1. Introduction
The use of mineral nitrogen (N) fertilizers in the European Union (EU) agricultural sector remains high, with 10.2 million tons of N consumed in 2018, corresponding to an increase of 1.9% since 2008 [
1]. Out of the 174 million tons of ammonia (NH
3) synthesized globally on an annual basis [
2], more than 85% is used in fertilizers [
3]. About 2% of the world’s energy consumption can be accounted for the synthesis of NH
3, based on the Haber-Bosch process [
4], which is also responsible for about 2.5% of the global fossil-fuel- based carbon dioxide emissions [
3].
Emphasis on reducing fossil-fuel dependency and mining of limited natural resources has driven an enhanced attention towards nutrient recovery from biomass streams such as animal manure, household and food waste, and sewage sludge [
5,
6,
7,
8,
9,
10]. The concept of a circular economy deeply highlights the significance of nutrient recovery, with an aim to prevent detrimental environmental effects such as soil salinity, eutrophication of water bodies, accumulation of nitrates in groundwater, heavy metal accumulation in soil, and release of greenhouse gases [
11,
12]. Commonly, the processing of the above-mentioned biomass streams often commence with anaerobic digestion (AD), followed by myriad nutrient recovery technologies (NRTs), resulting in process-dependent end-products, referred to as recycling-derived fertilizers (RDFs) in the context of this research.
NRTs such as stripping and scrubbing are established in different industrial settings, with its implementation dating back to the 1970s in wastewater treatment [
13]. NH
3 stripping from the liquid fraction (LF) of digestate is a process involving the transfer of aqueous ammonium (NH
4+) to the gaseous NH
3 phase, achieved mainly by the adjustment of pH and temperature, among other factors such as air-to-liquid ratio, hydraulic loading rate, etc. [
14]. Studies have shown an N separation of 17–33% from the LF of digestate utilizing the stripping-scrubbing technology [
15,
16]. The end-product of the technology can be ammonium nitrate (AN) or ammonium sulphate, depending on the acid used in the scrubber. Likewise, evaporation can be used to up-concentrate nutrients in the LF of digestate, obtaining an N-rich condensate (ammonium water (AW)), and an NK-rich concentrate (concentrate after evaporation (CaE)). Similar to the principle of stripping-scrubbing, the process of evaporation functions by phase shift of the water-soluble NH
4+ towards the gaseous NH
3, which is then recovered by condensation as AW. Volatilization of NH
3 can be prevented by pH adjustment in the evaporator. Since AW is obtained after evaporation followed by condensation, the RDF is devoid of any free suspended particles, metals, and pathogens. Moreover, since other elements such as phosphorus (P), potassium (K), etc., are not volatilized, AW is a pure N-rich end-product. The current use of AW is limited to industrial applications; however, due to its N-rich characteristic, a valorization pathway in agriculture might be possible.
Being in the developmental stages, there is a scarcity of research on the effects of the above-mentioned RDFs on agronomic performance and soil quality. Previously, these recovered products also lacked legal compliance for use in the market. In tandem with the Circular Economy Action Plan, RDFs from animal manure are now receiving an opportunity to replace synthetically manufactured nutrients, provided that these RDFs have a similar or better overall environmental performance relative to the synthetic fertilizers they aim to replace [
17]. Concurrently, EU’s Fertilizing Products Regulation (FPR, 2019/1009) aims to place secondary raw materials and enable recycled fertilizers (e.g., RDFs from digestate processing) access to the EU internal market, so they can compete on the same pedestal as synthetic mineral fertilizers. Both these legislative amendments could give RDFs such as AN and AW, respectively, the much necessary nudge towards influencing an increased market-uptake of these products, provided they exhibit comparable agricultural and environmental performance to their synthetic counterparts. In comparison to raw or primarily-separated biomass, RDFs comprise an up-concentrated nutrient content, i.e., their nutrient availability and content is enhanced, thus improving and increasing their agricultural value [
18,
19,
20,
21,
22]. A study in the past showed that the certainty of nutrient content of RDFs is a crucial parameter that led to generating the acceptance of these novel products among the farming community [
23]. RDFs, in general, are known for their highly variable nutrient composition [
22,
24,
25], making it difficult to apply their correct dosages during fertilization. Unlike the uncertainty of nutrient content of RDFs, synthetic fertilizer composition is known to the farmers [
23], which could influence the farmers’ choice and give an upper hand to synthetic fertilizers. Along with uncertain nutrient composition, RDFs obtained from different NRTs might also comprise only a particular nutrient (e.g., AN and AW that contains only N), or contain a mixture of nutrients (e.g., CaE containing N and K), but in insufficient nutrient ratios. An optimal ratio of macronutrients (N, P, and K) is desirable for fertilization, which can be generated by RDF blending. Tailor-made RDF blends might be of interest to achieve desired nutrient compositions, thus reducing the reliance on synthetic fertilizers.
Therefore, this study aimed to determine the potential use of novel RDFs such as AN, AW, CaE, and two tailor-made blends (blend 1: AN + CaE and blend 2: AW + CaE) as substitutes to the synthetic mineral N fertilizer calcium ammonium nitrate (CAN). All the tested RDFs were recovered via NRTs after processing the LF of digestate obtained from AD of different types of biomass such as animal manure/food and other organic wastes. An extensive characterization of the RDFs was performed, and their performance was compared against the synthetic N fertilizer in two experimental set-ups with the aim to:
(i) assess the RDF N dynamics in a soil incubation experiment;
(ii) evaluate the crop performance and N fertilizer replacement value (NFRV) of the RDFs in a plant experiment with lettuce.
2. Materials and Methods
2.1. RDF Sampling, Characterization and Blend Formulation
The AN was obtained from the stripping/scrubbing unit of a pig farm with an AD plant located in Gistel, Belgium. The farm has a capacity of 11,000 fattening pigs with a manure treatment capacity of 60,000 tons y−1. The input for AD treatment included different types of animal manure (65% pig manure, 17% solid fraction (SF) of pig manure, and 9% horse manure) and food waste (9%). The resultant digestate was separated into SF and LF by centrifugation, after which the LF was subjected to NH3 stripping and scrubbing, recovering N in the form of AN. The AW and CaE, both, were collected from an AD plant in Belgium that treats a variety of organic wastes such as wastewater sludge from the agro-food industry, supermarket waste, waste from the biodiesel industry, rejected food products, animal by-products from slaughterhouses, etc. As a technique to recover nutrients from the processed biomass, the company employs the process of evaporation of the LF of digestate that was initially separated by the centrifugation process. Evaporation resulted in two end-products: NH4+ condensate and CaE. The condensate of evaporation passed through an NH3 stripper resulted in AW, whereas the CaE was the residue after evaporation. All the RDFs were collected in air-tight polyethylene sampling bottles of 1 L each. AN and CaE were stored in the refrigerator at a temperature of 4 °C, whereas AW was stored under the fume-hood of the laboratory where the average temperature was between 15–20 °C, according to the instructions from the producer.
The blends formed for this study were formulated to fit the nutrient recommendations of the test crop, lettuce (Lactuca sativa L., cv. Cosmopolia) (200 kg N ha−1, 125 kg P2O5 ha−1 and 240 kg K2O ha−1; personal communication with Inagro vzw). CaE was chosen for blend preparation after analyzing a total of 20 different RDFs (such as digestate, ashes, struvites, composts, etc.). The choice was made based on the consideration that CaE brought about the least amount of P (since Flemish soils are already P-rich), simultaneously fulfilling the K requirements needed as per the nutrient recommendation for lettuce growth. CaE also comprised of negligible heavy metal content in comparison to the other tested RDFs. The blends were assessed by adding them individually into the pots and soil-incubation tubes in the ratio 1:1, rather than actual physical blending. For future research perspectives, it is recommended to produce blends by physically mixing the RDFs to ascertain any potential reactions such as precipitation, foaming, change in pH, etc.
2.1.1. Physicochemical Characterization of RDFs
The determination of dry matter (DM) content of RDFs was conducted by drying them at 105 °C to a constant weight for 48 h and calculating the DM as a percentage of its wet weight. Organic matter (OM) was analyzed only for CaE, since AN and AW are mineral N products. This was conducted by incineration of the oven-dried (at 105 °C) CaE at 550 °C in the muffle furnace (Nabertherm, Lilienthal, Lower Saxony, Germany) for 4 h, and the subsequent loss of mass on ignition was considered as the result. Total and inorganic carbon (C) (also, only for CaE) were analyzed using a CN analyzer (Primacs100, Skalar, The Netherlands) and organic C (OC) was calculated after deducting the inorganic C from the total C. For pH (pH- potassium chloride (KCl), a suspension of CaE was prepared by adding 25 mL of 1 M KCl to 10 g of fresh sample (stirred well and equilibrated for 10 min), after which the pH was measured using a pH meter (Orion Star A211, Thermo Fisher Scientific, Waltham, MA, USA). For the electrical conductivity (EC) analysis of CaE, a water extract was prepared by mixing 10 g of fresh sample in 50 mL of de-mineralized water and shaking the solution for 60 min. The solution was filtered using a Whatman filter paper of pore size 125 mm, and the extracts were analyzed on an EC meter (WTW Tetra Con 96, Xylem Analytics, Weilheim in Oberbayern, Bavaria, Germany). The pH and EC of AN and AW were measured directly using the respective meters. The total N of AW and CaE was determined by Kjeldahl digestion of samples (Gerhardt Vapodest, Königswinter, North Rhine-Westphalia, Germany) followed by distillation using a Kjeltec-1002 distilling unit (Kjeltec, FOSS, Hillerød, Capital (Hovedstaden), Denmark) whereas the total N of AN was determined as a sum of NH4+-N and nitrate-N (NO3−-N). The NH4+-N and NO3−-N were determined by analyzing extracts prepared in 1M KCl using a continuous flow auto-analyzer (Chemlab System 4, Skalar, Breda, North Brabant, The Netherlands). Since AW and CaE do not contain NO3−-N, organic N was calculated as the difference between total and NH4+-N. For AN, the sum of NH4+-N and NO3−-N equaled to total N, as AN is 100% a mineral RDF. Total elemental analysis of the RDFs was performed by digesting the products (CaE = 0.3 to 0.4 g; AN and AW = 3 to 4 mL; in 10 mL nitric acid ((HNO3) (65%)) in a closed microwave (CEM MARS 5, Drogenbos, Flemish Brabant, Belgium) (total 60 min; 20 min each spent in three stages: ramping the temperature to 180 °C, holding at 180 °C, and cooling down of the tubes) and analyzing them on the Inductively coupled plasma-Optical emission spectroscopy (ICP-OES) (Vista MPX, Varian, Inc., Palo Alto, CA, USA) to determine other macro- and micronutrients.
2.1.2. Microbiological Characterization of RDFs and the Presence of Pathogens
Microbiological tests were performed on RDFs to investigate their associated microbial loads and the presence of potential pathogens that can give rise to health and safety concerns, particularly Salmonella spp., Escherichia coli, Listeria spp., and Campylobacter spp. The tested RDFs were sent to the Institute of Technology Carlow, Ireland, for analysis of active biological agents. RDFs were stored at 4 °C in air-tight containers before testing. Tests were performed on single batches. Concentrations of mesophilic microorganisms present in the RDFs were estimated by performing aerobic plate counts using the pour-plate technique. RDFs serially diluted in sterile ¼ Strength Ringers solution (Oxoid™) were used to inoculate Plate Count Agar (Neogen®) supplemented with 10 µg mL−1 cycloheximide (ACROS Organics™) and Malt Extract Agar (Neogen®) supplemented with 50 µg mL−1 kanamycin sulphate (Fisher BioReagents™) in triplicate for bacterial and fungal counts, respectively. All agars and diluents were autoclaved at 121 °C, 15 psi to achieve sterilization. Bacterial colony forming units (CFUs) were observed after incubation at 37 °C for 48 h. Fungal CFUs were observed after five days of incubation at 30 °C. Sterile water was used as the control.
RDFs were outsourced to another external accredited laboratory (Agrihealth Laboratory Services, Co., Monaghan, Ireland) to investigate the presence of
Salmonella spp.,
Escherichia coli,
Listeria spp., and
Campylobacter spp. The detection of
Salmonella spp. per 25 g was performed by the selective enrichment technique in a modified semi-solid Rappaport-Vassiliadis agar [
26].
Listeria spp. per 25 g of sample was detected by means of an enzyme-linked fluorescence assay using the VIDAS
® UP Listeria method [
27]. Colony count techniques were employed for the enumeration of
Campylobacter spp. [
28] and β-glucoronidase-positive
Escherichia coli [
29] per g mL
−1 of RDF sample.
2.2. Soil Sampling and Characterization
The soil used for the study was collected from the surface layer (0 to 30 cm) of an arable field in Wingene, Belgium. This part of Flanders has a predominantly sandy soil, and according to the Belgian soil map, the field’s soil profile is characterized as a Z.c.h. soil type (soil with sandy texture and a moderately poor drainage class with signs of rust deeper than 60 cm and a post-podzol B-horizon) [
30]. The field was part of a mixed cattle-extensive vegetable farm, and the crops mainly grown on this farm in 2017 and 2018 were
Zea mays (maize) and
Solanum tuberosum (early potatoes), respectively. The organic fertilizer mainly used was farmyard manure (with straw at a low N and P content). Italian ryegrass was the catch crop grown mostly in this field.
The soil was sampled before fertilizer application and sowing of the field for an impending trial, approximately 4 months before the start of the first experiment. After air-drying in the greenhouse, the soil was sieved using 2-mm sieves and mixed thoroughly before analyses. The air-dried soil was stored in the greenhouse in plastic bags until the start of the experiment. A sub-sample of the soil was taken, and the soil OC, pH-KCl, EC, and total N were measured. Soil OC was measured in two steps: first, the soil OM was measured using a muffle furnace (Nabertherm, Lilienthal, Lower Saxony, Germany) for 4 h at 550 °C, and secondly, the OC was obtained by dividing the calculated OM by a factor of two [
31]. pH-KCl determined the soil potential acidity, and it was measured using a pH meter (Orion Star A211, Thermo Fisher Scientific, Waltham, MA, USA) by adding 25 mL of 1 M KCl to 10 g of air-dried soil and letting it equilibrate for 10 min [
32]. For the EC analysis, water extracts of 10 g of air-dried soil were prepared using de-mineralized water (after shaking for 60 min), and these extracts were filtered using a Whatman filter paper (pore size 125 mm). An EC meter (WTW Tetra Con 96, Xylem Analytics, Weilheim in Oberbayern, Bavaria, Germany) was used to determine the conductivity of the extracts. Total N was determined by the Kjeldahl digestion of samples (Gerhardt Vapodest, Königswinter, North Rhine-Westphalia, Germany), followed by distillation using a Kjeltec-1002 distilling unit (Kjeltec, FOSS, Hillerød, Capital (Hovedstaden), Denmark), and total C by the CN analyzer (Primacs100, Skalar, Breda, North Brabant, The Netherlands). NH
4+-N and NO
3−-N [
33,
34] were extracted in 1 M KCl by mixing 10 g of soil in 50 mL of KCl. These extracts were prepared after 30 min on a rotary shaker, and the supernatant was filtered out using a Whatman filter paper of pore size 125 mm. It was then analyzed using a continuous flow auto-analyzer (Chemlab System 4, Skalar, Breda, North Brabant, The Netherlands). Total elemental analysis of the soil was performed, first by digesting the soil (1 g soil + 2.5 mL demineralized water) in a solution of aqua regia (2.5 mL HNO
3 (65%) + 7.5 mL hydrochloric acid (47%)) on a hot plate (150 °C at 100 W for 2 h), then by analyzing the samples for macro- and micronutrients using the ICP-OES (Varian Vista MPX, Palo Alto, CA, USA).
Table 1 presents the physicochemical characterization of the soil.
2.3. RDF Assessment in Comparison to Synthetic N Fertilizer
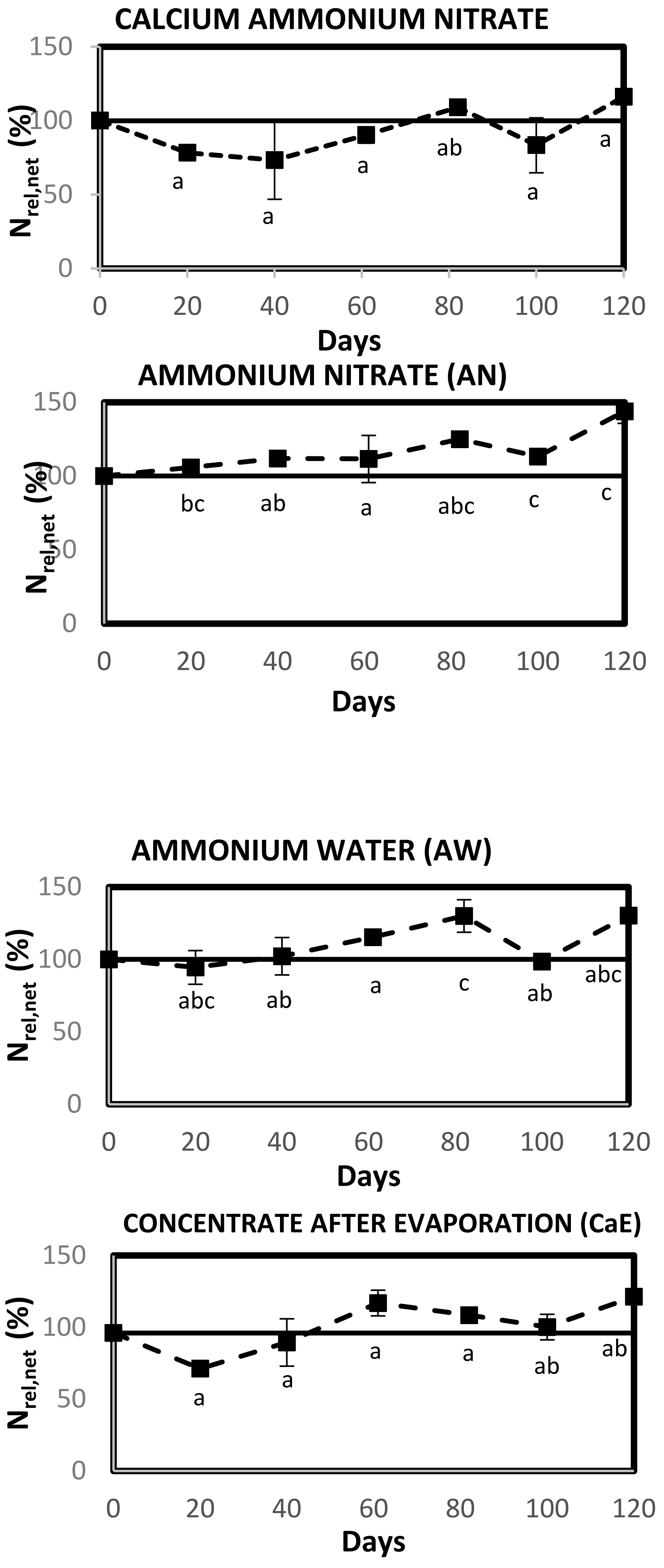
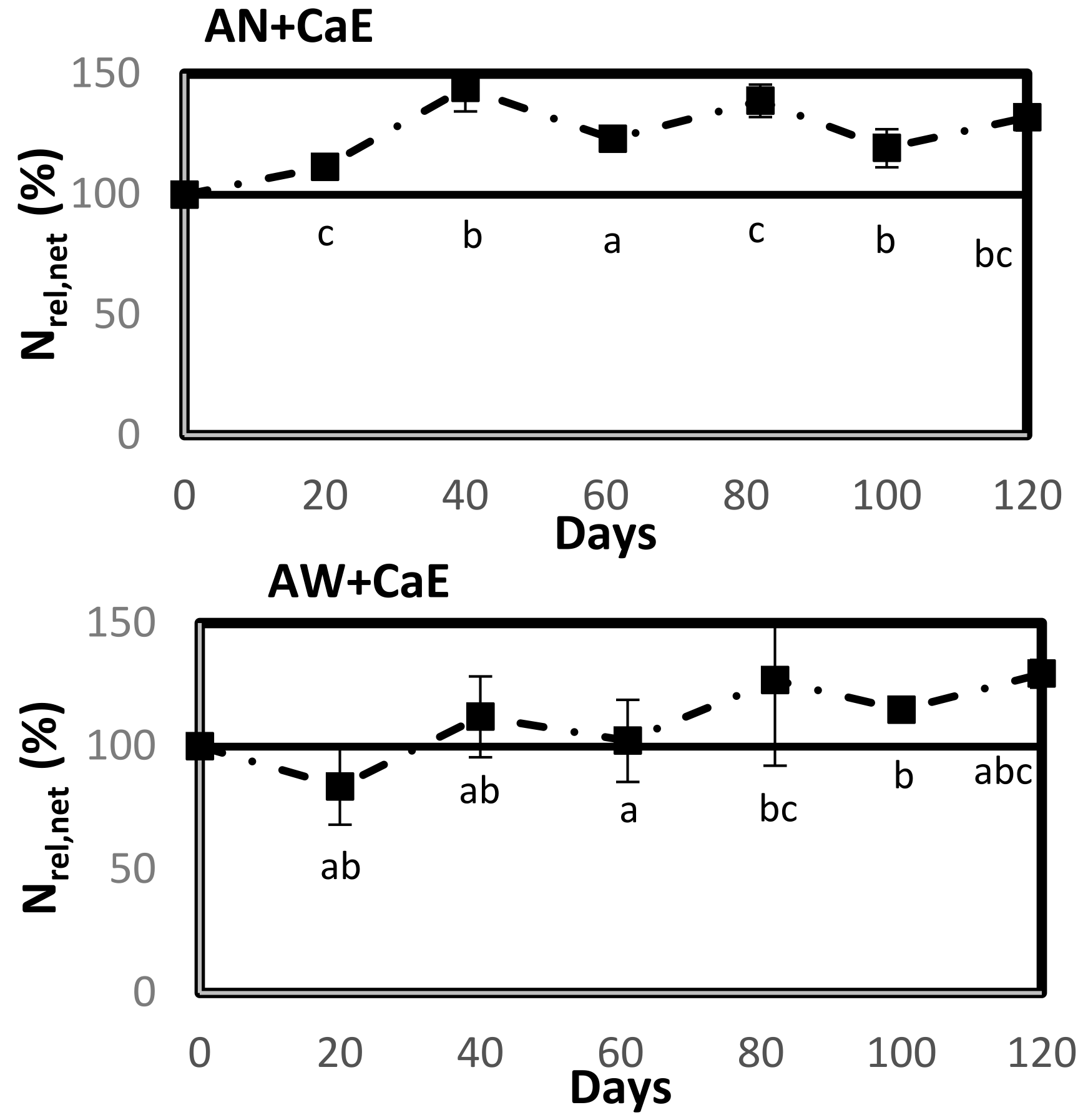
2.3.1. Experiment to Test Soil N Dynamics of RDFs
The RDFs were tested in a soil incubation experiment to determine their N release potential in comparison to the synthetic fertilizer CAN (N = 30%). The air-dried and sieved soil was pre-incubated at 35% water-filled pore space (WFPS). This pre-incubated soil was placed in the dark at 21 °C for one week after covering it with a pin-holed parafilm to prevent the loss of moisture by evaporation. All the products to be tested were mixed with 259 g of pre-incubated soil at a rate of 200 kg N ha
−1, which was the same dose of N applied to the soil in the pot cultivation of lettuce. For each treatment with an amendment, a homogenized mixture of soil and product was placed in polyvinyl chloride tubes of 18 cm length and 4.6 cm diameter. For the unamended control, plain soil was placed in the tubes. The soil in the tube was brought to a bulk density of 1.4 g cm
−3 by compacting it using a wooden cylindrical block to a height of 10 cm. The moisture content in the soil was adjusted to 50% WFPS to ensure that no denitrification occurred, since WFPS > 60% can cause denitrification [
35]. Additionally, the homogenous mixing of the product and soil and the lack of airflow at the soil surface during the incubation will ensure that no NH
3 is volatilized during the experimental duration [
35,
36]. The tubes were covered with a pin-holed gas-permeable parafilm to avoid the loss of moisture by evaporation. The treatments tested were: (i) control (unamended soil); (ii) CAN; (iii) AN; (iv) AW; (v) CaE; (vi) AN + CaE; (vii) AW + CaE. The treatments with the two tailor-made blends were tested by the addition of an individual RDF (ratio of 1:1) to form the blend in the soil, rather than the actual mixing of two products. Hence, tubes containing AN + CaE were considered as blend 1, and those containing AW + CaE were considered as blend 2. Since CAN is in granular form, it was first ground to a fine powder consistency for the ease of homogenization with soil. This powder was then dissolved in demineralized water to reach the desired concentration, and the solution was applied to the soil. In total, 126 tubes (seven treatments × three replicates × six sampling moments) were randomized for their treatments and incubated in the dark at an average temperature of 21 °C for a total experimental duration of 120 days. The moisture content was monitored every fortnight by weighing the tubes, and it was adjusted to 50% WFPS for the tubes where loss was observed. Three replicates of each treatment were sampled on days 20, 40, 61, 82, 100, and 120. The soil from the intact tubes was removed, mixed thoroughly, and 10 g from this mixture was taken to prepare KCl extracts to be analyzed for NH
4+-N and NO
3−-N (described in
Section 2.2). The mineral N in the unfertilized control at day zero was determined again to include the effects of drying and re-wetting of soil.
2.3.2. Experiment to Assess Crop Response of RDFs
A pot experiment with lettuce was performed to test the performance of the RDFs relative to the commercial synthetic fertilizer CAN. The experiment was conducted using seedlings of lettuce obtained from Inagro vzw. The seedlings were placed in the growth chamber of the laboratory for 9 days before the commencement of the experiment, under 8 h of artificial light (Brite-grow bio-growth light) of 2000 lux. The watering of seedlings was performed five days before the experiment, where approximately 5 L of water was added uniformly to the entire set of seedlings (conditions of seedlings maintained as per personal communication with Inagro). Soil and sand (river sand) were mixed in 1:1 ratio to be filled into the pots. The sand used in the experiment was rinsed with water thoroughly to eliminate any possible contaminants and air-dried before use. Each pot (height = 18 cm; top diameter = 12.6 cm) contained 1.65 kg of the soil-sand mixture. One day prior to setting up the experiment, 160 mL of demineralized water was added per 1 kg of air-dried soil-sand mixture, in order to ensure homogenous mixing of the medium with the RDFs. First, 1 kg of soil-sand mixture was added directly to each pot. RDFs were added to the remaining 650 g of the soil-sand mixture in a container and thoroughly mixed for homogeneity.
The experimental design comprised treatments with three RDFs, two blends, one synthetic reference, and an unfertilized control, similar to the soil incubation experimental set-up. Each treatment except the unfertilized control was tested at two dosages, i.e., 100% and at 50% recommended NPK dosage, in quadruplicate pots. The material application rates were calculated as per the nutrient requirements for lettuce growth (
Table 2). To supplement the growth of crops and ensure an equal application of P and K in all treatments, synthetic fertilization was conducted using triple super phosphate (TSP; 46% P
2O
5) and potassium sulphate (PAT; 30% K
2O, 10% MgO, and 42.5% SO
3). An important point to be considered here is the additional application (2.2 times the recommended dosage) of K
2O from the treatment with CaE, which was a consequence of satisfying the N requirements of lettuce with this RDF. Hence, to ensure that every treatment received an equal application, additional K
2O was added to all other fertilized treatments, accordingly, using PAT. For application in the current pot trial, a very small quantity of the RDFs AN and AW was needed (
Table 2) due to their high N content, which necessitated mixing AN and AW, each with demineralized water (to reach the required concentration), thus enabling that a substantial quantity of product (6.25 mL of each product) could be pipetted out and mixed with the soil homogenously.
After fertilization, the seedlings were transplanted into each pot, and an additional 100 mL of demineralized water was added to reach a water holding capacity of 60%. Watering was performed thrice per week or as per loss of moisture, and the position of pots was randomized once every week.
Plant and Soil Analysis after Harvest
After 54 days of growth in the pots, the lettuce was harvested using trimming scissors. The plants were clipped from the shoot above the soil level, and any remnant soil particles were cleaned off the plants by wiping it with tissues. This was followed by the fresh weight (FW) determination. The DM determination was conducted by oven drying the samples at 50 °C for 72 h. The dried material was ground using a mortar and pestle, and these ground samples were used for all further analyses. The total N was analyzed using a CN analyzer (Primacs100 Skalar, Breda, North Brabant, The Netherlands). Macro- and micronutrients such as P, K, and Na (0.2 g sample) were analyzed following the same methodology described in
Section 2.3.1. The plant nitrate analysis was performed using ion chromatography (Metrohm, Herisau, Switzerland) from an external certified laboratory (Innolab, Oostkamp, Belgium).
The entire soil from each pot was transferred to a plastic bag, roots removed, mixed thoroughly, and a sub-sample was taken and frozen for mineral N analysis. Moisture content was also determined at this point by drying a sub-sample of the soil at 105 °C for 24 h. The remaining soil was air-dried and sieved using a 1 mm sieve to remove smaller roots. The physicochemical characterization of the soil samples was performed following the same methods as described in
Section 2.2.
2.4. Calculations and Statistical Analysis
In the soil incubation experiment, net N release and net N mineralization were determined.
Net N release (Nrel,net) presented the difference between the mineral N measured in the amended soil (soil treated with fertilizer) from the mineral N measured in the unamended soil (the control). N
rel,net was calculated by the formula [
37]:
At t = 0, the Nrel,net (%) equals the product Nmineral/Ntotal ratio × 100.
Net N mineralization (Nmin,net) gave the N mineralized from the organic fraction of the product (expressed as a percentage of the total N in the product) and was calculated by deducting the amount of mineral N present in the product at time zero (t = 0) from the amount of mineral N at time t as [
38]:
A positive Nmin,net value indicates net N mineralization, and a negative Nmin,net indicates net N immobilization. Statistical analysis was performed using the SPSS statistical software IBM SPSS version 27.0. A parametric one-way ANOVA test was performed to evaluate significant differences in Nrel,net (%) between different tested treatments for each sampling moment, followed by Tukey’s post-hoc test to identify differences between treatments. Normality within and variance between treatments were determined by the Shapiro-Wilk test and Levene’s test, respectively.
The analyses of plant and soil samples from the pot experiment gave insights into various parameters, including fresh and dry yield of the plant, nutrient uptake, apparent N recovery (ANR), and NFRV. The calculation of ANR and NFRV [
39,
40,
41] was conducted as follows:
A non-parametric Mann–Whitney test was performed to observe significant differences in treatments for plant FW, DM, nutrient concentration in plants, ANR and NFRV, and soil parameter analysis, since the data for these results did not meet the assumptions of the parametric one-way ANOVA. The Mann–Whitney was performed to compare each treatment with the unfertilized control and synthetic fertilizer references (p = 0.05).