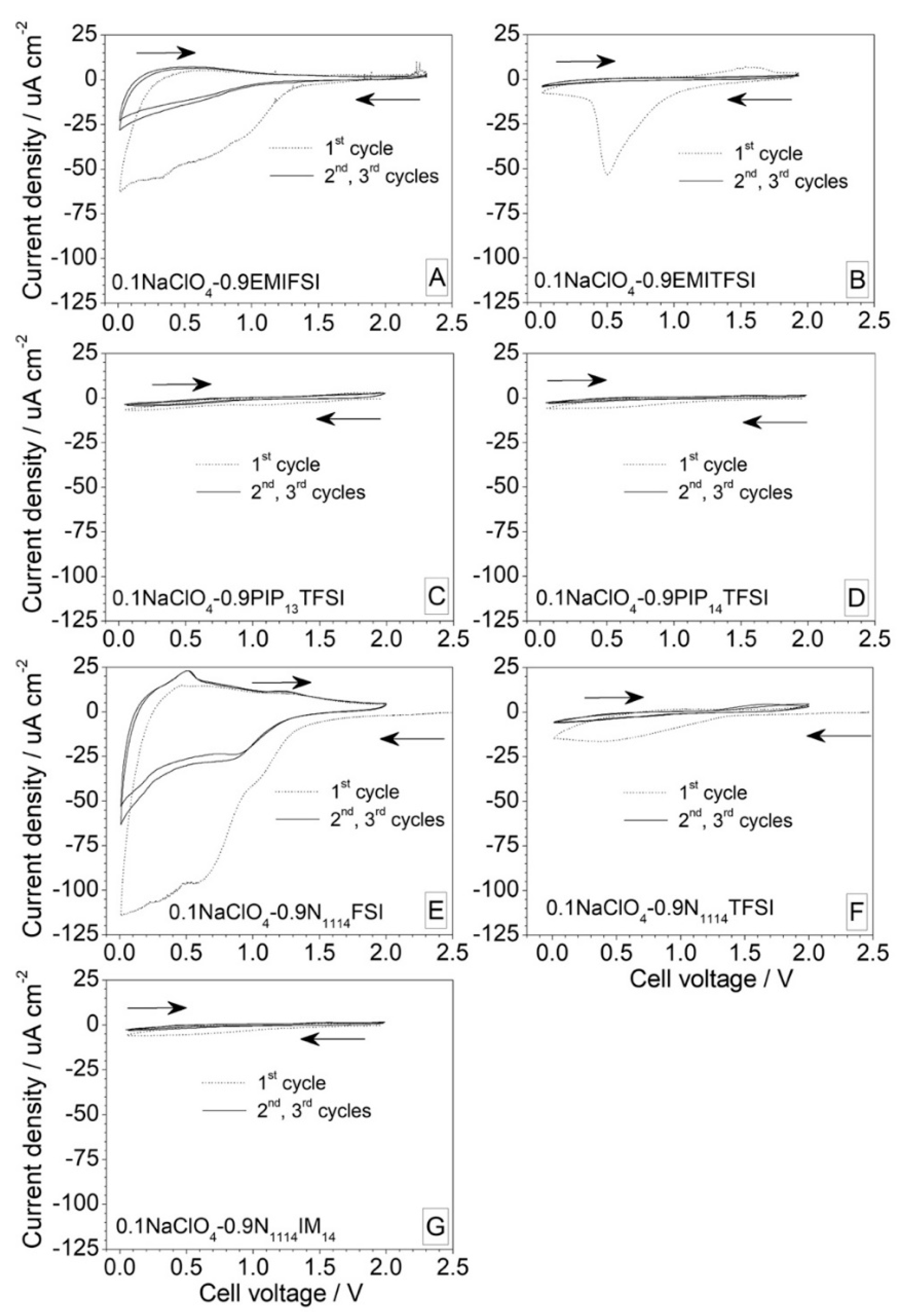
3.1.4. Cathodic Stability
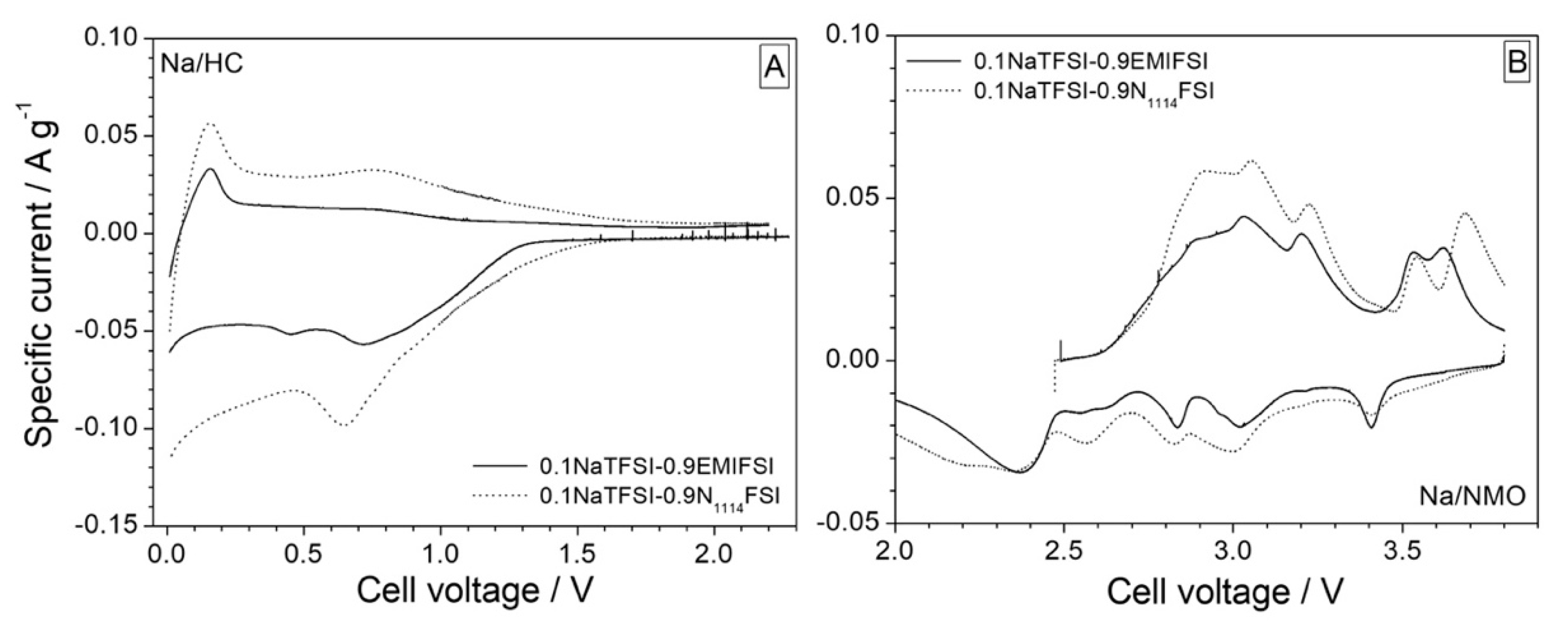
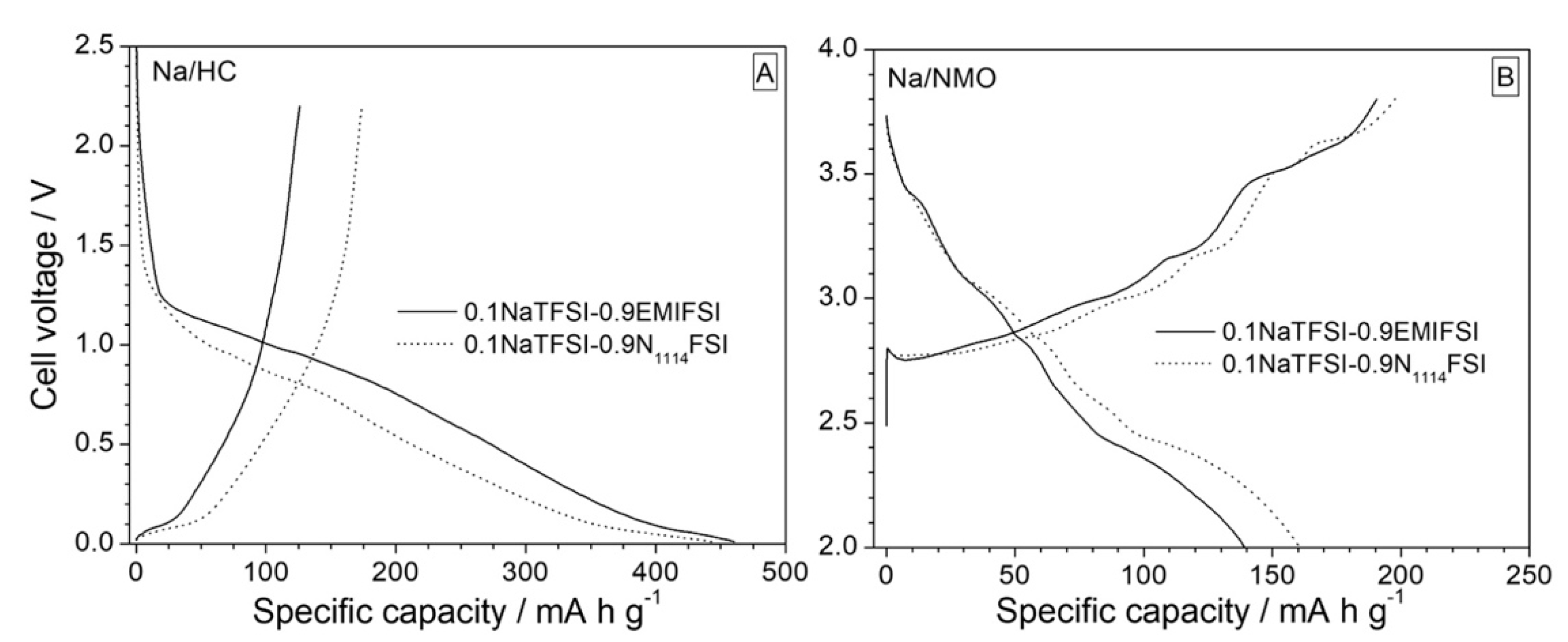
3.2. Preliminary Test in Na Half Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 2001, 1, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Tarascon, J.-M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef]
- Carmichael, R.S. Practical Handbook of Physical Properties of Rocks and Minerals; Choice Reviews Online; CRC: Boca Raton, FL, USA, 1989; pp. 27–1288. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.Á.; Saurel, D.; Gómez-Cámer, J.L.; Casas-Cabanas, M.; Castillo-Martínez, E.; Rojo, T. Na-Ion Batteries for Large Scale Applications: A Review on Anode Materials and Solid Electrolyte Interphase Formation. Adv. Energy Mater. 2017, 7, 1700463. [Google Scholar] [CrossRef]
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials. A growing research path. Energy Environ. Sci. 2013, 6, 2312–2337. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Montanino, M.; Passerini, S. Ionic Liquid-Based Electrolytes for High Energy, Safer Lithium Batteries. In Ionic Liquids: Science and Applications; ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2012; pp. 67–128. [Google Scholar]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Navarra, M.A. Ionic liquids as safe electrolyte components for Li-metal and Li-ion batteries. MRS Bull. 2013, 38, 548–553. [Google Scholar] [CrossRef]
- Bellusci, M.; Simonetti, E.; De Francesco, M.; Appetecchi, G.B. Ionic Liquid Electrolytes for Safer and More Reliable Sodium Battery Systems. Appl. Sci. 2020, 10, 6323. [Google Scholar] [CrossRef]
- Carboni, M.; Manzi, J.; Armstrong, A.R.; Billaud, J.; Brutti, S.; Younesi, R. Analysis of the Solid Electrolyte Interphase on Hard Carbon Electrodes in Sodium-Ion Batteries. ChemElectroChem 2019, 6, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- Manzi, J.; Paolone, A.; Palumbo, O.; Corona, D.; Massaro, A.; Cavaliere, R.; Muñoz-García, A.; Trequattrini, F.; Pavone, M.; Brutti, S. Monoclinic and Orthorhombic NaMnO2 for Secondary Batteries: A Comparative Study. Energies 2021, 14, 1230. [Google Scholar] [CrossRef]
- Montanino, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. Water-based synthesis of hydrophobic ionic liquids for high-energy electrochemical devices. Electrochim. Acta 2013, 96, 124–133. [Google Scholar] [CrossRef]
- De Francesco, M.; Simonetti, E.; Giorgi, G.; Appetecchi, G.B. About the Purification Route of Ionic Liquid Precursors. Challenges 2017, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Montanino, M.; Moreno, M.; Alessandrini, F.; Appetecchi, G.; Passerini, S.; Zhou, Q.; Henderson, W. Physical and electrochemical properties of binary ionic liquid mixtures: (1 − x) PYR14TFSI-(x) PYR14IM14. Electrochim. Acta 2012, 60, 163–169. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Montanino, M.; Carewska, M.; Moreno, M.; Alessandrini, F.; Passerini, S. Chemical–physical properties of bis(perfluoroalkylsulfonyl)imide-based ionic liquids. Electrochim. Acta 2011, 56, 1300–1307. [Google Scholar] [CrossRef]
- Jeong, S.; Li, S.; Appetecchi, G.B.; Passerini, S. Asymmetric ammonium-based ionic liquids as electrolyte components for safer, high-energy, electrochemical storage devices. Energy Storage Mater. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Brutti, S.; Simonetti, E.; De Francesco, M.; Sarra, A.; Paolone, A.; Palumbo, O.; Fantini, S.; Lin, R.; Falgayrat, A.; Choi, H.; et al. Ionic liquid electrolytes for high-voltage, lithium-ion batteries. J. Power Sources 2020, 479, 228791. [Google Scholar] [CrossRef]
- Randström, S.; Montanino, M.; Appetecchi, G.B.; Lagergren, C.; Moreno, A.; Passerini, S. Effect of water and oxygen traces on the cathodic stability of N-alkyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide. Electrochim. Acta 2008, 53, 6397–6401. [Google Scholar] [CrossRef]
- Doblinger, S.; Donati, T.J.; Silvester, D.S. Effect of Humidity and Impurities on the Electrochemical Window of Ionic Liquids and Its Implications for Electroanalysis. J. Phys. Chem. C 2020, 124, 20309–20319. [Google Scholar] [CrossRef]
- Jafta, C.J.; Sun, X.; Lyu, H.; Chen, H.; Thapaliya, B.P.; Heller, W.T.; Cuneo, M.J.; Mayes, R.T.; Paranthaman, M.P.; Dai, S.; et al. Insight into the Solid Electrolyte Interphase Formation in Bis(fluorosulfonyl)Imide Based Ionic Liquid Electrolytes. Adv. Funct. Mater. 2021, 31, 2008708. [Google Scholar] [CrossRef]
- Girard, G.M.A.; Hilder, M.; Dupre, N.; Guyomard, D.; Nucciarone, D.; Whitbread, K.; Zavorine, S.; Moser, M.; Forsyth, M.; MacFarlane, D.R.; et al. Spectroscopic Characterization of the SEI Layer Formed on Lithium Metal Electrodes in Phosphonium Bis(fluorosulfonyl)imide Ionic Liquid Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803–A811. [Google Scholar] [CrossRef]
- Irisarri, E.; Ponrouch, A.; Palacin, M.R. Review—Hard Carbon Negative Electrode Materials for Sodium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2476–A2482. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Ceder, G. Electrochemical Properties of Monoclinic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307–A1312. [Google Scholar] [CrossRef]
- Thomas, P.; Billaud, D. Electrochemical insertion of sodium into hard carbons. Electrochim. Acta 2002, 47, 3303–3307. [Google Scholar] [CrossRef]
| Ionic Liquid | Thermal Stability/°C | σ/ S cm−1 | |
|---|---|---|---|
| −20 °C | 20 °C | ||
| EMIFSI | 150 | (1.1 ± 0.1) × 10−3 | (8.6 ± 0.5) × 10−3 |
| EMITFSI | 275 | (3.8 ± 0.2) × 10−4 | (3.8 ± 0.2) × 10−3 |
| N1114FSI | 150 | (2.8 ± 0.2) ×10−7 | (1.8 ± 0.1) × 10−3 |
| N1114TFSI | 225 | (1.6 ± 0.1) × 10−7 | (5.3 ± 0.3) × 10−4 |
| N1114IM14 | 225 | (7.0 ± 0.4) × 10−7 | (8.2 ± 0.5) × 10−5 |
| PIP13TFSI | 250 | (5.6 ± 0.3) × 10−7 | (5.0 ± 0.3) × 10−4 |
| PIP14TFSI | 225 | (9.9 ± 0.5) × 10−8 | (3.1 ± 0.2) × 10−4 |
| Electrolyte Sample | EA1 | EA2 | EA1 | EA2 |
|---|---|---|---|---|
| 0.9EMIFSI:0.1NaClO4 | 3.984 | 4.142 | 4.311 | 4.589 |
| 0.9EMITFSI:0.1NaClO4 | 3.453 | 3.789 | 3.166 | 4.093 |
| 0.9N1114FSI:0.1NaClO4 | 4.092 | 4.372 | 4.514 | >4.6 |
| 0.9N1114TFSI:0.1NaClO4 | 4.464 | >4.6 | >4.8 | >4.6 |
| 0.9N1114IM4:0.1NaClO4 | 4.381 | >4.6 | 4.598 | >4.6 |
| 0.9PIP13TFSI:0.1NaClO4 | 3.947 | >4.6 | 4.479 | >4.6 |
| 0.9PIP14TFSI:0.1NaClO4 | 3.248 | 3.489 | 4.212 | >4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maresca, G.; Casu, P.; Simonetti, E.; Brutti, S.; Appetecchi, G.B. Sodium-Conducting Ionic Liquid Electrolytes: Electrochemical Stability Investigation. Appl. Sci. 2022, 12, 4174. https://doi.org/10.3390/app12094174
Maresca G, Casu P, Simonetti E, Brutti S, Appetecchi GB. Sodium-Conducting Ionic Liquid Electrolytes: Electrochemical Stability Investigation. Applied Sciences. 2022; 12(9):4174. https://doi.org/10.3390/app12094174
Chicago/Turabian StyleMaresca, Giovanna, Paolo Casu, Elisabetta Simonetti, Sergio Brutti, and Giovanni Battista Appetecchi. 2022. "Sodium-Conducting Ionic Liquid Electrolytes: Electrochemical Stability Investigation" Applied Sciences 12, no. 9: 4174. https://doi.org/10.3390/app12094174
APA StyleMaresca, G., Casu, P., Simonetti, E., Brutti, S., & Appetecchi, G. B. (2022). Sodium-Conducting Ionic Liquid Electrolytes: Electrochemical Stability Investigation. Applied Sciences, 12(9), 4174. https://doi.org/10.3390/app12094174








