Abstract
The incorporation of coal fly ash (CFA) in fired clay bricks (FCBs), as a clay replacement, contributes toward cleaner production practices. CFA disposal is an important issue worldwide due to its huge volume and to its potential negative environmental impacts, and currently does not have a recovery route due to its high concentration in unburned materials. In this study, the impact of the incorporation of two CFA, with different content of unburned carbon, FAA (low LOI) and FAB (high LOI) in FCBs, from a technical and environmental point of view was conducted. Unburned carbon plays an important role on the final properties of FCBs. The thermal decomposition during the firing process promotes an increase of water absorption, decreasing the flexural strength as the porosity increases, although the technical and mechanical properties of samples containing up to 30% FAA and percentages of 20% FAB are acceptable. The leaching behaviour showed an immobilisation of Cr and Se in FCBs while Mo reduced its mobility to values below non-hazardous limits. Acid gas emission values do not exceed the reference emission value, except for SO2 emissions while the level of CO2 emissions must be estimated based on the total annual production of the ceramic factory.
1. Introduction
Development of innovative solutions of resource efficiency in the construction sector, both in products and in infrastructure works, may include huge amounts of waste and thus promotes the circular economy for a sustainable future and also contributes to achieving the long-term United Nations Sustainable Development Goals [1]. Clay minerals, mainly aluminosilicates, are a resource widely used in industry for their well-known properties such as plasticity, fire resistance, and their wide distribution, but from an environmental engineering point of view, the relevant properties are cation exchange capacity, structure, texture, and surface area. The use of clay minerals as potential sorbent material for organic and inorganic contaminants has increased in recent decades [2]. For example, a sustainable application is the capture of carbon through carbon dioxide adsorption by modified clays [3].
On the other hand, among the many industrial processes in which clay is used is the manufacture of fired clay bricks (FCBs), one of the most extended construction materials [4]. FCBs are frequently produced from non-renewable materials or cementitious materials at high firing temperatures, which implies environmental damage due to the extraction of raw material, GHG emissions, and air pollution globally [5].
The incorporation of industrial wastes in clay based-ceramics, as a clay replacement, contributes toward cleaner production practices, and has been addressed by many scientific works during the last decade [6]. Four groups of waste can mainly be determined according to their nature [7,8]: sludges from different industrial processes [9,10,11]; inorganic waste, mainly from mining and metallurgy waste processes [12,13]; organic waste from paper manufacturing plants and also biomass of different biological activities [14,15,16] and ashes, mainly from coal energy production, and municipal solid waste and biomass incineration [9,17,18,19]. Among all of them, it is important to underline coal fly ash (CFA), which is generated in thermal power plants, typically constituting around 80% of the total ash, and approximately 30% of the mass of coal consumed [20]. It has been estimated that the world’s 2300 coal-fired power station fleet creates approximately one billion tonnes of new fly ash each year [21]. Therefore, the incorporation of CFA in the production of FCBs can be an important application, bearing in mind the large quantities of raw materials needed for FCB production [22]. The valorisation of CFA contributes to the reduction in natural resources extracted as its composition is very similar to that of clay, SiO2, Al2O3, and Fe2O3 with some minor constituents such as CaO and MgO and other inorganic elements. Amorphous material and crystalline phases as well as unburned or partially burned carbon residues represent its mineralogical composition [23]. CFA is considered “non-hazardous” waste according to different regulations (EPA and EU) despite containing metals and metalloids such as, As, Pb, Zn, Ni, Cu, Mn, Cd, Cr, and Se at trace levels [24]. However, due to serious environmental accidents, agencies such as the EPA have questioned the need to include them in a new classification such as special waste (Resource Conservation and Recovery Act, 2008) [25]. Therefore, CFA disposal is an important issue worldwide due to its huge volume and to its potential negative environmental impacts, which requires great disposal fees and the consequent negative Public Relations (PR) of the sector. On the other hand, unburned carbon in CFA is an indicator of defective combustion of the original fuel, mainly due to the ageing of the boilers [26]. CFA can be valuable as a source of activated carbon or a source of coal to return it to the boiler. However, an unburned carbon content greater than 10% considerably reduces its pozzolanic capacity, preventing its recovery in cement and concrete industries [20]. For this reason, in practice, only 30% of the total ash produced in the world is re-used [27]. These limitations in the recovery of ashes in the manufacture of concrete reinforce the need for a more rigorous study of its recovery in other industrial processes, specifically in the ceramic industry. Many studies have investigated the properties of clay-based bricks containing CFA to evaluate the technical performance of FCBs [9,17,28,29,30,31,32,33]. Specifically, the study of the percentage of unburned in CFA on the final properties of FCBs, only Vasic et al. [34] considered it as an input parameter in an artificial neural network model.
On the other hand, there is no harmonisation to determine the environmental behaviour of ceramic products incorporating waste; the most widely used leaching tests to evaluate them at the end of their useful life, as construction and demolition waste (C&DW), whether deposited in landfills or used as secondary raw material, are the compliance tests: TCLP (Toxicity Characteristic Leaching Procedure) [32,35,36] and the Equilibrium Leaching Test EN 12457 [37,38,39,40,41,42,43]. Specifically, in the case of ceramic products manufactured with coal ash, only very few authors [29,32,43] have reported equilibrium leaching test data of heavy metal mobility with different proportions CFA. Only Gupta et al. (2019) [44] showed comparative results of batch and column leaching test of metal release, and the difference between bricks with/without CFA was minimal.
Furthermore, from the point of view of the environmental performance of the process, firing is a critical stage due to emissions into the atmosphere [45]. For this reason, the ceramic industry is included in the pollutant release and transfer register (PRTR), establishing the best available technologies (MTDs) as well as the emission limit values of the process. Considering that the incorporation of waste can affect gaseous emissions, some authors have studied these effects on an industrial scale [37,41,42,46,47,48] and also on a laboratory scale [49,50,51]. However, only very limited information has been obtained on the effects of the composition, mineral transformation, and firing parameters on these emissions.
As highlighted in the review of Zanelli et al. [52], it is essential for a technological transfer to the industrial practice of a correct evaluation of the technical performance of the FCBs incorporating waste as well as an assessment of the environmental behaviour through the leaching test of the final products and gas emissions during the firing process. In this way, there are no works that have performed an integral study of the incorporation of CFAs into FCBs. Therefore, this paper proposes the substitution of part of a virgin raw material such as clay for a residual material that currently does not have a recovery route due to its high concentration in unburned materials. In this research, the incorporation of two coal fly ashes with different content of unburned carbon, with additions varying from 0 to 100 wt% in FCBs was conducted. The technological properties, leaching behaviour, and microstructural characterisation of the final products as well as the estimation of the gas emissions during the firing process were performed with the aim to study the impact of influence of unburned carbon on CFA-FCBs from a technical and environmental point of view.
2. Materials and Methods
2.1. Materials
Two illitic clays (clay A and clay B), currently used as traditional raw materials in two Spanish industrial ceramic processes (face bricks), and two combustion by-products, low calcium fly ashes (ASTM class F), with different contents of unburnt carbon, low (FAA) and high content (FAB) were used in this study. Quarry clays were collected from a two different industrial ceramic companies and fly ashes were supplied by a coal combustion boilers. All are located in Cantabria region, Spain.
2.2. Processing Method
The methodology used in this work is represented in Figure 1. Both clays were subjected to a grinding process to achieve a particle size exceeding 0.5 mm clay A and 1 mm clay B. The mixture of raw materials, fly ash, and clay was realised with a laboratory mixer Raimondi, Iperbet model. The proportions of raw materials were calculated by weight and the necessary water was added to obtain a plastic mixture (between 5 and 10% of moulding water). The mixture was processed by pressing in a hydraulic press Nanetti Mignon model SS/EA, which reaches 200 bar pressure and prismatic moulds with dimensions of 80 × 40 × 20 mm. The samples were dried for 30 h at 105 °C before the firing cycle, using a laboratory muffle furnace Hobersal, PR Model 12/300 capable of ramp temperature versus time of up to 16 points. Industrial firing cycle was provided by the ceramic companies reaching temperatures of 1021 °C for clay A and 1058 °C for clay B.
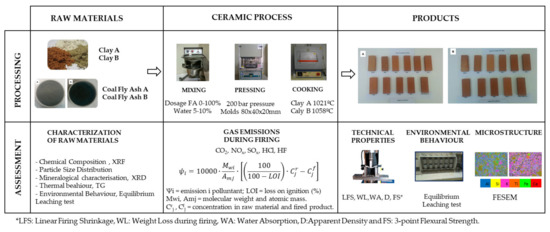
Figure 1.
Methodology carried out in the development of this study.
Table 1 shows the experimental formulations developed. Samples were prepared with the addition of coal fly ash, varying from 0 to 100 wt%. These formulations were studied for both types of coal fly ashes and clays.

Table 1.
Coal fly ash-clay brick formulation.
2.3. Characterisation of Raw Materials
Elemental analysis and major oxide composition were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) with an ARL Fisons 3410 spectrometer and X-ray fluorescence, respectively, in Activations Laboratories (Ancaster, ON, Canada). The particle size distribution were determined by sieving (ISO series sieves) and weighing. The mineralogical characterisation of all materials was carried out by powder X-ray diffraction (XRD) using a D8 Advance Automatic Diffractometer in Bragg-Brentano geometry. Cu Kα1 radiation (λ = 1.5406 Å) was employed with 0.03° 2θ steps and constant 8 s acquisition time in the 10–90° 2θ range. The effect of temperature was studied on the clays and coal fly ashes at 10 °C/min up to 1200 °C using thermogravimetry (Setaram Setsys Evolution Thermogravimetric Analyser).
2.4. Characterisation of Fired Samples
Fired ceramic products of all replicates were characterised in terms of linear firing shrinkage (using a precision calliper), weight loss during firing (UNE 772–13), water absorption (UNE 67027), bulk density (UNE-EN 772–13:2001), and 3-point flexural strength (UNE-EN 843–1).
Leaching behaviour of potentially hazardous pollutants was determined by applying the equilibrium leaching test EN12457-2 proposed by European regulation. This test was realised for raw materials and fired specimens (three replicates). Samples were milled to below 4 mm and contacted with deionised water, used as the leachant, at a liquid/solid ratio of 10 and 24 h stirring. At the end of the test, samples were filtered, and the leachates analysed. The pH was measured and the concentrations of As, Ba, Cd, Cr, Cu, Mo, Ni, Pb, and Zn in the leachates were determined by inductively coupled plasma mass spectrometry (ICP-MS). These concentrations were compared with the limits established for open landfill conditions (landfill Directive 2003/33/EC) to assess the environmental performance of the construction products at the end of life when they become demolition waste.
The mineralogical characterisation was obtained, as described earlier for the raw materials, by XRD. Microstructural analysis of the clays, coal fly ashes, and fired bricks was carried out by field emission scanning electron microscopy (FESEM) using an accelerating voltage of 20 kV. The microstructural porosity study was performed on polished fracture surfaces, while phase distribution observations were carried out on polished samples, which were subsequently etched for 10 s in a 5% HF solution. Before observation, all samples were coated with a layer of C. The ion distribution between the different crystalline phases was determined by digital X-ray mapping. The mineralogical characterisation was obtained, as described earlier for the raw materials, by XRD.
Finally, the emission of potentially harmful species (HCl, HF, SOx, NOx, CO2) into the atmosphere during the firing of ceramic bricks were determined according to the standard methodology described in the BREF Document on Ceramic Manufacturing Industry. The method involves analysing the raw materials and the fired specimens for potentially polluting elements. The concentration of carbon, sulphur, and nitrogen was determined using a FISONS EA 1108 elemental analyser, and the concentration of halogens (Cl and F) was determined by neutron activation (INAA) and an ion-selective electrode (ISE), respectively, at Activations Laboratories (Canada). Emission values (Ψ) for each element were determined by simple mass balance according to Equation (1). This equation assumes that elemental Cl, F, S, N, and C are released into the atmosphere during the firing process in the form of HCl, HF, SO2, NO2, and CO2, respectively:
where i is the polluting compounds (HCl, HF, SO2, NO2, CO2) and j is the constituting elements (Cl, F, S, N, and C) of the polluting compounds. Ψi = emission (in mg of compound i per kg of brick produced). LOI = loss on ignition (%). Mwi = molecular weight of the polluting compounds. Amj = atomic mass of the element. Crj = concentration of element (j) in the raw material and Cfj = concentration of element (j) in the fired product.
3. Results and Discussion
3.1. Characterisation of Raw Materials
Table 2 shows the results of the main oxides and elements in all of the raw materials used to form bricks. Clays and FAA had three major oxides (silicon, aluminium, and iron), which represent over 75% of raw materials, whereas FAB represents 61%. The minor fraction consists of eight oxides: manganese, magnesium, calcium, sodium, potassium, titanium, and phosphorus. LOI values obtained for each type of clay were related to the content of unburned carbon. Furthermore, FAA and FAB had a higher content of alkaline, earth alkaline, and iron oxides, which make them suitable for their valorisation in fired clay ceramic bricks due to its melting character.

Table 2.
Chemical composition of raw materials.
Particle size of quarry clays was less than 0.5 mm in clay A, and 1 mm in clay B, with average particle sizes of 180 µm and 220 µm, respectively. The particle size distribution of fly ashes is given in Figure 2. FAA had a lower particle size than FAB (average particle size of 65 µm of FAA versus 111 µm of FAB). FAA showed 4% ash particles above 100 microns; 23% of particles between 63 and 100 microns; and 73% of fly ash with a particle size below 63 microns. Instead, FAB had 25% of particles with sizes greater than 100 microns; 23% with sizes between 63 and 100 microns; and 52% of particles with sizes lower than 63 microns.
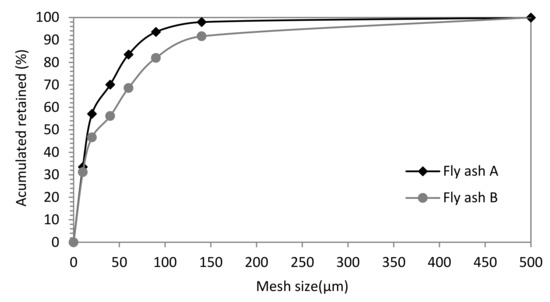
Figure 2.
Particle size distribution of coal fly ashes A and B.
Mineralogical analysis of raw materials is shown in Figure 3. The mineralogical analysis of clays A and B showed an amorphous fraction in the form of maximum width between 15 and 30 degrees. On the other hand, the crystal phases present were mainly quartz (SiO2), muscovite (KAl3Si3O10(OH)1.8F0.2), dickite (Al2Si2O5(OH)4), and kaolinite (Al2Si2O5(OH)4). These phases can constitute approximately 90% of the total clay, respectively. Furthermore, minority phases are iron oxides (Fe3O4). FAA and FAB showed an amorphous fraction in the form of maximum width, much larger than clays, between 15 and 30 degrees. On the other hand, the crystal phases present were mainly quartz (SiO2), mullite (Al4+2xSi2-2xO10-x being x≈0.4), and anhydrous aluminium silicate (Al2O7Si2). These phases can constitute approximately 70 and 50% of FAA and FAB, respectively. Furthermore, minority phases were dolomite CaMg(CO3)2, calcium aluminate (xCaO yAl2O3 being x and y the number of molecules), calcium silicate (2CaO.SiO2), and iron oxides (Fe3O4).
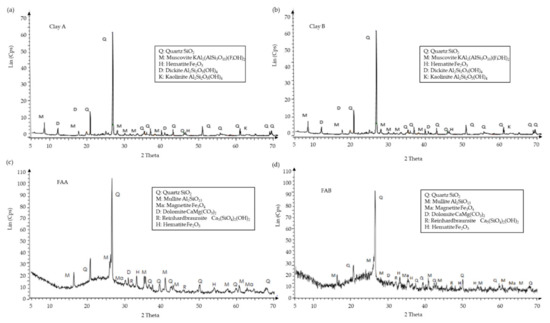
Figure 3.
XRD pattern of clay A (a), clay B (b), FAA (c), and FAB (d).
Figure 4a shows the thermal behaviour during firing (10 °C/min) of clays A and B up to 1200 °C. Thermogravimetric analysis showed the typical clay dehydroxylation process (water of hydration) with a mass loss of 2.33% and 3.57% up to 100 °C, respectively. The second step was around 500–550 °C, which corresponds with the dehydroxylation of clays (aluminosilicate decomposition) with a mass loss of 5.70% and 5.84%, respectively.
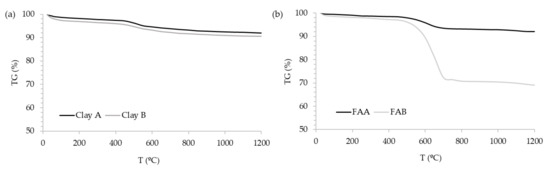
Figure 4.
Thermogravimetric behaviour of (a) clay A and clay B; (b) FAA and FAB.
Figure 4b represents the thermogravimetry of both FA. The mass loss of 1.55% for FAA and 2.93% for FAB between 100 and 400 °C corresponded to the thermal decomposition of water present in ashes. Between 500 and 700 °C, where the mass loss for FAA was 5.69% and for FAB was 27.91%, this corresponded to the thermal decomposition of carbonates and unburned carbon. In this case, the decomposition of carbonates was due to the presence of dolomite MgCa(CO3)2, which is decomposed into calcium and magnesium oxides and carbon dioxide. The total mass loss due to thermal decomposition of water, carbonates, and unburned carbon for FAA was 7.95% and 30.84% for FAB, much higher due to the high contents of unburned carbon of FAB. The Compliance Leaching test EN 12457-2 was performed in triplicate for raw materials and the results are presented as an average and standard deviation of concentrations in Table 3. For all the pollutants considered on the European landfill regulation, the concentrations in clays A and B were below the inert threshold limit, but for clay A, As was close to the inert limit. Most of the pollutants considered for the FAA and FAB were below the inert threshold limit, except for Cr and Mo, which were below the non-hazardous limit for both ashes. Only Se exceeded the non-hazardous limits and was well below the limit for landfilling hazardous waste landfills.

Table 3.
pH values and leaching of major elements from raw materials according to the compliance leaching test EN 12457-2.
3.2. Technological Properties of Fired Products
The effect of unburned carbon of fly ash on the linear shrinkage is shown in Figure 5. FAB/clay ceramics present a higher value of linear shrinkage for percentages of fly ash greater than 50%. There was a difference over three units between FAA and FAB for both clays. For concentrations between 10–50% of fly ash, fired clay ceramics with FAB had a linear shrinkage close to the bricks with FAA. The difference between ashes was lower than 0.5 units and presented a linear shrinkage close to the bricks with 100% clay. The same tendency of increasing the linear shrinkage, due to the addition of waste, has been observed in other studies [53].
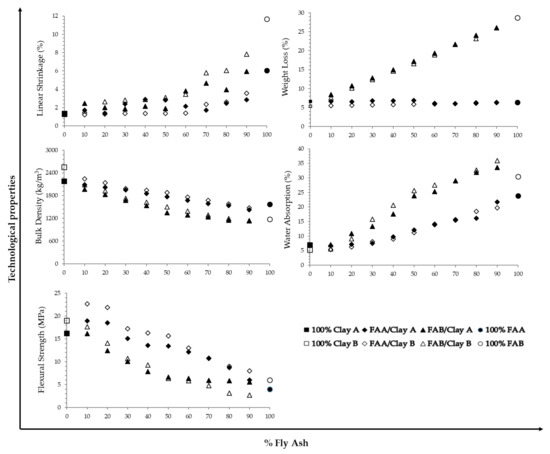
Figure 5.
Main technological properties of fired clay bricks.
Weight loss increases as the percentage of FAB increases due to the high carbon contents [31]. Samples were fired up to 1058 °C and at this range, FAB experiences a thermal decomposition of carbonates and unburned carbon [54]. This decomposition supposes a loss of weight and was confirmed with the thermogravimetric analysis (Figure 4). Fired clay bricks with FAA presented results of weight loss close to the bricks made with 100% clay. Percentages of FAA above 60% had slightly higher values due to the thermal decomposition of water and carbonates. The difference between fired bricks was 5 units for 10% of fly ash, while 15 and 20 units for 60% and 90% of FAB, respectively, so unburned carbon significantly influenced the weight loss of fired clay bricks.
Fly ash is less dense than the clays used, thereby lowering the density with increasing the ash content in the bricks. The percentage of ash used considerably affects the densification. The presence of unburned carbon in the fly ash provides less dense bricks. Bricks with 10% of fly ash presented 100 units of difference between FAA and FAB and with 50% had 400 units of difference. With percentages up to 20% of fly ash, results of bulk density were very close between FAA and FAB, regardless of the clay used.
FAB/clay bricks presented higher results of water absorption than FAA. The difference in results between bricks 100% clay and bricks incorporating fly ash was due to the porosity caused by the thermal decomposition of water and carbonates. In this case, FAB is greater due to the higher contents of unburned carbon, which also experiences a thermal decomposition. This thermal decomposition releases gases that generate more porous materials. Bricks with 20% FAB had the same results as bricks with FAA. Bricks up to 20% FAA and 10% FAB had water absorption close to 100% clay bricks.
Flexural strength is related to water absorption and density, which are associated with the porosity of the ceramic brick. As FAB/clay bricks presented the highest results of water absorption and the lowest density, in flexural strength, these bricks showed results related to these properties. Bricks with 10% of fly ash presented two units of difference between FAA and FAB and bricks with 50% of fly ash had seven units of difference. It can be concluded that the higher the unburned carbon content, the worse the results of flexural strength, but at percentages of 10, the obtained values were close to fired clay bricks with FAA and from 80% ash, the values were close again.
3.3. Leaching Behaviour of Fired Bricks
Once a leaching test was applied, the elements that exceeded the limit of the inert waste landfill on raw materials were studied (Table 3). The contaminants associated with fly ash were Cr, Mo, and Se, as shown in Figure 6a. Introducing fly ash into fired clay bricks in a dosage of 50% reduced the mobility of Mo and immobilised Cr and Se below the inert waste limit. The concentration of As for fly ash was below the limit of inert waste landfill, but when incorporated into the ceramic bricks in 50% of FAB, it exceeded it. The 100% clay B was above the inert waste limit, so clay B was the raw material, which favours that the 50% FAB/clay B bricks exceeded this limit. The content of unburned carbon did not influence the mobility of contaminants.
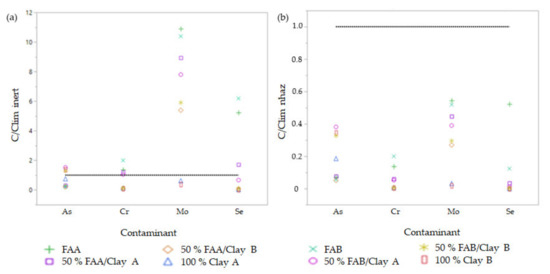
Figure 6.
Concentration of contaminants of fly ash/clay bricks regarding the inert waste landfill limits (a) and the non-hazardous waste landfill limits (b) according to Decision 2003/33/EC.
We also studied the limits of non-hazardous waste landfill for these contaminants, as shown in Figure 6b. All the contaminants were below the limit of landfill for non-hazardous waste, and the unburned carbon content did not influence the mobility of elements. As mobility is due to its presence in clays, as concentrations of 100% clay bricks were higher than 50% fly ash/clay bricks. In conclusion, the unburned carbon content did not affect the leaching of contaminants, so the obtained results are very interesting.
Oxyanions such as Mo can be adsorbed on the surface of amorphous iron oxides that many alkaline wastes contain. Furthermore, the complexation of these compounds has been studied and a relation between the specific surface area and the extension of the complexation process found for MoO−24. In his work, McKenzie concluded that the decrease in the specific surface led to higher Mo leaching [55].
3.4. Microstructural Characterisation of Fired Clay Bricks
From the results obtained on the technological and environmental properties of the fired products, clay B was chosen to carry out a microstructure study of the bricks obtained and the gaseous emissions generated during the firing process.
3.4.1. XRD Characterisation
Figure 7 shows the XRD patterns of ceramic brick compositions with 10–50% FAA, 10–50% FAB, and without fly ash, only 100% clay B that were fired at 1058 °C. The mineral phases of the bricks were identified as quartz, sillimanite, microcline, kyanite, and iron oxide (hematite).
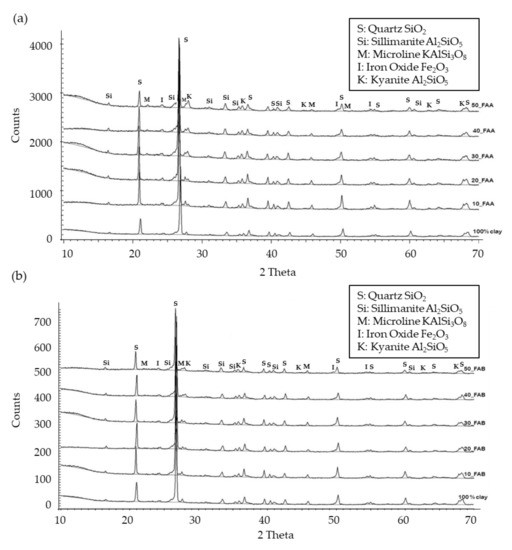
Figure 7.
XRD patterns of fired clay bricks prepared from clay B. (a) XRD patterns of FAA/clay; (b) XRD patterns of FAB/clay.
As can be seen, XRD patterns of FAA/clay and FAB/clay were close to the clay ones. An increase in peaks of quartz and Fe-containing phases such as hematite, with the fly ash amount in the ceramic matrix, could be observed. Mullite and dolomite, a significant phase in both fly ash samples, was not detected in bricks incorporating both FAA and FAB, which were mainly composed of quartz and hematite and other characteristic aluminosilicates of clay bricks. The presence of high amounts of unburned carbon in FAB samples did not imply differences in the final mineral phases of the bricks.
3.4.2. SEM
Figure 8a,b shows the microstructure observed by FESEM on the polished surfaces of FAA/clay and FAB/clay bricks, respectively. The main microstructural effect was the increase in porosity as the content of coal fly ash in the brick body increased. The increase in porosity was mainly associated with the presence of dolomite and unburned carbon in the mineralogical composition of the fly ashes (Figure 3).
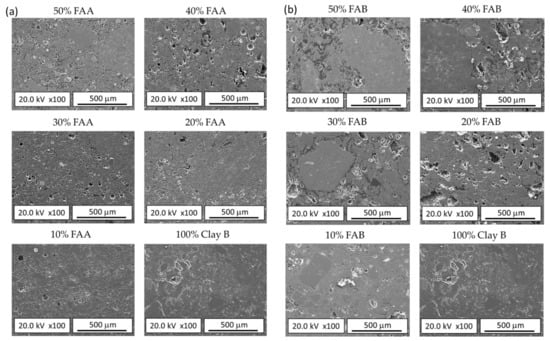
Figure 8.
FESEM images of fired clay bricks: (a) FAA–Clay fired bricks; (b) FAB–Clay fired bricks.
However, the incorporation of FAA and FAB led to different impacts on the porosity. For further discussion, Figure 9 presents details of the polished surface microstructure of samples prepared exclusively from clay B (100% Clay B) and those prepared with 50% fly ash incorporation (50% FAA/Clay B and 50% FAB/Clay B). The microstructure of the 100% clay B sample was characterised by the presence of open porosity consisting of interconnected and irregular cavities with a thickness of fewer than 5 μm. This porosity is the result of the dehydroxylation of the clay during firing, which causes a volume reduction. The increase in porosity observed when fly ash was incorporated into the brick body is probably due to the combined action of two factors, namely the larger particle size of fly ash compared to clay, and the dolomite and unburned carbon content in fly ash. Thus, due to its larger average size, the inclusion of fly ash in the brick paste will result in green-shaped specimens with different porosity. According to Amorós et al. [56], at equal pressing variables, a higher content of larger particles results in compacted specimens of higher bulk density (lower porosity), which, however, contain larger pores. As the pore size in the fired bodies depends more on the pore size in the green body than on the pore volume, the inclusion of fly ash in the brick paste will result in less densified fired bodies, and to achieve maximum densification of the product, a higher firing temperature would be required. On the other hand, fly ash contains dolomite and unburned carbon, which during the firing process will decompose with the consequent release of gases, causing the size of the open porosity to increase. FAA presents a low dolomite and unburned carbon content, as can be inferred from its LOI value (Table 2), which was even lower than that of clay B. Therefore, the major effect induced by the incorporation of FAA in the body of the brick was a slight increase in porosity associated with its larger particle size (Figure 9). In contrast, FAB contained much higher dolomite and unburned carbon than the clays, so that during firing, it will generate a high increase in porosity due to the release of the gases generated during its decomposition. This increase in porosity, which is evident in Figure 9, is associated with a significant increase in the water absorption of the brick specimens manufactured with this fly ash (Figure 5).
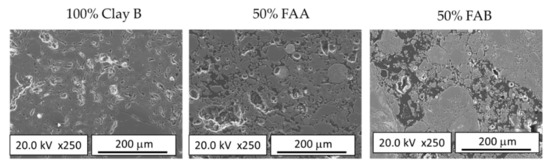
Figure 9.
FESEM images of 100% clay B and 50% FAA and FAB/clay B fired clay bricks.
The incorporation of fly ash into the brick composition affects not only the porosity of the fired pieces but also their crystalline composition and the distribution of crystalline phases. Figure 10 presents the FESEM images of polished and etched fracture surfaces of samples of 100% clay B and 50% FAB/clay B compositions, together with the mapping of the elements Al, Si, K, Ti, Fe, and Ca. The different crystalline phases that comprise the mineralogical composition of the fired bricks were clearly distinguished by the colour differences caused by the varying concentrations of these ions in each of the crystalline phases. The 100% clay B sample shows the conventional phase distribution in traditional fired clay materials composed mainly of quartz grains embedded in a ceramic matrix enriched in aluminium oxide.
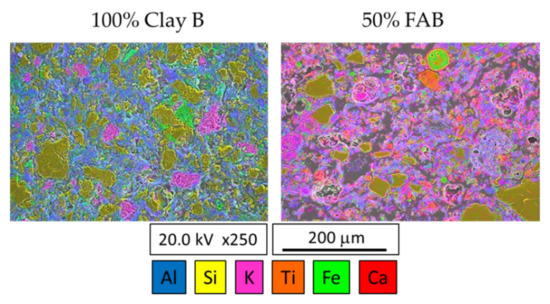
Figure 10.
FESEM images and mapping of elements of samples 100% clay B and 50% FAB/clay B.
Figure 11a depicts FESEM images of different regions observed in the microstructure of 100% clay B brick. Some of the quartz grains showed micro cracks, which were likely formed as a result of the relaxation of micro stresses caused by the difference between the coefficients of thermal expansion of the quartz grains and the surrounding ceramic phase as stated in Romero et al. [57]. In addition to quartz particles, in the ceramic matrix, regions with high potassium oxide content with crystals of irregular morphology were identified in Figure 11b, which must correspond to the microcline phase, as observed in the XRD of the fired bricks (Figure 7). Isolated iron oxide particles were also clearly distinguishable in the mapping image 100% clay B sample. Regarding the morphology of the ceramic matrix, FESEM observations at high magnification (Figure 11c) allowed us to distinguish regions with elongated crystals, which likely correspond to the sillimanite phase (as observed in Figure 7). Regarding the microstructure of the 50% FAB/clay B bricks, Figure 10 shows the presence of quartz grains to a smaller extent. The quartz grains showed fewer micro-cracks, probably because they were surrounded by a much more porous matrix that could absorb the stresses caused by the differences in expansion coefficients. The microcline crystals were not distinguishable and potassium oxide was dispersed throughout the matrix, which was more enriched in calcium oxide.
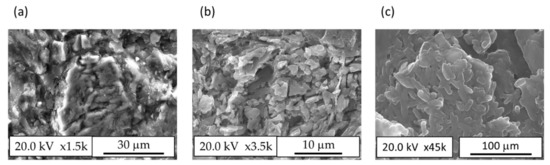
Figure 11.
Microstructure FESEM images taken at different regions of 100% clay B. (a) The FESEM images of different regions observed in the microstructure of 100% clay B brick; (b) The high potassium ox-ide content area in ceramic matrix; (c) FESEM observations at high magnification.
3.5. Estimation of Gas Emissions during Firing Process
The content of Cl, F, S, C, and N for clay B and fly ash A (low LOI) and B (high LOI), as well as their corresponding acid gas emissions HCl, HF, SO2, and CO2 after the firing stage at the temperature of 1058 °C simulating the industrial processes and estimated by mass balance (Equation (1)) are summarized in Table 4. The content of N was below the detection limit of the equipment used. Chlorine emissions are related to its content in the samples. The chlorine in the clay comes from micas, halite, and organic matter, while in the ash, it mainly forms chlorinated compounds associated with its organic matter. The significant increase in chlorine emissions in clay compared to fly ash, despite having the same initial content, may be due to the presence of chlorine as sodium chloride (halite), which is very unstable during the firing process. While the difference in emissions between the two types of ashes is directly related to the unburned content, the higher the LOI, the higher the decomposition of chlorinated compounds. Fluorine emissions also depend on the initial concentration of this element in the samples. The difference observed between the clay and the ashes may be due to the decomposition of phyllosilicates present in the clay while the difference between both ashes may be due to the formation of thermally stable compounds (CaF2) in samples with low carbonate content [58]. Sulphur emissions depend on the mineralogy of the samples. Due to the oxidation and decomposition of pyrite and organic matter during the firing process, high values of sulphur emissions are obtained for ashes with a high content of unburned carbon [59]. Finally, in the case of CO2 emissions, a direct relationship can be observed between the initial carbon content in the raw materials and the level of emissions.

Table 4.
Content and pollutants emissions of clay B and fly ashes A and B.
Gas emission for the mixtures of FAA/clay and FAB/clay fired at the temperature of 1058 °C simulating the industrial processes and estimated by mass balance (Equation (1)) are summarized in Figure 12. Emission limit values for HF, HCl, and SO2 proposed by Gonzalez et al. (2011) [58], aimed to include them in the Andalusian-Spain legislation in response to the indications of the IPPC, have also been considered. From a study conducted with 40 ceramic factories in Andalusia, Spain by means, median, and the 90th percentile values of 180 samples, three ranges of emission values were established: “acceptable”, “recommended intervention”, and “compulsory intervention”.
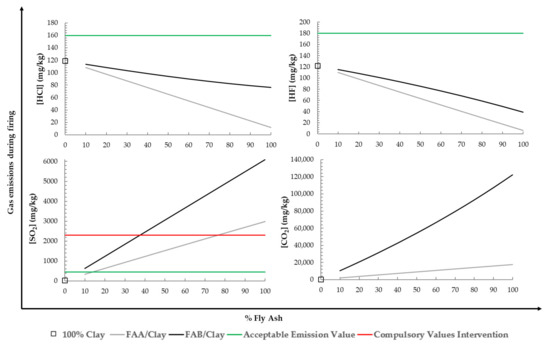
Figure 12.
Estimation of HCl, HF, SO2, and CO2 emissions for mixtures performed with FAA/clay B and FAB/clay B during the firing process including reference emission values proposed by I. Gonzalez et al. [58].
The introduction of fly ash in the mixtures reduces the emissions of HCl due to low chlorine emissions from fly ash. Incorporating up to 90% of FAA and FAB, a reduction in emissions of HCl was achieved compared with the brick of 100% clay. The percentage of reduction obtained was 61% for FAA and 25% for FAB. The reduction in emissions by introducing FAA was higher due to the lower value of emission with respect to FAB. As indicated above, it is probably due to a greater decomposition of chlorinated compounds associated with organic matter, that is, the content of unburned matter. The introduction of fly ash in the mixtures of FAA/clay and FAB/clay reduced the emissions of HF due to the low contents of F that fly ash present. The reduction in HF emissions was higher for fly ash due to the lower contents of F before the firing process compared to clay. This is due to the fact that clay contains one of the most important sources of fluorine such as phyllosilicates, therefore, the less clay in the mixture, the greater the reductions in fluorine emissions. Incorporating up to 90% of FAA and FAB reduced the emissions of HF by 42% and 60% compared to the 100% clay brick.
The introduction of fly ash in the mixtures produced an increase in SO2 emissions due to the high content of S present in the fly ash. The FAB/clay mixtures showed extremely high levels due to the presence of the combination of different sulphur associated with inorganic, pyritic, and organic carbon.
The introduction of fly ash in the mixtures FAA/clay and FAB/clay increases the emissions of CO2 due to the high contents of unburnt carbon that fly ash present. The increase in CO2 emissions was higher for FAB due to the high contents of unburnt carbon (27.03%) before the firing process compared to FAA (5.43%) and clay. In this case, the CO2 emissions from inorganic carbon were not important in an overall assessment.
Considering the technological properties to market this type of brick according to AENOR Fired Clay Piece HD Clinker R-55 and the leaching behaviour, it could be possible to incorporate up to 20% of FAA and 10% of FAB. The reduction in emissions of HCl with these additions was 13% and 5%, respectively, according to reference emission values. As the HCl emission limit value was <160 ppm, the addition of 20% FAA and 10% FAB did not exceed the acceptable emission value proposed. In the case of HF, the introduction of FA supposes a reduction of 10–11%, respectively. The HF emission limit value was <180 ppm, therefore, the addition of 20% FAA and 10% FAB did not exceed the acceptable emission value. In the case of SO2, the introduction of FA produced an increase of SO2 emissions was 61% and 65%, respectively. The SO2 emission limit value was <450 ppm, therefore, it exceeds the acceptable emission value, and an intervention to review the value would be advisable. For 20% of FAA and 10% of FAB in the mixtures, the increase in CO2 emissions was 62% and 87%, respectively. It is important to note that the level of CO2 emissions must be estimated based on the total annual production of the ceramic factory.
4. Conclusions
The influence of the amount of unburned carbon present in coal fly ash on the properties of fly ash-fired clay bricks was studied through the dosage of increased amounts of fly ashes with different unburned carbon content: FAA (low LOI) and FAB (high LOI).
The main microstructural effect was the increase in porosity as the content of coal fly ash (FAB) in the brick body increased. The porosity was associated with the release of the gases generated during the decomposition of dolomite and the content of unburned carbon of the fly ash. This increase in porosity corresponded with a significant increase in the water absorption and therefore, a bulk density and flexural strength reduction. Although unburned carbon plays an important role on the final properties, no detriment was observed in the properties of ceramic bricks containing up to 30% FAA and percentages of 20% FAB.
The leaching behaviour of critical contaminants associated with the presence of fly ash showed an immobilisation of Cr and Se in fired clay bricks while Mo reduced its mobility to values close to the inert landfill limit values. No influence of porosity on Mo leaching was observed, but a higher retention, in samples with FAB, due to the adsorption of Mo on the surface of amorphous iron oxides. HF and HCl emissions were below the emissions of the 100% clay bricks, so introducing fly ash would reduce the emissions of these pollutants in the firing process. The decomposition of chlorinated compounds associated with the unburned material was the main difference in the emission reduction values between both ashes. SO2 emissions of FAB/clay mixtures showed high levels due to the presence of the combination of different sulphur associated with unburned carbon, mainly.
On the other hand, the level of CO2 emissions should be estimated based on the total annual production of the company. Therefore, unburned carbon separation option from fly ash would be advisable to obtain acceptable emission values in the CFB process.
Author Contributions
Conceptualisation, A.A. and E.C.; Methodology, J.D. and M.R.; Formal analysis, and investigation, J.D., E.C., M.R., T.L. and A.A.; Resources, A.A. and E.C.; Data curation, J.D. and T.L.; Writing—original draft preparation, J.D. and M.R.; Writing—review and editing, A.A. and E.C.; Project administration and funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project “Valorisation of fly ash in ceramic products and fly ash based products” funded by Solvay Química SL.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
This work was presented at the workshop “Engineering and circular economy: the road to sustainability” funded as a part of the ECO-MET-AL Project (PID2019-109520RB-I00), “Can industrial and mining metalliferous wastes produce green lightweight aggregates? Applying the Circular Economy” funded by the Spanish Ministry of Science, Innovation, and Universities and ERDF funds, framed in the “Grants for “R&D&I Projects” in the framework of the State Programmes for the Generation of Knowledge and Scientific and Technological Strengthening of the R&D&I System and R&D&I oriented to the Challenges of Society, Call 2019”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vincevica-gaile, Z.; Teppand, T.; Kriipsalu, M.; Krievans, M.; Jani, Y.; Klavins, M.; Hendroko Setyobudi, R.; Grinfelde, I.; Rudovica, V.; Tamm, T.; et al. Towards Sustainable Soil Stabilization in Peatlands: Secondary Raw Materials as an Alternative. Sustainability 2021, 13, 6726. [Google Scholar] [CrossRef]
- Ozola, R.; Krauklis, A.; Burlakovs, J.; Klavins, M.; Vincevica-Gaile, Z.; Hogland, W. Surfactant-Modified Clay Sorbents for the Removal of p-Nitrophenol. Clays Clay Miner. 2019, 67, 132–142. [Google Scholar] [CrossRef]
- Krūmiņš, J.; Kļaviņš, M.; Ozola-Davidāne, R.; Ansone-Bērtiņa, L. The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review. Minerals 2022, 12, 349. [Google Scholar] [CrossRef]
- European Commission, Joint Research Centre; Borchardt, S.; Buscaglia, D.; Barbero Vignola, G.; Maroni, M.; Marelli, L. A Sustainable Recovery for the EU: A Text Mining Approach to Map the EU Recovery Plan to the Sustainable Development Goals; Publications Office of the European Union: Luxembourg, 2020; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC122301 (accessed on 19 March 2022).
- Del Rio, D.D.F.; Sovacool, B.K.; Foley, A.M.; Griffiths, S.; Bazilian, M.; Kim, J.; Rooney, D. Decarbonizing the ceramics industry: A systematic and critical review of policy options, developments and sociotechnical systems. Renew. Sustain. Energy Rev. 2022, 157, 112081. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Horpibulsuk, S. A Review of Studies on Bricks Using Alternative Materials and Approaches. Constr. Build. Mater. 2018, 188, 1101–1118. [Google Scholar] [CrossRef]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muñoz Velasco, L. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Contreras, M.; Gázquez, M.J.; Romero, M.; Bolívar, J.P. Recycling of industrial wastes for value-added applications in clay-based ceramic products: A global review (2015–19). In New Materials in Civil Engineering; Samui, P., Kim, D., Iyer, N.R., Chaudhary, S., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 155–219. ISBN 9780128189610. [Google Scholar]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef]
- Beshah, D.A.; Tiruye, G.A.; Mekonnen, Y.S. Characterization and recycling of textile sludge for energy-efficient brick production in Ethiopia. Environ. Sci. Pollut. Res. 2021, 28, 16272–16281. [Google Scholar] [CrossRef]
- Amin, S.H.K.; Elmahgary, M.G.; Abadir, M.F. Preparation and characterization of dry pressed ceramic tiles incorporating ceramic sludge waste. Ceram. Silik. 2019, 63, 11–20. [Google Scholar] [CrossRef]
- Belmonte, L.J.; Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E.; Vestbø, A.P. Screening of heavy metal containing waste types for use as raw material in Arctic clay-based bricks. Environ. Sci. Pollut. Res. 2018, 25, 32831–32843. [Google Scholar] [CrossRef]
- Sharif, N.M.; Lim, C.Y.; Teo, P.T.; Seman, A.A. Effects of body formulation and firing temperature to properties of ceramic tile incorporated with electric arc furnace (EAF) slag waste. AIP Conf. Proc. 2017, 1865, 1–7. [Google Scholar] [CrossRef]
- Sena Da Fonseca, B.; Vilão, A.; Galhano, C.; Simão, J.A.R. Reusing coffee waste in manufacture of ceramics for construction. Adv. Appl. Ceram. 2014, 113, 159–166. [Google Scholar] [CrossRef]
- Demir, I.; Baspinar, M.S.; Orhan, M. Utilization of kraft pulp production residues in clay brick production. Build. Environ. 2005, 40, 1533–1537. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Corpas-Iglesias, F.A.; Pérez-Villarejo, L.; Iglesias-Godino, F.J. Recycling of sawdust, spent earth from oil filtration, compost and marble residues for brick manufacturing. Constr. Build. Mater. 2012, 34, 275–284. [Google Scholar] [CrossRef]
- Sutcu, M.; Erdogmus, E.; Gencel, O.; Gholampour, A.; Atan, E.; Ozbakkaloglu, T. Recycling of bottom ash and fly ash wastes in eco-friendly clay brick production. J. Clean. Prod. 2019, 233, 753–764. [Google Scholar] [CrossRef]
- Brunner, P.H.; Rechberger, H. Waste to Energy—Key Element for Sustainable Waste Management. Waste Manag. 2015, 37, 3–12. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase–Mineral and Chemical Composition and Classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- CEDEX Catálogo de Residuos Utilizables en Construcción. Available online: http://www.cedexmateriales.es/catalogo-de-residuos/24/diciembre-2011/ (accessed on 16 January 2021).
- Vecor Australia Pty Ltd. Company Profile/Rozelle, New South Wales, Australia/Competitors, Financials & Contacts—Dun & Bradstreet. Available online: https://www.dnb.com/business-directory/company-profiles.vecor_australia_pty_ltd.aefefd25a2d9ed847d91b85bf5085cfc.html (accessed on 20 March 2021).
- Xu, G.; Shi, X. Characteristics and Applications of Fly Ash as a Sustainable Construction Material: A State-of-the-Art Review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-Derived Unburned Carbons in Fly Ash: A Review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Jayaranjan, M.L.D.; van Hullebusch, E.D.; Annachhatre, A.P. Reuse options for coal fired power plant bottom ash and fly ash. Rev. Environ. Sci. Biotechnol. 2014, 13, 467–486. [Google Scholar] [CrossRef]
- EPA, Environmental Protection Agency. Coal Combustion Residuals—Proposed Rule. 2008. Available online: http://www.epa.gov/osw/nonhaz/industrial/special/fossil/ccr-rule/ (accessed on 18 June 2021).
- Elías, X. Reciclaje de Residuos Industriales: Residuos Solidos Urbanos y Fangos de Depuradora. Residuos Sólidos Urbanos y Fangos de Depurador; Ediciones Díaz de Santos: Madrid, Spain, 2009. [Google Scholar]
- ECOBA European Coal Combustion Products Association. Available online: https://www.gem.wiki/European_Coal_Combustion_Products_Association (accessed on 16 January 2021).
- Akhtar, M.N.; Hattamleh, O.; Akhtar, J.N. Feasibility of coal fly ash based bricks and roof tiles as construction materials: A review. In Proceedings of the MATEC Web of Conferences, Taichung, Taiwan, 4 August 2017; Volume 120. [Google Scholar]
- Leiva, C.; Arenas, C.; Alonso-Fariñas, B.; Vilches, L.F.; Peceño, B.; Rodriguez-Galán, M.; Baena, F. Characteristics of fired bricks with co-combustion fly ashes. J. Build. Eng. 2016, 5, 114–118. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Sun, Z.; Ma, E. New Technology and Application of Brick Making with Coal Fly Ash. J. Mater. Cycles Waste Manag. 2016, 18, 763–770. [Google Scholar] [CrossRef]
- Kockal, N.U. Utilisation of Different Types of Coal Fly Ash in the Production Of Ceramic Tiles; Sociedad Española de Cerámica y Vidrio: Madrid, Spain, 2012; Volume 51, pp. 297–304. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Sandalio-Pérez, J.A.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Sánchez-Soto, P.J. Investigation of Use of Coal Fly Ash in Eco-Friendly Construction Materials: Fired Clay Bricks and Silica-Calcareous Non Fired Bricks. Ceram. Int. 2018, 44, 4400–4412. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Leonelli, C.; Manfredini, T. Recycling of Industrial Wastes in Ceramic Manufacturing: State of Art and Glass Case Studies. Ceram. Int. 2016, 42, 13333–13338. [Google Scholar] [CrossRef]
- Vasić, M.V.; Goel, G.; Vasić, M.; Radojević, Z. Recycling of Waste Coal Dust for the Energy-Efficient Fabrication of Bricks: A Laboratory to Industrial-Scale Study. Environ. Technol. Innov. 2021, 21, 101350. [Google Scholar] [CrossRef]
- Garcia-Ubaque, C.A.; Giraldo, L.; Moreno-Piraján, J.C. Quality Study of Ceramic Bricks Manufacture with Clay and Ashes from the Incineration of Municipal Solid Wastes. Afinidad 2013, 561, 60–66. [Google Scholar]
- Singh, I.B.; Chaturvedi, K.; Morchhale, R.K.; Yegneswaran, A.H. Thermal Treatment of Toxic Metals of Industrial Hazardous Wastes with Fly Ash and Clay. J. Hazard. Mater. 2007, 141, 215–222. [Google Scholar] [CrossRef]
- Galán-Arboledas, R.J.; Álvarez de Diego, J.; Dondi, M.; Bueno, S. Energy, Environmental and Technical Assessment for the Incorporation of EAF Stainless Steel Slag in Ceramic Building Materials. J. Clean. Prod. 2017, 142, 1778–1788. [Google Scholar] [CrossRef]
- Fernández-Pereira, C.; de La Casa, J.A.; Gómez-Barea, A.; Arroyo, F.; Leiva, C.; Luna, Y. Application of Biomass Gasification Fly Ash for Brick Manufacturing. Fuel 2011, 90, 220–232. [Google Scholar] [CrossRef]
- Alonso-Santurde, R.; Andrés, A.; Viguri, J.R.; Raimondo, M.; Guarini, G.; Zanelli, C.; Dondi, M. Technological Behaviour and Recycling Potential of Spent Foundry Sands in Clay Bricks. J. Environ. Manag. 2011, 92, 994–1002. [Google Scholar] [CrossRef]
- Alonso-Santurde, R.; Coz, A.; Quijorna, N.; Viguri, J.R.; Andrés, A. Valorization of Foundry Sand in Clay Bricks at Industrial Scale. J. Ind. Ecol. 2010, 14, 217–230. [Google Scholar] [CrossRef]
- Quijorna, N.; Miguel, G.S.S.; Andrés, A. Incorporation of Waelz Slag into Commercial Ceramic Bricks: A Practical Example of Industrial Ecology. Ind. Eng. Chem. Res. 2011, 50, 5806–5814. [Google Scholar] [CrossRef]
- Quijorna, N.; Coz, A.; Andres, A.; Cheeseman, C. Recycling of Waelz Slag and Waste Foundry Sand in Red Clay Bricks. Resour. Conserv. Recycl. 2012, 65, 1–10. [Google Scholar] [CrossRef]
- Little, M.R.; Adell, V.; Boccaccini, A.R.; Cheeseman, C.R. Production of Novel Ceramic Materials from Coal Fly Ash and Metal Finishing Wastes. Resour. Conserv. Recycl. 2008, 52, 1329–1335. [Google Scholar] [CrossRef]
- Gupta, N.; Gedam, V.V.; Moghe, C.; Labhasetwar, P. Comparative Assessment of Batch and Column Leaching Studies for Heavy Metals Release from Coal Fly Ash Bricks and Clay Bricks. Environ. Technol. Innov. 2019, 16, 100461. [Google Scholar] [CrossRef]
- European Comission. European Comission Reference Document on Best Available Techniques in the Ceramic Manufacturing Industry; European Comission: Brussels, Belgium, 2007. [Google Scholar]
- Hamer, K.; Karius, V. Brick Production with Dredged Harbour Sediments. An Industrial-Scale Experiment. Waste Manag. 2002, 22, 521–530. [Google Scholar] [CrossRef]
- Anderson, M.; Elliott, M.; Hickson, C. Factory-scale proving trials using combined mixtures of three by-product wastes (including incinerated sewage sludge ash) in clay building bricks. J. Chem. Technol. Biotechnol. 2002, 77, 345–351. [Google Scholar] [CrossRef]
- García-Ubaque, C.A.; Moreno-Piraján, J.C.; Giraldo-Gutierrez, L.; Sapag, K. Stabilization/Solidification of Ashes in Clays Used in the Manufacturing of Ceramic Bricks. Waste Manag. Res. 2007, 25, 352–362. [Google Scholar] [CrossRef]
- Dondi, M.; Guarini, G.; Raimondo, M.; Ruffini, A. Orimulsion Fly Ash in Clay Bricks—Part 3: Chemical Stability of Ash-Bearing Products. J. Eur. Ceram. Soc. 2002, 22, 1749–1758. [Google Scholar] [CrossRef]
- Souza, V.P.; Toledo, R.; Holanda, J.N.F.; Vargas, H.; Faria, R.T. Pollutant Gas Analysis Evolved during Firing of Red Ceramic Incorporated with Water Treatment Plant Sludge. Ceramica 2008, 54, 351–355. [Google Scholar] [CrossRef]
- Coronado, M.; Andrés, A.; Cheeseman, C.R. Acid Gas Emissions from Structural Clay Products Containing Secondary Resources: Foundry Sand Dust and Waelz Slag. J. Clean. Prod. 2016, 115, 191–202. [Google Scholar] [CrossRef]
- Zanelli, C.; Conte, S.; Molinari, C.; Soldati, R.; Dondi, M. Waste recycling in ceramic tiles: A technological outlook. Resour. Conserv. Recycl. 2021, 168, 105289. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E. Fly ash addition in clayey materials to improve the quality of solid bricks. Constr. Build. Mater. 2009, 23, 1178–1184. [Google Scholar] [CrossRef]
- Naganathan, S.; Mohamed, A.Y.O.; Mustapha, K.N. Performance of bricks made using fly ash and bottom ash. Constr. Build. Mater. 2015, 96, 576–580. [Google Scholar] [CrossRef]
- McKenzie, R.M. The Adsorption of Molybdenum on Oxide Surfaces. Aust. J. Soil Res. 1983, 21, 505–513. [Google Scholar] [CrossRef]
- Amorós, J.L.; Orts, M.J.; García-Ten, J.; Gozalbo, A.; Sánchez, E. Effect of the green porous texture on porcelain tile properties. J. Eur. Ceram. Soc. 2007, 27, 2295–2301. [Google Scholar] [CrossRef]
- Romero, M.; Pérez, J.M. Relation between the microstructure and technological properties of porcelain stoneware. A review. Mater. Constr. 2015, 65, e065. [Google Scholar] [CrossRef]
- González, I.; Galán, E.; Miras, A.; Vázquez, M.A. CO2 emissions derived from raw materials used in brick factories. Applications to Andalusia (Southern Spain). Appl. Clay Sci. 2011, 52, 193–198. [Google Scholar] [CrossRef]
- Uaciquete, D.L.E.; Sakusabe, K.; Kato, T.; Okawa, H.; Sugawara, K.; Nonaka, R. Influence of unburned carbon on mercury chemical forms in fly ash produced from a coal-fired power plant. Fuel 2021, 300, 120802. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).