Production of Docosahexaenoic Acid and Odd-Chain Fatty Acids by Microalgae Schizochytrium limacinum Grown on Waste-Derived Volatile Fatty Acids
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microorganism
2.2. Shake Flask Culture Conditions
2.3. Fed Batch Culture Conditions
2.4. Analytical Methods
2.4.1. Harvesting and Cell Dry Weight Determination
2.4.2. VFA Determination
2.4.3. Lipids and Fatty Acid Profile
3. Results and Discussion
3.1. Schizochytrium Limacinum Growth on Synthetic VFAs and Resulting Fatty Acid Profile
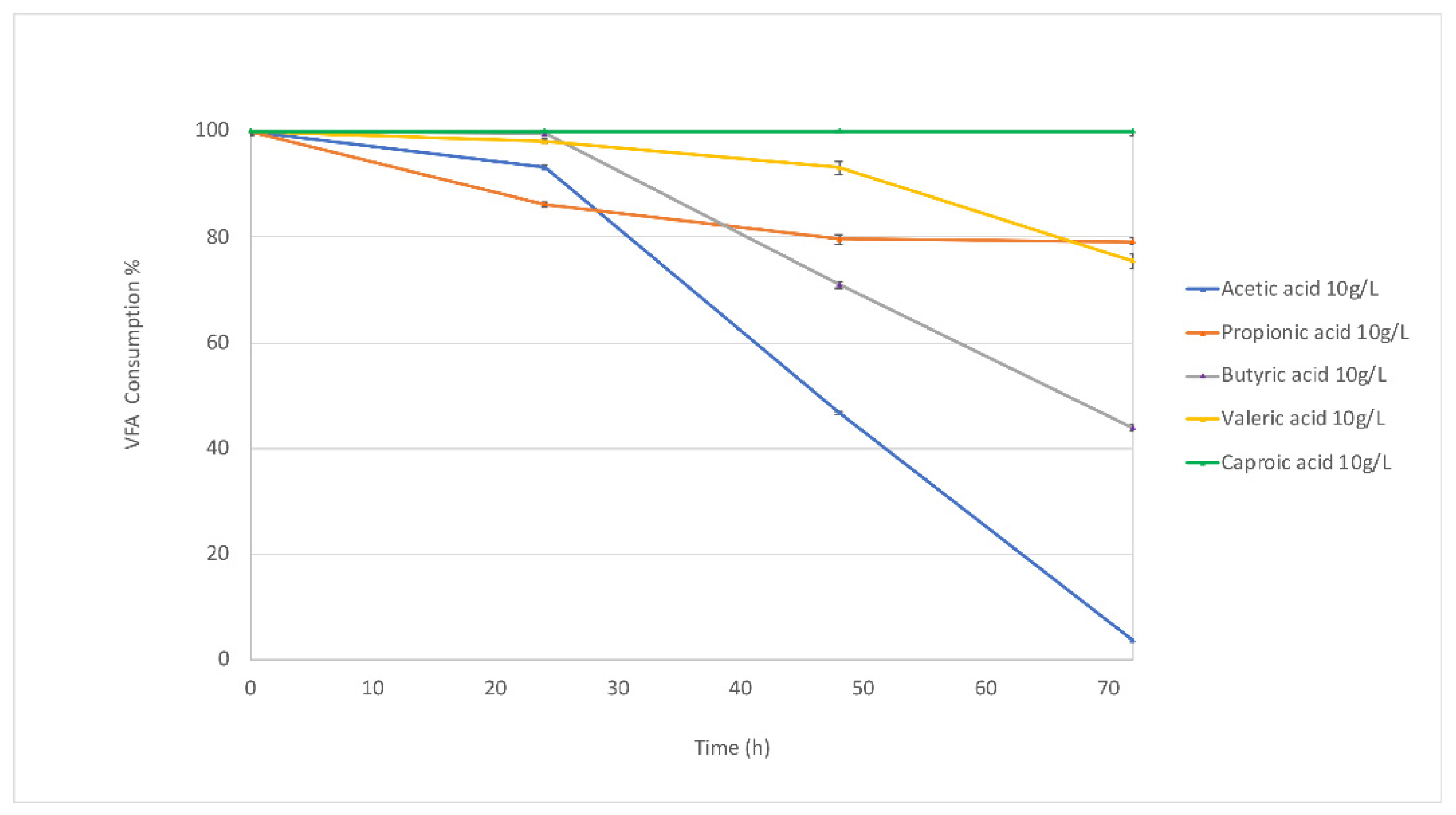
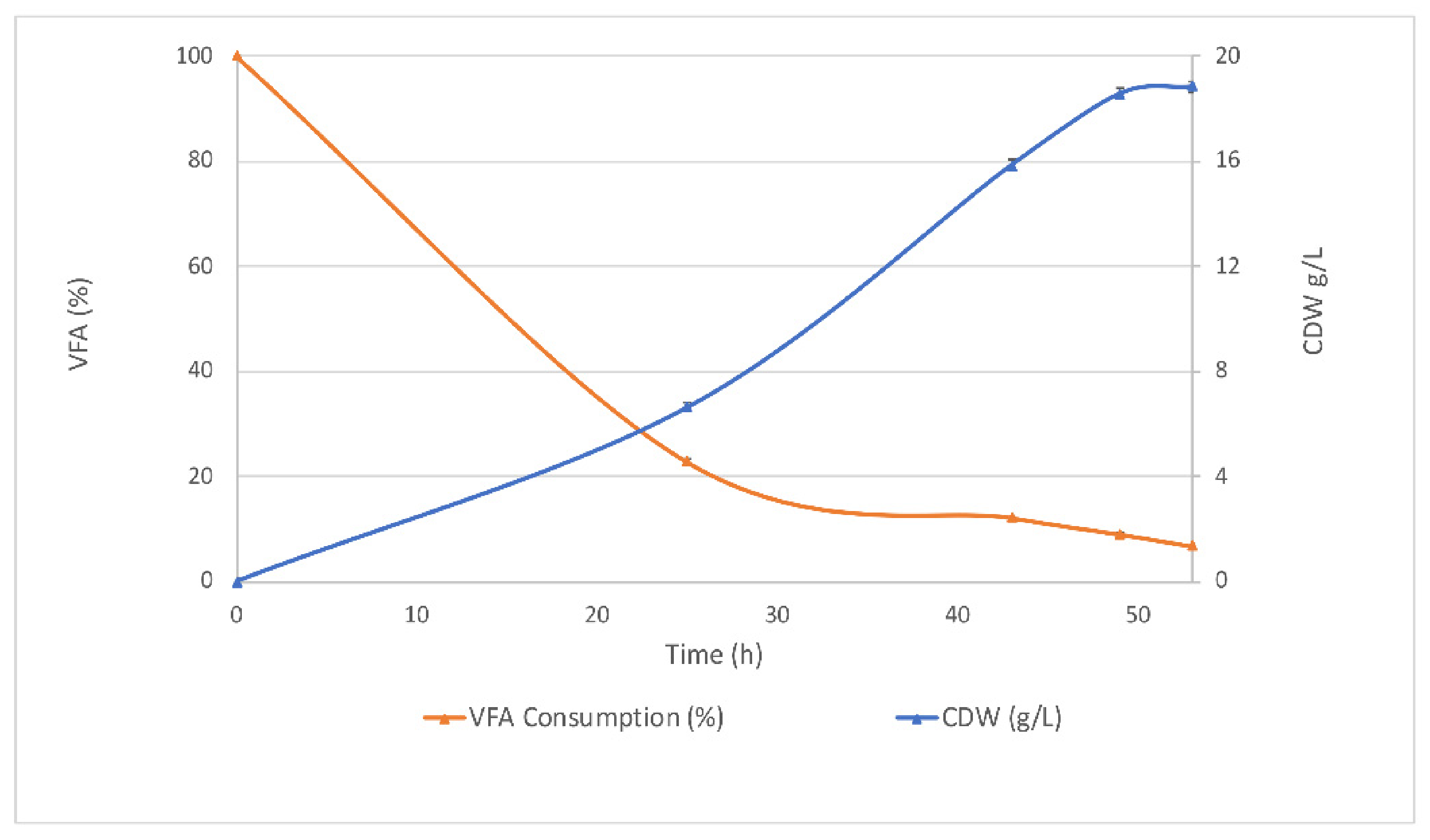
3.1.1. Biomass Production and VFA Consumption in Batch Fermentations
3.1.2. Effect on Fatty Acid Production
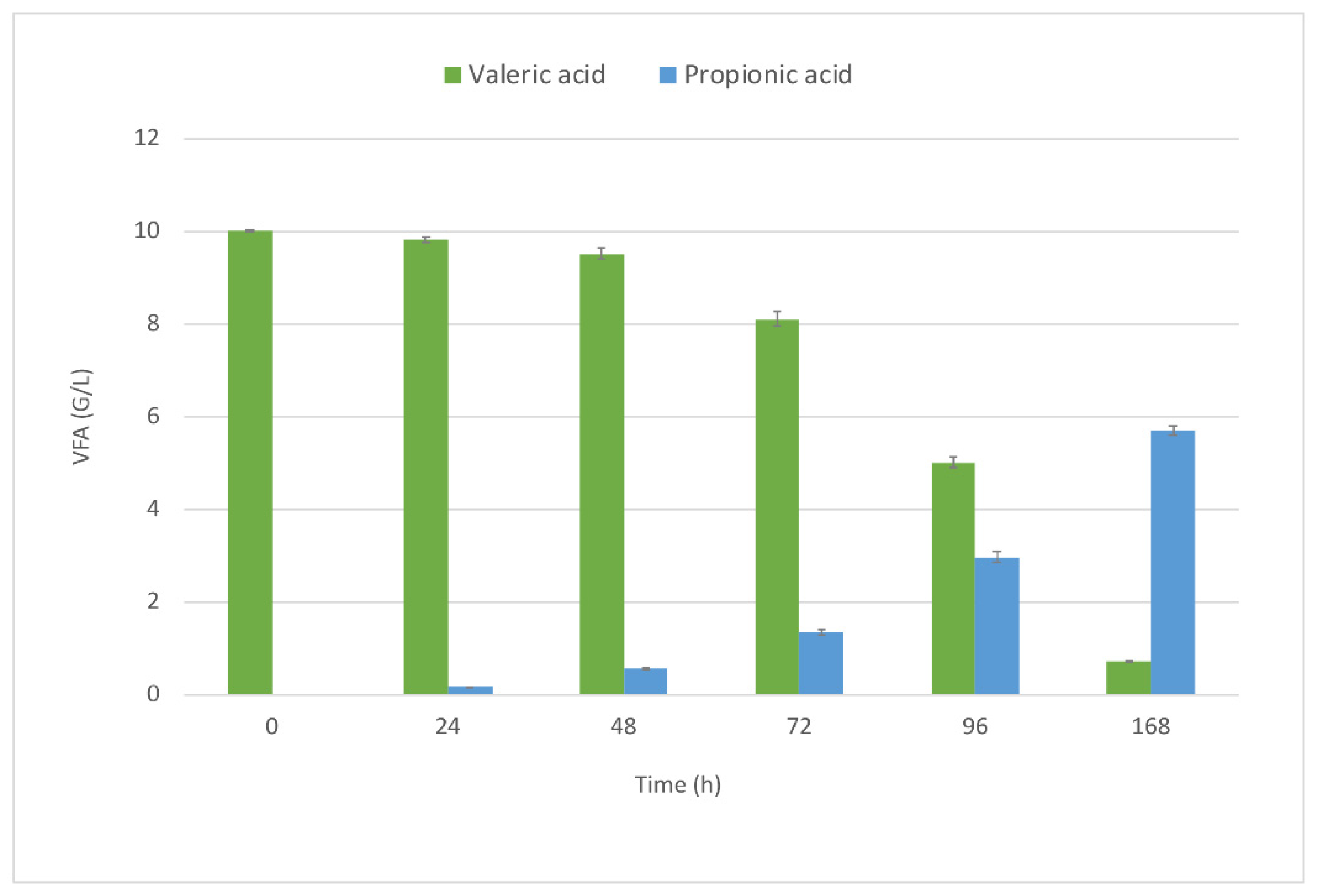
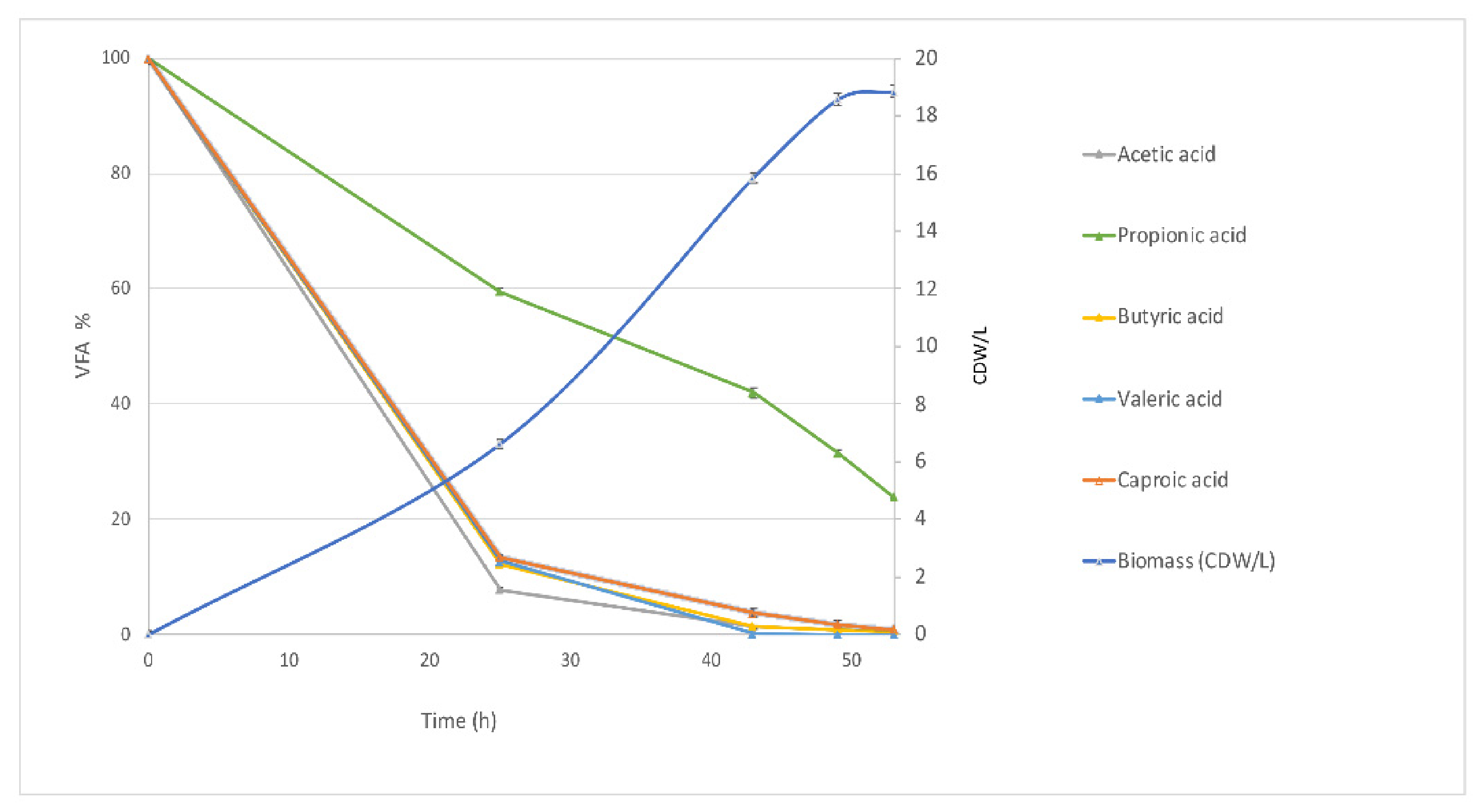
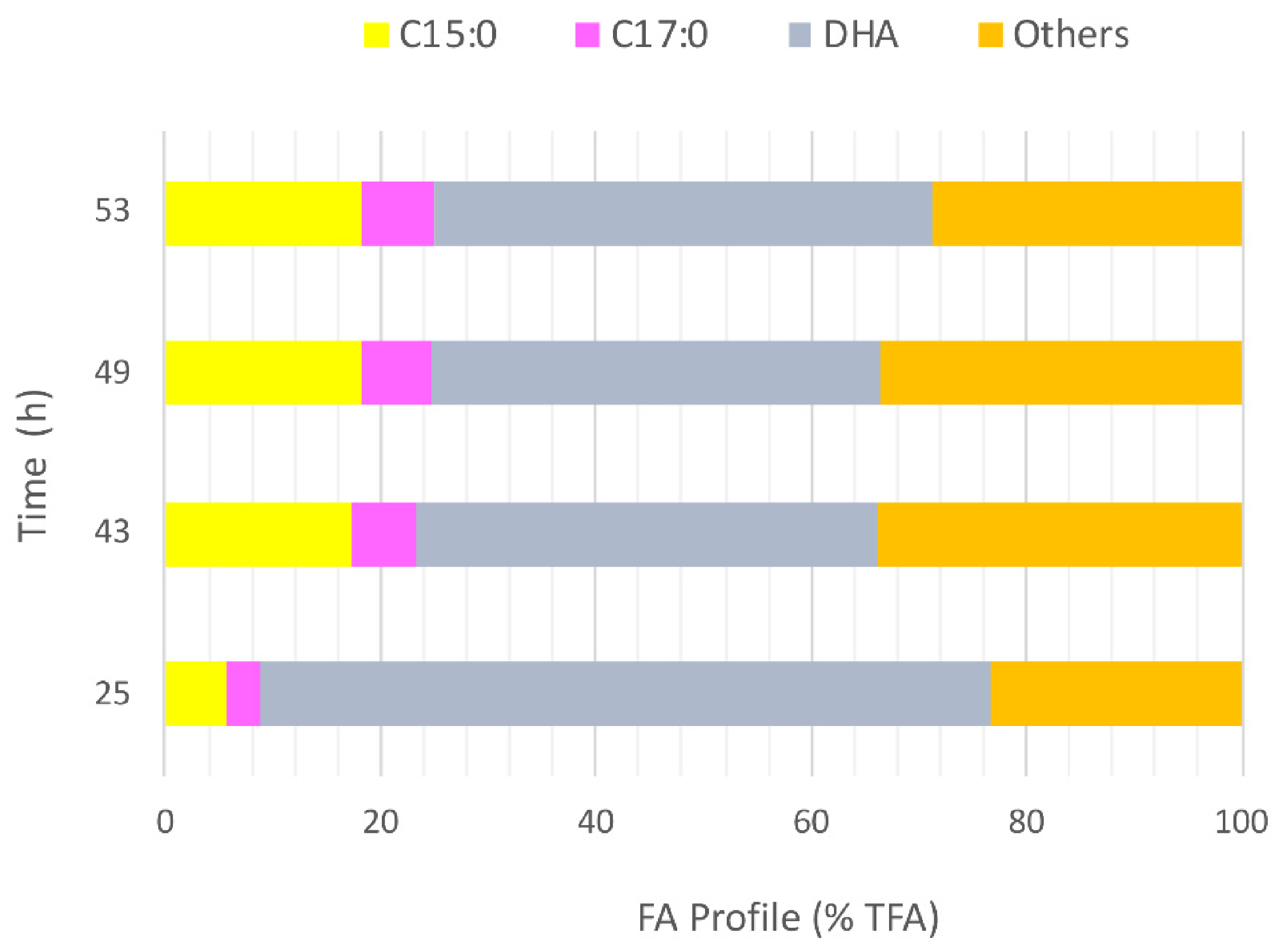
3.2. Waste-Derived VFA for DHA and OCFA Production in Fed-Batch Fermentations
Biomass Production and VFA Consumption Pattern
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a potential natural functional food source—A review. Pol. J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Borowitzka, M. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- Finco, A.M.; Mamani, L.D.G.; de Carvalho, J.; Pereira, G.; Soccol, V.T.; Soccol, C.R. Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit. Rev. Biotechnol. 2017, 37, 656–671. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua- and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; Macías-Sánchez, M.D.; Cerón-García, M.C.; Alarcón, F.J.; Molina-Grima, E. Microalgae as a potential ingredient for partial fish meal replacement in aquafeeds: Nutrient stability under different storage conditions. J. Appl. Phycol. 2018, 30, 1049–1059. [Google Scholar] [CrossRef]
- Byreddy, A.R. Thraustochytrids as an alternative source of omega-3 fatty acids, carotenoids and enzymes. Lipid Technol. 2016, 28, 68–70. [Google Scholar] [CrossRef]
- Chang, K.J.L.; Mansour, M.P.; Dunstan, G.A.; Blackburn, S.I.; Koutoulis, A.; Nichols, P.D. Odd-chain polyunsaturated fatty acids in thraustochytrids. Phytochemistry 2011, 72, 1460–1465. [Google Scholar] [CrossRef]
- Chalima, A.; Taxeidis, G.; Topakas, E. Optimization of the production of docosahexaenoic fatty acid by the heterotrophic microalga Crypthecodinium cohnii utilizing a dark fermentation effluent. Renew. Energy 2020, 152, 102–109. [Google Scholar] [CrossRef]
- Prokop, A.; Bajpai, R.K.; Zappi, M.E. (Eds.) Algal Biorefineries; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20199-3. [Google Scholar]
- Chen, J.; Li, Q.; Chang, C.; Bai, J.; Liu, L.; Fang, S. Techno-economic analysis of biodiesel production from microalgae: A review. Trends Renew. Energy 2017, 3, 141–152. [Google Scholar] [CrossRef][Green Version]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Patel, A.; Liefeldt, S.; Rova, U.; Christakopoulos, P.; Matsakas, L. Co-production of DHA and squalene by thraustochytrid from forest biomass. Sci. Rep. 2020, 10, 1992. [Google Scholar] [CrossRef]
- Espinosa-Gonzalez, I.; Parashar, A.; Bressler, D.C. Heterotrophic growth and lipid accumulation of Chlorella protothecoides in whey permeate, a dairy by-product stream, for biofuel production. Bioresour. Technol. 2014, 155, 170–176. [Google Scholar] [CrossRef]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Hu, H.-Y.; Yu, Y.; Zhang, T.-Y.; Zhu, S.-F.; Zhuang, L.-L.; Zhang, X.; Lu, Y. Microalgal species for sustainable biomass/lipid production using wastewater as resource: A review. Renew. Sustain. Energy Rev. 2014, 33, 675–688. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Su, C.-H.; Yu, Y.-K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crop. Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Chalima, A.; Oliver, L.; De Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Assessment of fatty acids profile and omega-3 polyunsaturated fatty acid production by the oleaginous marine thraustochytrid Aurantiochytrium sp. T66 cultivated on volatile fatty acids. Biomolecules 2020, 10, 694. [Google Scholar] [CrossRef]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From sewage sludge and agri-food waste to VFA: Individual acid production potential and up-scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Christophe, G.; Fontanille, P.; Larroche, C. Biological upgrading of volatile fatty acids, key intermediates for the valorization of biowaste through dark anaerobic fermentation. Bioresour. Technol. 2013, 145, 166–174. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fayet, A.; Fouilland, E.; Steyer, J.-P. Raw dark fermentation effluent to support heterotrophic microalgae growth: Microalgae successfully outcompete bacteria for acetate. Algal Res. 2015, 12, 119–125. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.-P. Growth of Chlorella sorokiniana on a mixture of volatile fatty acids: The effects of light and temperature. Bioresour. Technol. 2015, 198, 852–860. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.-P. Potentialities of dark fermentation effluents as substrates for microalgae growth: A review. Process Biochem. 2016, 51, 1843–1854. [Google Scholar] [CrossRef]
- Kurotani, K.; Sato, M.; Yasuda, K.; Kashima, K.; Tanaka, S.; Hayashi, T.; Shirouchi, B.; Akter, S.; Kashino, I.; Hayabuchi, H.; et al. Even- and odd-chain saturated fatty acids in serum phospholipids are differentially associated with adipokines. PLoS ONE 2017, 12, e0178192. [Google Scholar] [CrossRef]
- Dornan, K.; Gunenc, A.; Oomah, B.D.; Hosseinian, F. Odd chain fatty acids and odd chain phenolic lipids (alkylresorcinols) are essential for diet. J. Am. Oil Chem. Soc. 2021, 98, 813–824. [Google Scholar] [CrossRef]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Sbaï, D.; Narcy, C.; Thompson, G.N.; Mariotti, A.; Poggi, F.; Saudubray, J.M.; Bresson, J.L. Contribution of odd-chain fatty acid oxidation to propionate production in disorders of propionate metabolism. Am. J. Clin. Nutr. 1994, 59, 1332–1337. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Rainger, G.E. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Holman, R.T.; Johnson, S.B.; Kokmen, E. Deficiencies of polyunsaturated fatty acids and replacement by nonessential fatty acids in plasma lipids in multiple sclerosis. Proc. Natl. Acad. Sci. USA 1989, 86, 4720–4724. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K. The role of selected bioactive compounds in the prevention of Alzheimer’s disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef]
- Mika, A.; Stepnowski, P.; Kaska, L.; Proczko, M.; Wisniewski, P.; Sledzinski, M.; Sledzinski, T. A comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight. Obesity 2016, 24, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1141-52; Standard Practice for Preparation of Substitute Ocean Water. Lake Products Company LLC: Florissant, MO, USA, 2021.
- Velghe, F.; De Wilde, F.; Snellinx, S.; Farahbakhsh, S.; Belderbos, E.; Peral, C.; Wiedemann, A.; Hiessl, S.; Michels, J.; Pierrard, M.-A.; et al. Volatile fatty acid platform—A cornerstone for the circular bioeconomy. FEMS Microbiol. Lett. 2021, 368, fnab056. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil Accumulation via Heterotrophic/Mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef]
- Wallace, J.S. Chemical Analysis of Firearms, Ammunition, and Gunshot Residue; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Wang, F.; Bi, Y.; Diao, J.; Lv, M.; Cui, J.; Chen, L.; Zhang, W. Metabolic engineering to enhance biosynthesis of both docosahexaenoic acid and odd-chain fatty acids in Schizochytrium sp. S31. Biotechnol. Biofuels 2019, 12, 141. [Google Scholar] [CrossRef]
- Fei, Q.; Fu, R.; Shang, L.; Brigham, C.J.; Chang, H.N. Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst. Eng. 2015, 38, 691–700. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]

| Parameters | Acetic Acid | Propionic Acid | Butyric Acid | Valeric Acid | Caproic Acid |
|---|---|---|---|---|---|
| Cell dry weight (g/L) | 3.2 ± 0.05 | 0.70 ± 0.03 | 3.0 ± 0.05 | 1.2 ± 0.03 | -- |
| Biomass productivity (g/L/h) | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | -- |
| Biomass yield (g·g−1substrate) | 0.32 ± 0.01 | 0.07 ± 0.01 | 0.30 ± 0.01 | 0.12 ± 0.01 | -- |
| Carbon Source | DCW (g/L) | Biomass Productivity (g/L/h) | Biomass Yield (g·g−1 Substrate) | Lipids (%) | DHA (%TFA) | C15:0 (%TFA) | C17:0 (%TFA) |
|---|---|---|---|---|---|---|---|
| Synthetic VFA | 12.3 ± 0.1 | 0.23 ± 0.1 | 1.23 ± 0.01 | 45.9 ± 1.0 | 18.3 ± 0.9 | 14.3 ± 0.7 | 4.9 ± 0.6 |
| Carbon Source | DCW (g/L) | Biomass Productivity (g/L/h) | Biomass Yield (g·g−1 Substrate) | Lipids (%) | DHA (% TFA) | C15:0 (% TFA) | C17:0 (% TFA) |
|---|---|---|---|---|---|---|---|
| Waste-derived VFA | 18.5 ± 0.1 | 0.35 ± 0.1 | 1.85 ± 0.01 | 54.0 ± 1.2 | 46.3 ± 0.8 | 18.1 ± 0.7 | 6.9 ± 0.7 |
| Parameters | VFA Synthetic Media 2L | Waste-Derived VFA Media 2L |
|---|---|---|
| Cell dry weight (g/L) | 12.3 ± 0.1 | 18.5 ± 0.1 |
| Biomass yield (g·g−1 substrate) | 1.23 ± 0.01 | 1.89 ± 0.01 |
| Lipid content (%) | 45.9 ± 1.0 | 54.0 ± 1.2 |
| C14:0 (%) | 2.2 ± 0.1 | 1,4 ± 0.1 |
| C15:0 (%) | 14.3 ± 0.7 | 18.1 ± 0.7 |
| C16:0 (%) | 36.9 ± 0.3 | 23.3 ± 0.3 |
| C17:0 (%) | 4.9 ± 0.6 | 6.9 ± 0.6 |
| C18:0 (%) | 6.8 ± 0.1 | 1.4 ± 0.1 |
| C18:3 (%) | 2.7 ± 0.1 | 0.3 ± 0.05 |
| EPA (%) | 1.2 ± 0.3 | 1.5 ± 0.3 |
| DPA (%) | 2.5 ± 0.3 | 2.2 ± 0.3 |
| DHA (%) | 18.3 ± 0.9 | 46.3 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver, L.; Fernández-de-Castro, L.; Dietrich, T.; Villaran, M.C.; Barrio, R.J. Production of Docosahexaenoic Acid and Odd-Chain Fatty Acids by Microalgae Schizochytrium limacinum Grown on Waste-Derived Volatile Fatty Acids. Appl. Sci. 2022, 12, 3976. https://doi.org/10.3390/app12083976
Oliver L, Fernández-de-Castro L, Dietrich T, Villaran MC, Barrio RJ. Production of Docosahexaenoic Acid and Odd-Chain Fatty Acids by Microalgae Schizochytrium limacinum Grown on Waste-Derived Volatile Fatty Acids. Applied Sciences. 2022; 12(8):3976. https://doi.org/10.3390/app12083976
Chicago/Turabian StyleOliver, Laura, Laura Fernández-de-Castro, Thomas Dietrich, Maria Carmen Villaran, and Ramón J. Barrio. 2022. "Production of Docosahexaenoic Acid and Odd-Chain Fatty Acids by Microalgae Schizochytrium limacinum Grown on Waste-Derived Volatile Fatty Acids" Applied Sciences 12, no. 8: 3976. https://doi.org/10.3390/app12083976
APA StyleOliver, L., Fernández-de-Castro, L., Dietrich, T., Villaran, M. C., & Barrio, R. J. (2022). Production of Docosahexaenoic Acid and Odd-Chain Fatty Acids by Microalgae Schizochytrium limacinum Grown on Waste-Derived Volatile Fatty Acids. Applied Sciences, 12(8), 3976. https://doi.org/10.3390/app12083976






