Characterization of the Different Oils Obtained through the Catalytic In Situ Pyrolysis of Polyethylene Film from Municipal Solid Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Catalysts
2.2.1. Textural Parameters
2.2.2. XRD
2.3. Catalytic Pyrolysis Tests
2.4. Physical Characterization of the Pyrolytic Oils
- Light oil: API gravity > 31.1°;
- Medium oil: API gravity between 22.3° and 31.1°;
- Heavy oil: API gravity < 22.3°;
- Extra-heavy oil: API gravity < 10°.
2.5. Chemical Characterization of the Pyrolytic Oils
2.5.1. Elemental Analysis and Calorific Value
2.5.2. Thermogravimetry Analysis (TGA)
2.5.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.5.4. Gas Chromatography-Mass Spectrometry (GC-MS)
3. Results and Discussions
3.1. Characterization of the Catalysts
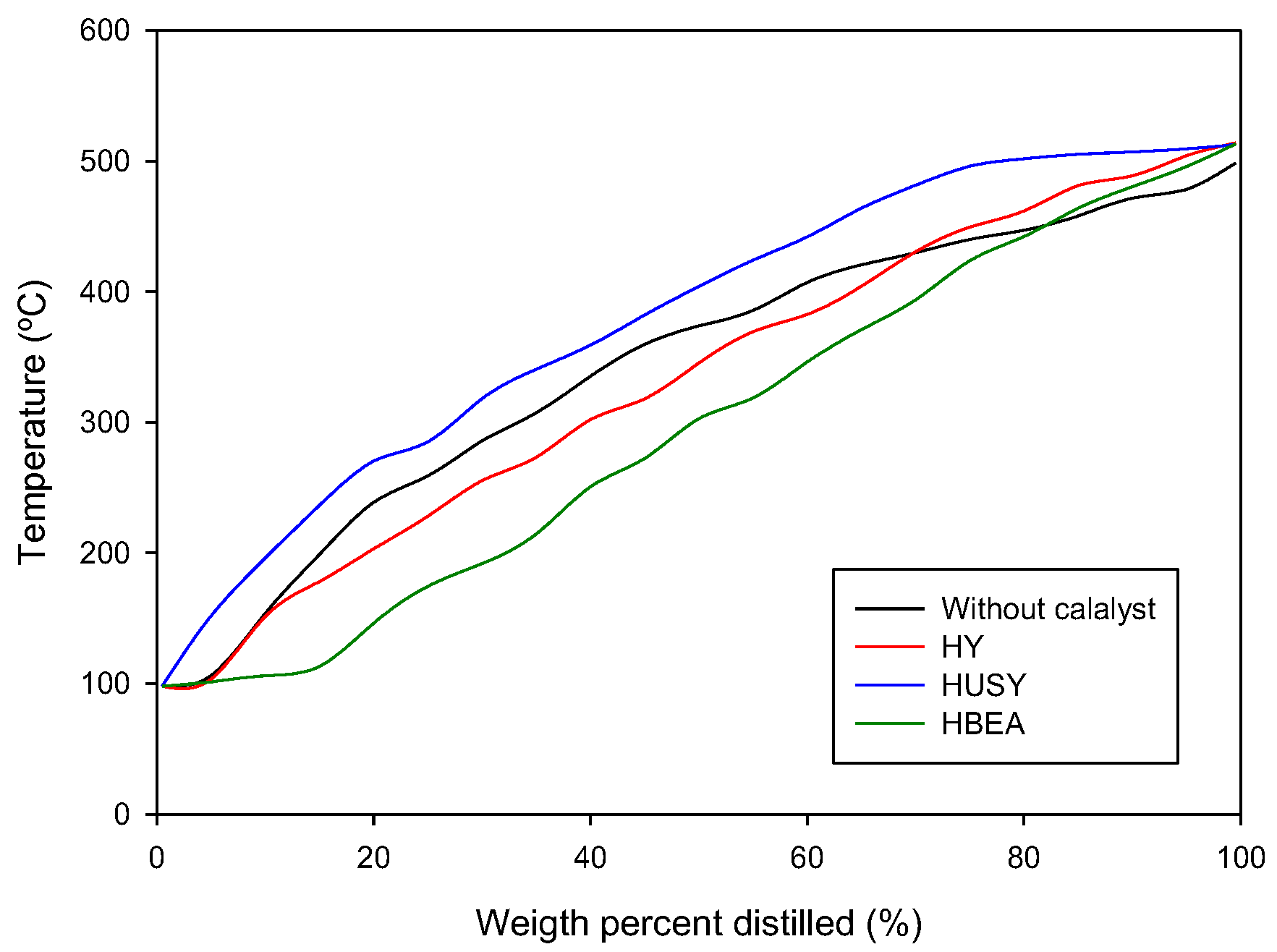
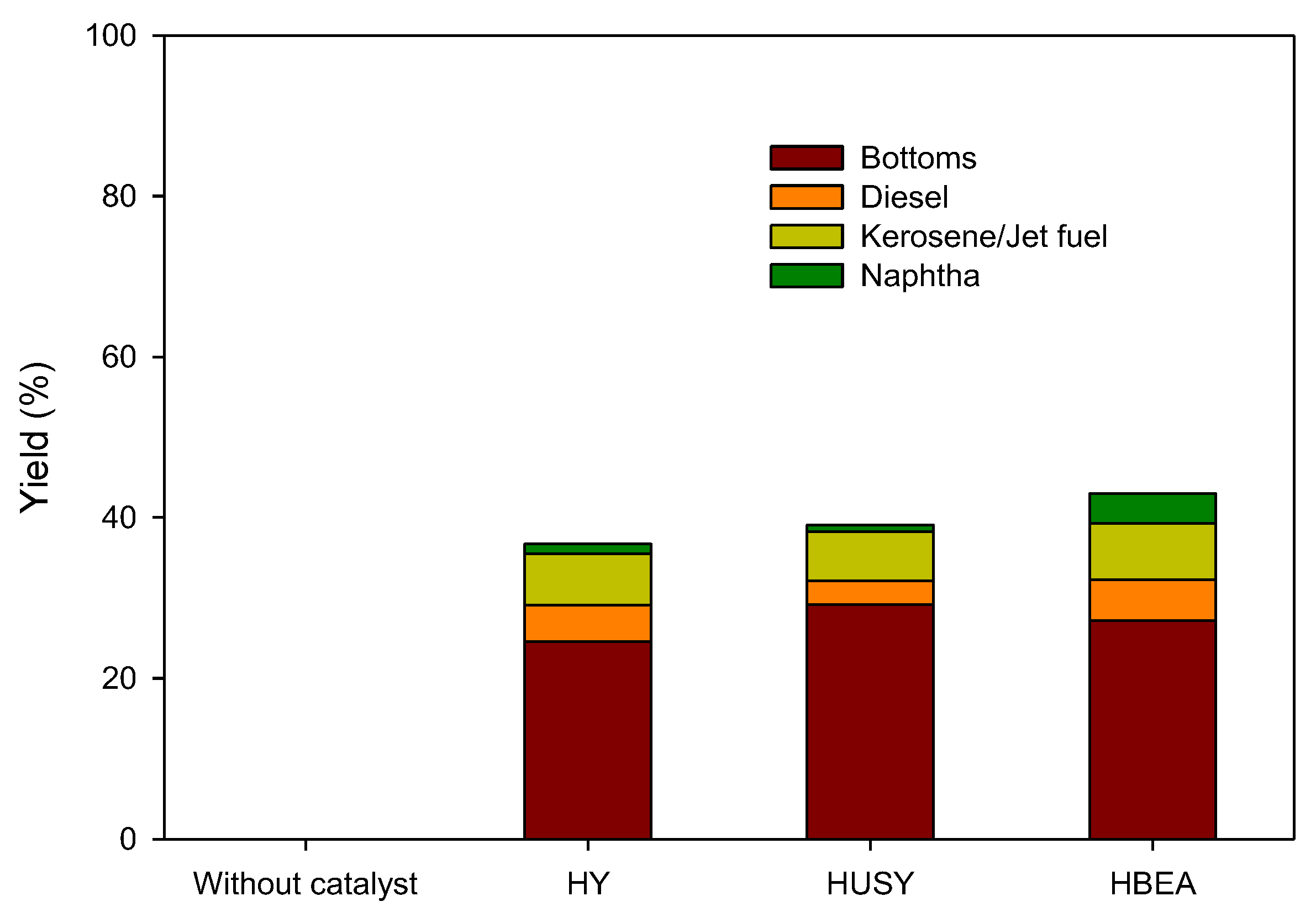
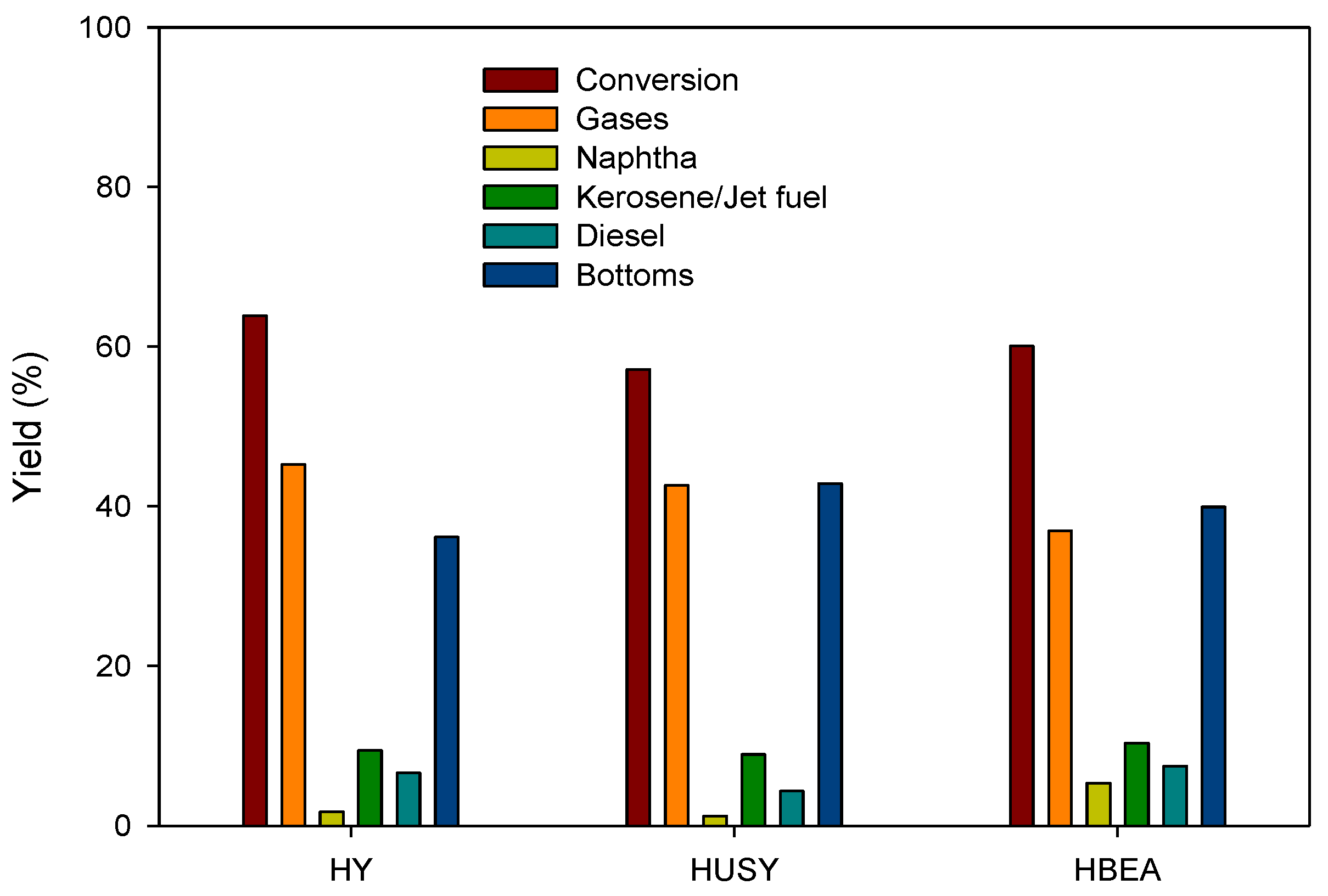
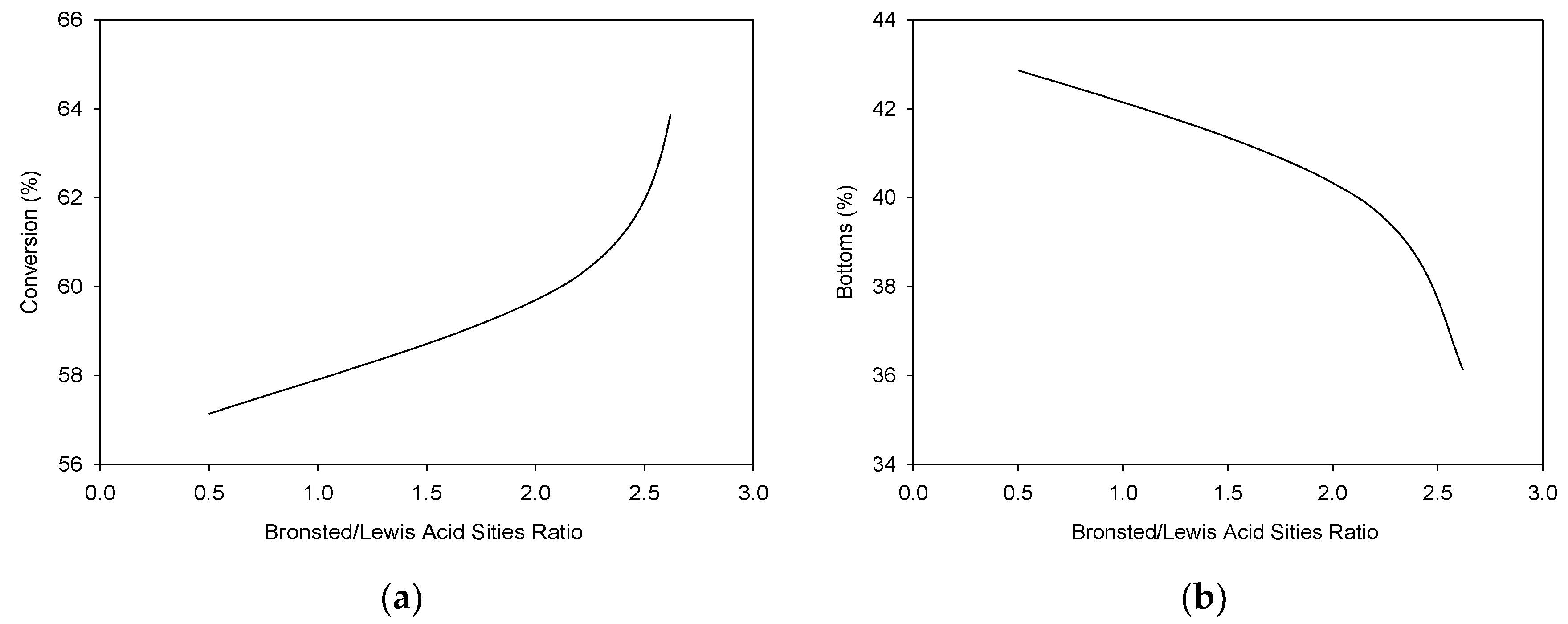
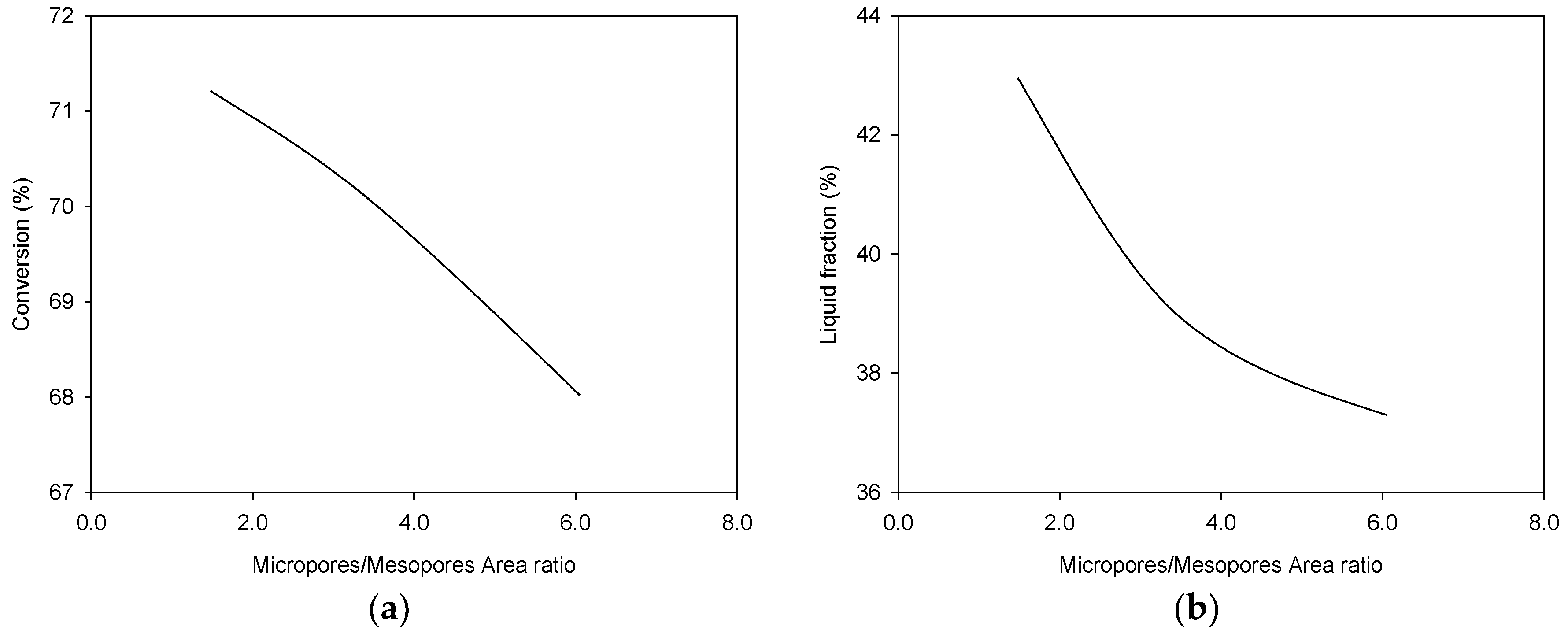
3.2. Physical Characterization of Pyrolytic Oil
3.3. Chemical Characterization of Pyrolytic Oil
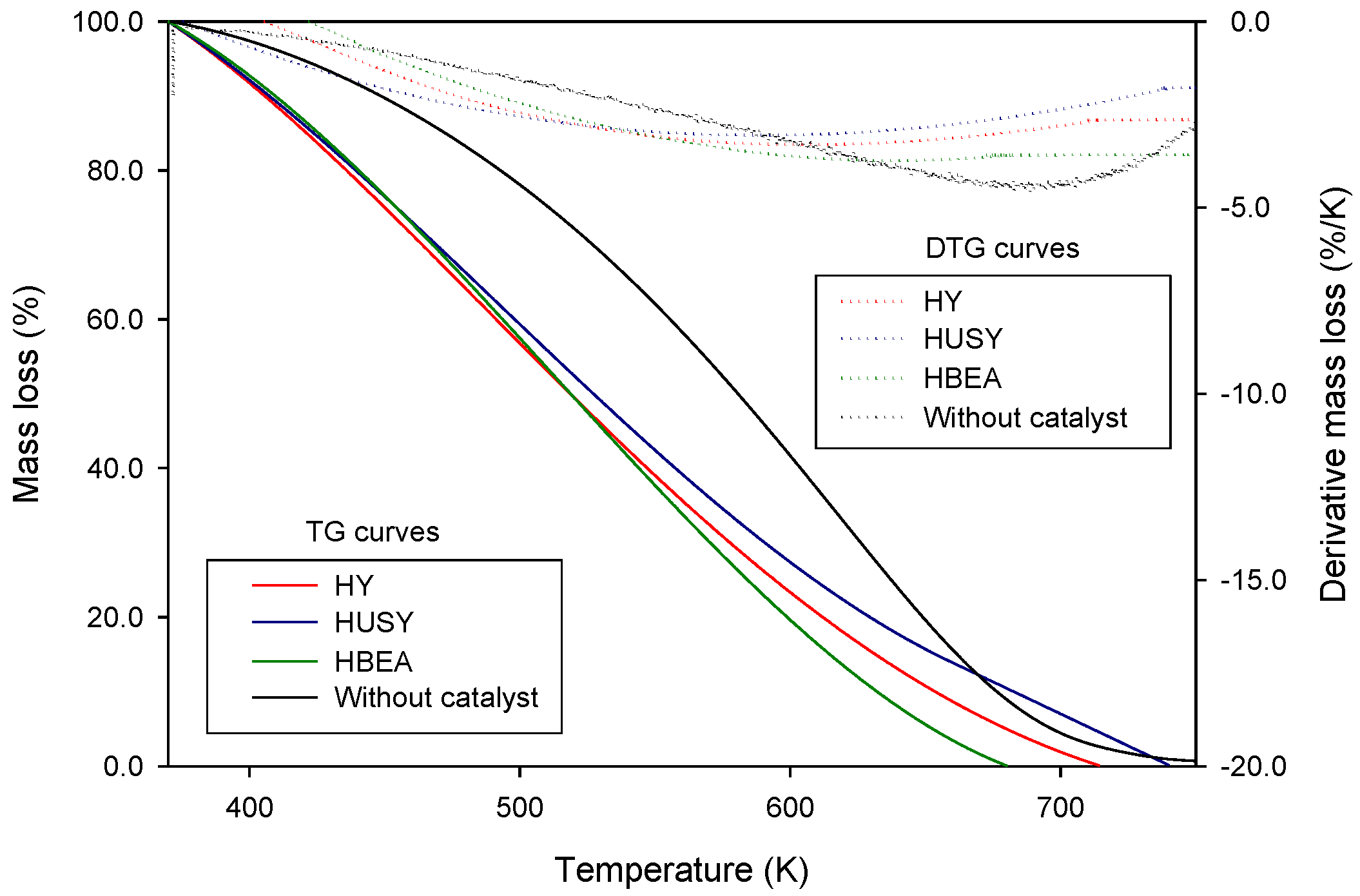
3.3.1. TGA Analysis
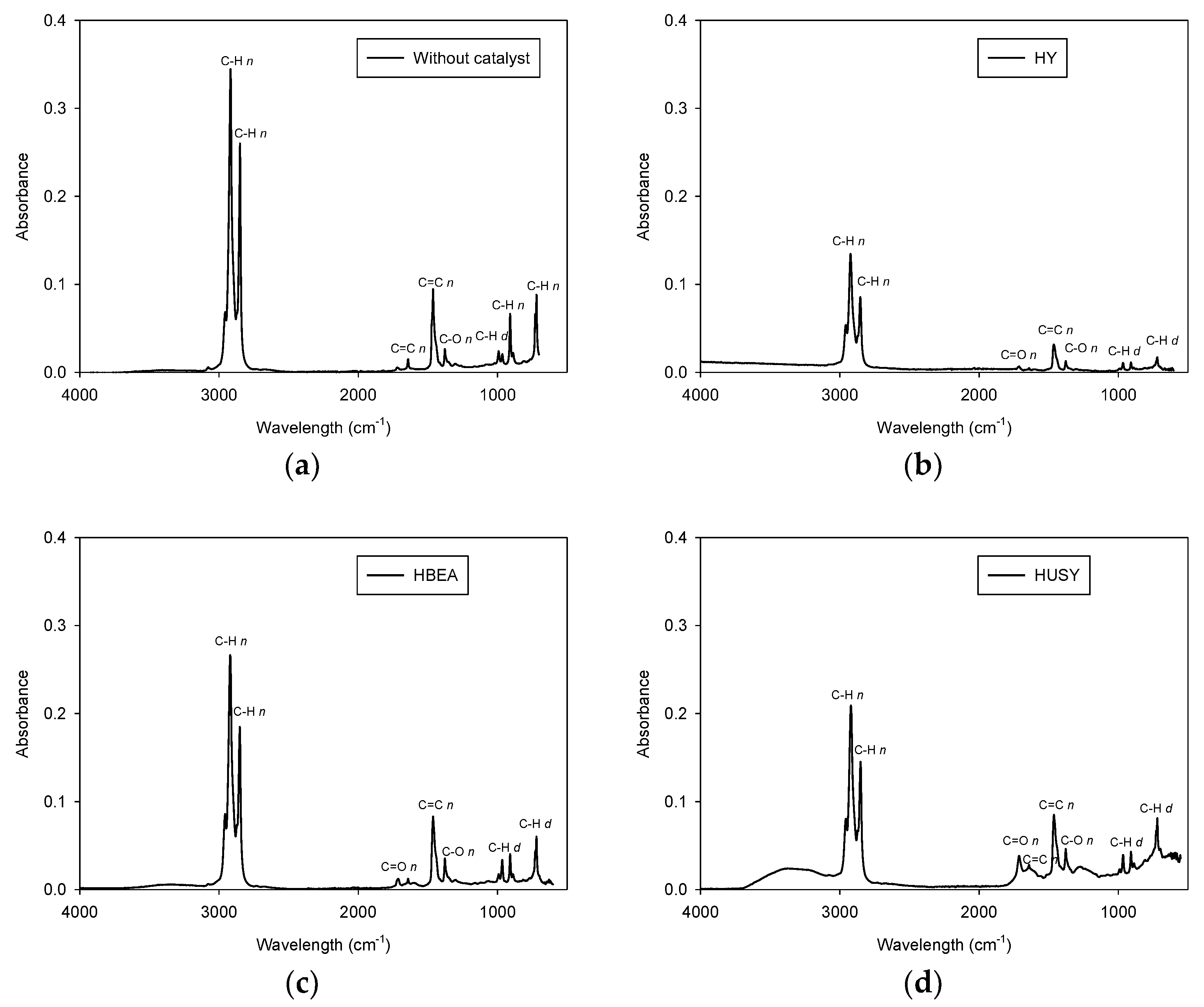
3.3.2. FTIR Analysis of the Pyrolytic Oil
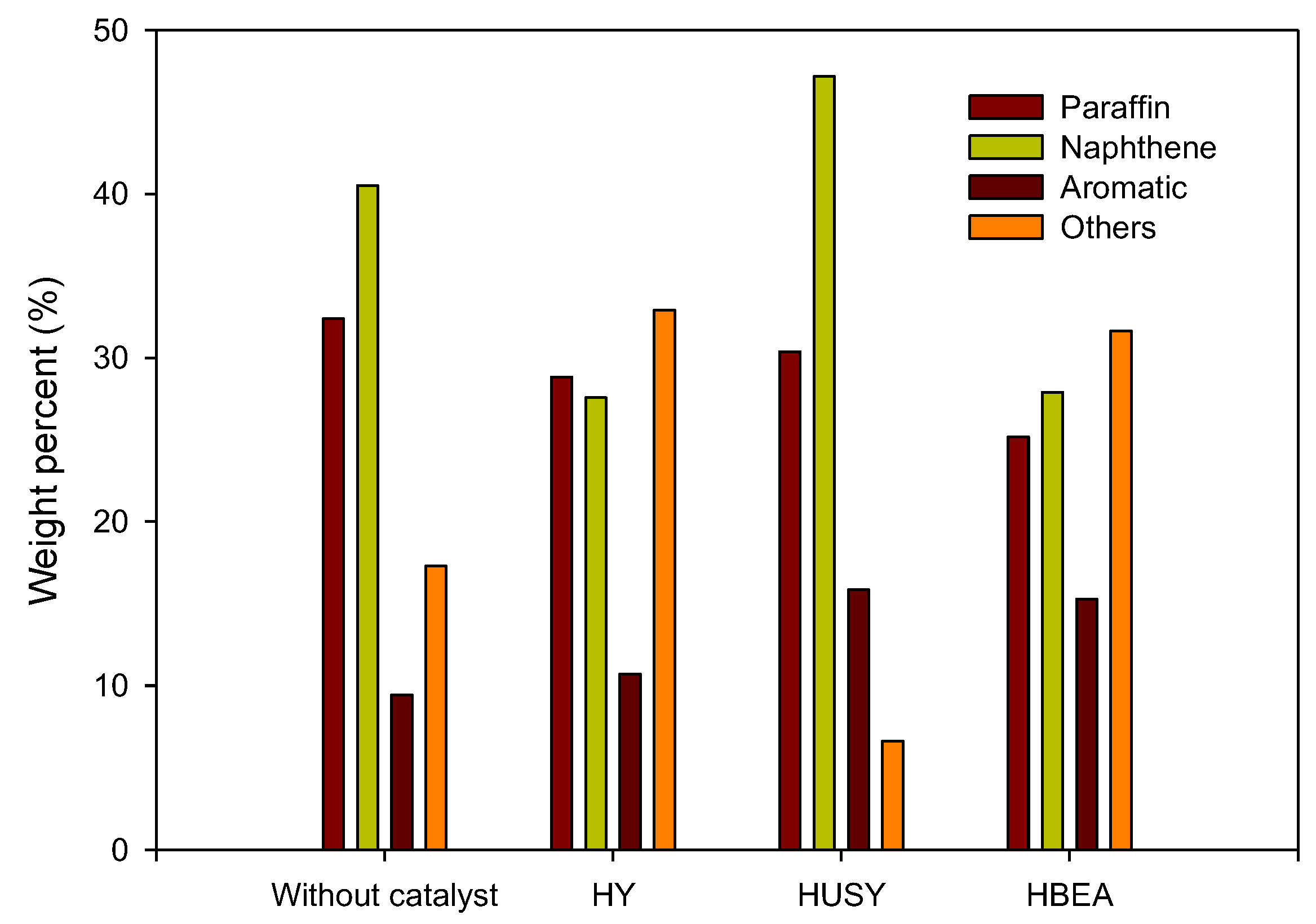
3.3.3. GC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- PlasticsEurope. An Analysis of European Plastics Production, Demand and Waste Data. Plastics—The Facts 2020 PlasticsEurope. 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 14 February 2022).
- Provincial Council of Granada. Municipal Waste Treatment Service. Municipal Waste Collection in the Province of Granada (Spain). 2015. Available online: https://www.resurgranada.es/datos_recogidas_RM.php (accessed on 14 February 2022).
- Calero, M.; Martin-Lara, M.A.; Godoy, V.; Quesada, L.; Martinez, D.; Peula, F.; Soto, J.M. Characterization of plastic materials present in municipal solid waste: Preliminary study for their mechanical recycling. Detritus 2018, 4, 104–112. [Google Scholar] [CrossRef]
- Rajendran, K.M.; Chintala, V.; Sharma, A.; Pal, S.; Pandey, J.K.; Ghodke, P. Review of catalyst materials in achieving the liquid hydrocarbon fuels from municipal mixed plastic waste (MMPW). Mater. Today Commun. 2020, 24, 100982. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic conversion of plastic waste to value-added products and fuels: A review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. J. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Li, N.; Lium, H.X.; Cheng, Z.N.; Yan, B.B.; Chen, G.Y.; Wang, S.B. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 2022, 424, 127460. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martin-Lara, M.A.; Pérez, A.; Blázquez, G. Characterization of fuel produced by pyrolysis of plastic film obtained of municipal solid waste. Energy 2019, 186, 115874. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehanb, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Benedetti, M.; Cafiero, L.; Angelis, D.D.; Dell’Era, A.; Pasquali, M.; Stendardo, S.; Tuffi, R.; Ciprioti, S.V. Pyrolysis of WEEE plastics using catalysts produced from fly ash of coal gasification. Front. Environ. Sci. Eng. 2017, 11, 11. [Google Scholar] [CrossRef]
- Kremer, I.; Tomic, T.; Katancic, Z.; Erceg, M.; Papuga, S.; Vukovic, J.P.; Schneider, D.R. Catalytic pyrolysis of mechanically non-recyclable waste plastics mixture: Kinetics and pyrolysis in laboratory-scale reactor. J. Environ. Manag. 2021, 296, 113145. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Serrano, D.P.; Miguel, G.S.; Escola, J.M.; Rodriguez, J.M. Catalytic activity of zeolitic and mesostructured catalysts in the cracking of pure and waste polyolefins. J. Anal. Appl. Pyrolysis 2007, 78, 153–161. [Google Scholar] [CrossRef]
- Awan, U.; Kraslawski, A.; Huiskonen, J. Progress from blue to the green world: Multilevel governance for pollution prevention planning and sustainability. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar]
- Soto, J.M.; Blázquez, G.; Calero, M.; Quesada, L.; Godoy, V.; Martin-Lara, M.A. A real case study of mechanical recycling as an alternative for managing of polyethylene plastic film presented in mixed municipal solid waste. J. Clean. Prod. 2018, 203, 777–787. [Google Scholar] [CrossRef]
- Lakiss, L.; Gilson, J.-P.; Valtchev, V.; Mintova, S.; Vicente, A.; Vimont, A.; Bedard, R.; Abdo, S.; Bricker, J. Zeolites in a good shape: Catalyst forming by extrusion modifies their performances. Microporous Mesoporous Mater. 2020, 299, 110114. [Google Scholar] [CrossRef]
- Nichterwitz, M.; Grätz, S.; Nickel, W.; Borchardt, L. Solvent-free hierarchization of zeolites by carbochlorination. J. Mater. Chem. A 2017, 5, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Batalha, N.; Comparot, J.-D.; Le Valant, A.; Pinard, L. In situ FTIR spectroscopy to unravel the bifunctional nature of aromatics hydrogenation synergy on zeolite/metal catalysts. Catal. Sci. Technol. 2022, 12, 1117–1129. [Google Scholar] [CrossRef]
- Awan, I.Z.; Beltrami, G.; Bonincontro, D.; Gimello, O.; Cacciaguerra, T.; Tanchoux, N.; Martucci, A.; Albonetti, S.; Cavani, F.; Di Renzo, F. Copper-nickel mixed oxide catalysts from layered double hydroxides for the hydrogen-transfer valorisation of lignin in organosolv pulping. Appl. Catal. A Gen. 2021, 609, 117929. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, S.; Raja, D.; Khusni, N.B.; Liu, J.; Zhang, J.; Abdulridha, S.; Xiang, H.; Guan, Y.; Jiao, Y.; et al. On the effect of mesoporosity of FAU Y zeolites in the liquid-phase catalysis. Microporous Mesoporous Mater. 2019, 278, 297–306. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. Production and characterization of pyrolysis oil using waste polyethylene in a semi batch reactor. Indian J. Chem. Technol. 2018, 25, 336–344. [Google Scholar]
- Kathiravale, S.; Yunus, M.N.M.; Sopian, K.; Samsuddin, A.H.; Rahman, R.A. Modeling the heating value of municipal solid waste. Fuel 2003, 82, 1119–1125. [Google Scholar] [CrossRef]
- Totten, G.; Westbrook, S.; Shah, R. Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing, 2nd ed.; American Society for Testing & Materials: Seattle, WA, USA, 2019; Available online: https://www.astm.org/mnl37-2nd-eb.html (accessed on 14 February 2022).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.Y.; Jin, L.J.; Wang, D.C.; Shi, H.; Hu, H.Q. Catalytic upgrading of lignite pyrolysis volatiles over modified HY zeolites. Fuel 2020, 259, 116234. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Amutio, M.; Artetxe, M.; Aguado, R.; Bilbao, J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2009, 85, 345–351. [Google Scholar] [CrossRef]
- Kenvin, J.; Mitchell, S.; Sterling, M.; Warringham, R.; Keller, T.C.; Crivelli, P.; Jagiello, J.; Pérez-Ramírez, J. Quantifying the complex pore architecture of hierarchical faujasite zeolites and the impact on diffusion. Adv. Funct. Mater. 2016, 26, 5621–5630. [Google Scholar] [CrossRef]
- Simon-Masseron, A.; Marques, J.P.; Lopes, J.M.; Ribeiro, F.R.; Gener, I.; Guisnet, M. Influence of the Si/Al ratio and crystal size on the acidity and activity of HBEA zeolites. Appl. Catal. A-Gen. 2007, 316, 75–82. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Quesada, L.; Calero, M.; Martin-Lara, M.A.; Luzón, G.; Blázquez, G. Performance of different catalysts for the in situ cracking of the oil-waxes obtained by the pyrolysis of polyethylene film waste. Sustainability 2020, 12, 5482. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martin-Lara, M.A.; Pérez, A.; Blázquez, G. Production of an alternative fuel by pyrolysis of plastic wastes mixtures. Energy Fuel 2020, 34, 1781–1790. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
- Singh, M.V.; Kumar, S.; Sarker, M. Waste HD-PE plastic, deformation into liquid hydrocarbon fuel using pyrolysis-catalytic cracking with a CuCO3 catalyst. Sustain. Energy Fuels 2018, 2, 1057–1068. [Google Scholar] [CrossRef]
- Lee, D.; Nam, H.; Wang, S.; Kin, H.; Kim, J.H.; Won, Y.; Hwang, B.W.; Kim, Y.D.; Nam, H.; Lee, K.-H.; et al. Characteristics of fractionated drop-in liquid fuel of plastic wastes from a commercial pyrolysis plant. Waste Manag. 2021, 126, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Singh, R.K. Experimental optimization of process for the thermo-catalytic degradation of waste polypropylene to liquid fuel. Adv. Energy Eng. 2013, 1, 74–84. [Google Scholar]
- Kusenberg, M.; Zayaud, A.; Roosen, M.; Thi, H.D.; Abbas-Abadi, M.S.; Eschenbacher, A.; Kresovic, U.; De Meester, S. A comprehensive experimental investigation of plastic waste pyrolysis oil quality and its dependence on the plastic waste composition. Fuel Process. Technol. 2022, 227, 107090. [Google Scholar] [CrossRef]
- Colantonio, S.; Cafiero, L.; De Angelis, D.; Ippolito, N.M.; Tuffi, R.; Ciprioti, S.V. Thermal and catalytic pyrolysis of a synthetic mixture representative of packaging plastics residue. Front. Chem. Sci. Eng. 2020, 14, 288–303. [Google Scholar] [CrossRef]
- Kasar, P.; Sharma, D.K.; Ahmaruzzaman, M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Clean. Prod. 2020, 265, 121639. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Ding, W.B.; Liang, J.; Anderson, L.L. Thermal and catalytic degradation of high density polyethylene and commingled post-consumer plastic waste. Fuel Process. Technol. 1997, 51, 47–62. [Google Scholar] [CrossRef]
- Manos, G.; Garforth, A.; Dwyer, J. Catalytic degradation of high-density polyethylene over different zeolitic structures. Ind. Eng. Chem. Res. 2000, 39, 1198–1202. [Google Scholar] [CrossRef]
- Attique, S.; Batool, M.; Jalees, M.I.; Shehzad, K.; Farooq, U.; Khan, Z.; Ashraf, F.; Shah, A.T. Highly efficient catalytic degradation of low-density polyethylene using a novel tungstophosphoric acid/kaolin clay composite catalyst. Turk. J. Chem. 2018, 42, 684–693. [Google Scholar]
- Mitchell, M.M.; Hoffman, J.F.; Moore, H.F. Residual feed cracking catalysts. In Studies in Surface Science and Catalysis. Fluid Catalytic Cracking: Science and Technology; Magee, J.S., Mitchell, M.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 293–338. [Google Scholar]
- Paucar-Sanchez, M.F. Study of the Activity of the Circulating Catalyst in the Fluidized Catalytic Cracking Unit (FCC) of the Esmeraldas State Refinery (REE). Master’s Dissertation, University of San Francisco, Quito, Ecuador, 2011. (In Spanish). [Google Scholar]
- Li, J.H.; Wang, Y.N.; Jia, W.Z.; Xi, Z.W.; Chen, H.H.; Zhu, Z.R.; Hu, Z.G. Effect of external surface of HZSM-5 zeolite on product distribution in the conversion of methanol to hydrocarbons. J. Energy Chem. 2014, 23, 771–780. [Google Scholar] [CrossRef]
- Olivera, M.; Musso, M.; De Leon, A.; Volonterio, E.; Amaya, A.; Tancredi, N.; Bussi, J. Catalytic assessment of solid materials for the pyrolytic conversion of low-density polyethylene into fuels. Heliyon 2020, 6, e05080. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Liu, R.H.; Rahman, M.M.; Li, C.; Chai, M.Y.; Nishu, N.; He, Y.F. Impact of acid-modified ZSM-5 on hydrocarbon yield of catalytic co-pyrolysis of poplar wood sawdust and high-density polyethylene by Py-GC/MS analysis. J. Energy Inst. 2020, 93, 2435–2443. [Google Scholar] [CrossRef]
| Zeolite | Molecular Formula | Nominal Cation Form | Si/Al Molar Ratio | Brønsted Acitidy (μmol/g) | Lewis Acidity (μmol/g) | Total Acidity (μmol/g) | Commercia Name Zeolyst |
|---|---|---|---|---|---|---|---|
| Zeolite HY | - | Hydrogen | 2.6 | 272 | 104 | 995 | CBV 600 |
| Zeolite HUSY | H11.3Al11.3Si181O384 | Hydrogen | 15 | 160 | 318 | CBV 720 | |
| Zeolite Ammonium Beta (HBEA) | (NH4)3.33Al3.33Si61O128 | Ammonium | 12.5 | 448 | 208 | 1030 | CP 814E |
| Catalyst | HY CBV 600 | HUSY CBV 720 | HBEA CP 814E |
|---|---|---|---|
| Micropore volume (cm3/g) | 0.19 | 0.24 | 0.13 |
| Mesopore volume (cm3/g) | 0.94 | 0.22 | 0.42 |
| Total volume (cm3/g) | 1.13 | 0.46 | 0.56 |
| Micropore area (m2/g) | 474 | 589 | 324 |
| External surface area (m2/g) | 78 | 174 | 218 |
| BET surface area (m2/g) | 553 | 762 | 542 |
| Catalyst | Without Catalyst | HY CBV 600 | HUSY CBV 720 | Ammonium Beta CP 814E |
|---|---|---|---|---|
| C (wt%) | 83.2 | 83.8 | 84.5 | 83.4 |
| H (wt%) | 14.0 | 13.5 | 13.8 | 12.8 |
| N (wt%) | 0.28 | 0.44 | 0.18 | 0.22 |
| O (wt%) | 2.61 | 2.24 | 1.55 | 3.58 |
| H/C (molar ratio) | 2.01 | 1.93 | 1.96 | 1.84 |
| O/C (molar ratio) | 0.024 | 0.020 | 0.014 | 0.032 |
| Density (g/cm3) | 0.823 | 0.816 | 0.825 | 0.817 |
| API gravity | 40.2 | 41.8 | 39.8 | 41.6 |
| Viscosity (cSt *) | 1352 | 137 | 209 | 89 |
| HHV (MJ/kg) | 47.6 | 47.2 | 48.0 | 45.8 |
| pH * | 5.9 | 6.0 | 6.0 | 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quesada, L.; Calero, M.; Martín-Lara, M.Á.; Pérez, A.; Paucar-Sánchez, M.F.; Blázquez, G. Characterization of the Different Oils Obtained through the Catalytic In Situ Pyrolysis of Polyethylene Film from Municipal Solid Waste. Appl. Sci. 2022, 12, 4043. https://doi.org/10.3390/app12084043
Quesada L, Calero M, Martín-Lara MÁ, Pérez A, Paucar-Sánchez MF, Blázquez G. Characterization of the Different Oils Obtained through the Catalytic In Situ Pyrolysis of Polyethylene Film from Municipal Solid Waste. Applied Sciences. 2022; 12(8):4043. https://doi.org/10.3390/app12084043
Chicago/Turabian StyleQuesada, Lucía, Mónica Calero, María Ángeles Martín-Lara, Antonio Pérez, Marco F. Paucar-Sánchez, and Gabriel Blázquez. 2022. "Characterization of the Different Oils Obtained through the Catalytic In Situ Pyrolysis of Polyethylene Film from Municipal Solid Waste" Applied Sciences 12, no. 8: 4043. https://doi.org/10.3390/app12084043
APA StyleQuesada, L., Calero, M., Martín-Lara, M. Á., Pérez, A., Paucar-Sánchez, M. F., & Blázquez, G. (2022). Characterization of the Different Oils Obtained through the Catalytic In Situ Pyrolysis of Polyethylene Film from Municipal Solid Waste. Applied Sciences, 12(8), 4043. https://doi.org/10.3390/app12084043










