Abstract
In biosciences and biotechnologies, it is recently critical to promote research regarding the regulation of the dynamic functions of proteins of interest. Light-induced control of protein activity is a strong tool for a wide variety of applications because light can be spatiotemporally irradiated in high resolutions. Therefore, synthetic, semi-synthetic, and genetic engineering techniques for photoactivation of proteins have been actively developed. In this review, the conventional approaches will be outlined. As a solution for overcoming barriers in conventional ones, our recent approaches in which proteins were chemically modified with biotinylated caging reagents are introduced to photo-activate a variety of proteins without genetic engineering and elaborate optimization. This review mainly focuses on protein caging and describes the concepts underlying the development of reported approaches that can contribute to the emergence of both novel protein photo-regulating methods and their killer applications.
1. Introduction
Methods for photo-regulating the function and quantity of proteins are attracting attention in a wide range of fields, from fundamental research in molecular biology [1,2,3,4,5] to drug delivery systems [5,6,7,8,9]. In these methods, proteins are activated [2,3,4,5,10,11,12] or released from their carriers in response to light irradiation in and around cells [6,7,8,9,13,14]. Due to the high spatial and temporal resolution of light, the function of the protein can be selectively exerted at the desired location and at the desired time. The amount of active protein expressed can be remotely controlled by light. Therefore, we can obtain spatiotemporal information on the function of proteins by controlling the location and timing of light irradiation, giving insight into the dynamic roles of proteins in biological systems. Furthermore, light-induced activation and release of proteins are expected to reduce the off-target effects of protein-based drugs, and moreover potentially enable therapeutic effects to be obtained by producing only the minimum amount of therapeutic proteins selectively in the affected area.
In recent years, optogenetics based on naturally occurring light-responsive proteins has been widely reported as a method for photo-regulation of proteins [3,4,15,16,17,18]. Light-dependent interactions and structural changes of light-sensing proteins such as cryptochrome-2 [16], vivid [17] and phototropin [18], which are derived from plants and fungi, have been used to control the activity of functional proteins, including membrane receptors [16], gene-editing enzymes [17] and antibodies [18]. In these methods, a light-responsive protein that is genetically engineered is expressed as a fusion protein consisting of a functional protein of interest and a light-sensing domain such as the LOV domain of phototropin, which is the most familiar in optogenetics [18]. Since all parts of the protein can be expressed genetically, it is simple to express the desired photo-responsive proteins in living cells by the introduction of the gene using plasmid DNA or a virus vector. In the case that the protein of interest is activated by dimerization or complexation, it is relatively easy to design the photoactivatable fusion proteins. However, for many other proteins, the proteins of interest must be engineered elaborately to introduce optogenetics through reversibly partitioning them, in which the partitioned proteins are inactive before light-induced dimerization or complexation [17]. In addition, after photoactivation, the optogenetically activated proteins are kept to fuse to light-responsive domains, and such fusion with exogenous ones may reduce the activity and limit the range of applications. Recently, a photocleavable protein has been reported as a new category of optogenetics [19,20]. In the pioneering report, the protein of interest was tethered to the membrane through the photocleavable protein, and after exposure to light, the active protein without any fusion partner was released from the membrane, leading to light-induced localization and activation [19]. This approach can simply design light-induced release of wild-type protein from specific organelle by fusion with organelle-localizing proteins through the photocleavable protein, but there are also many proteins whose activity cannot be suppressed before exposure to light by control of localization alone. Thus, although optogenetics is an extremely attractive tool for intracellular protein photoactivation, it may not be suitable for some applications in terms of convenience and versatility. The present review mainly focuses on chemical modification-based methodologies for protein photoactivation. As a complementary approach to optogenetics, the progress in the synthetic and semi-synthetic approaches for protein photoactivation are systematically introduced to help the researchers in a variety of fields to simply design and realize a suitable protein photoactivation system for their goals.
2. Caged Proteins
Methods for converting proteins to photo-responsive ones by reversible chemical modification have also been used as a complementary approach in which intact proteins with no modification are produced after photoactivation [10,11,12,21,22,23]. In the field of chemical biology, inactivation of biomolecules by modification with photodegradable protective groups is called “caging”, and such protected compounds are called caged compounds [10,21]. Caging have been employed for activating biomolecules of interest at desired timing and location by exposure to light (Figure 1A). For example, a caged glutamine was reported to be introduced into a neuron, and then one of the dendrites was site-specifically exposed to light. As a result, the shape change of the light-exposed dendrites clearly indicated the molecular system in which glutamine affects the shape memory of neural spines [24]. Thus, caging has been utilized as a powerful research tool for giving new insight into molecular mechanisms in cell biology. Such a caging approach has also been applied to proteins, and caged proteins have been reported for more than a quarter of a century [22,23]. The simplest strategy for preparing caged proteins is to randomly introduce a photodegradable protecting group to the amino group on the protein surface through the reaction with an active carbonyl group (Figure 1B) [25,26,27,28]. In this method, in principle, almost all proteins can be caged without any pretreatment simply by mixing a reactive photodegradable protecting reagent (a caging reagent). After the protective group is degraded by light, the caged lysine residue returns to the original lysine one without leaving any trace, so there is no need to consider the effect of caging on the protein function after photoactivation. This is a great advantage when studying the function of a protein or using it as a drug. Actually, the polymerization activity of G-actin was photo-regulated by this caging strategy [25]. The selective binding function of antibodies were demonstrated to be remotely controlled by light exposure [27]. In a similar way, the carboxylic acid moiety on protein surfaces was reported to be randomly blocked with the diazo derivatives of photolytic protection groups [29]. This method realized photoactivation of hemoglobin. However, unlike small caged compounds, such random introduction of a small photodegradable protecting group onto the surface of a large protein often fails to fully inactivate the function of the protein (Figure 1C). In the case of enzymes, for example, this simple strategy based on random amine modification can function only on the enzymes that happen to have lysine residues at sites involved in catalytic activity or substrate binding (Figure 1B). Therefore, caging of proteins often requires the strategy of introducing the photodegradable protecting group specifically at the sites involved in their activity.
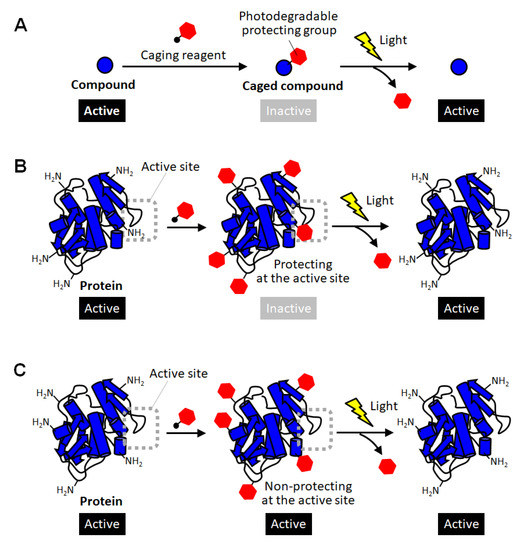
Figure 1.
Schematic illustration of caging of compounds and proteins. (A) Caging of small molecular compounds. (B) Successful and (C) unsuccessful caging of proteins through random modification of lysine residues.
3. Site-Specific Protein Caging
As a method for site-specific introduction of the photodegradable protecting group, the use of the cysteine residue of the proteins has been utilized [11,30,31,32,33,34]. The selective reactivity of the thiol group realized the site-specific caging of the cysteine residue of which there are often one or a few on protein surfaces. Similar to the random amine coupling, the function of intact proteins was reported to be photo-regulated by caging with thiol-reactive protecting reagents when the cysteine residues were at the sites involved in protein functions [30,31]. By this approach, the enzymatic activities of myosin [30] and β-galactosidase [31] were demonstrated to be activated by light exposure. As another method using the thiol-based reaction, the caging of thiophosphorylated proteins was also reported [32]. In this method, the threonine residue of a protein was thiophosphorylated using 3-phosphoinositide-dependent kinase, and then modified with a thiol-reactive caging reagent. After photolysis, the caged protein converted to the thiophosphorylated protein which exerted almost the same activity as the phosphorylated one. This kinase-coupled approach may be versatile for caging a variety of phosphorylated proteins. To apply such thiol-based site-specific caging approaches to any other proteins, the use of cysteine-substituted mutants has been widely utilized (Figure 2A) [11,33,34]. In this approach, the function-related site is replaced with cysteine, and a caging reagent that selectively reacts with the thiol group is applied. Based on this approach, the actin polymerization activity of cofilin, which was unknown so far, was exerted by light exposure in living cells, giving new insights into the role of cofilin [11]. Furthermore, light-induced depolymerization of actin was also achieved by caged cofilin on the glass substrate [33], and the pore-formation on cellular membranes was photo-induced by caged α-hemolysin [34]. Although this approach is simple to introduce a protecting group at the desired active site, the proteins produced after photolysis are only the cysteine-substituted mutant protein on which the mutation is introduced at a critical site involved in function (Figure 2A). Therefore, there is a high risk that the light-induced activity of the mutant protein will be inferior to that of the native one. Thus, the caging strategy based on post-translational chemical modification often requires the site-specific replacement with reactive amino acids for selective caging, and therefore, almost exclusively applied to mutant proteins.
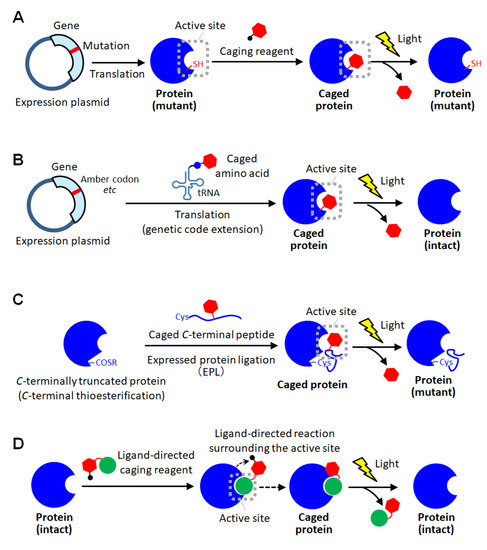
Figure 2.
Schematic illustration of site-specific caging of proteins. (A) Caging based on cysteine mutagenesis. (B) Caging based on unnatural amino acid introduction using genetic code extension technologies. (C) Caging based on protein semi-synthesis using expressed protein ligation (EPL). (D) Caging based on ligand-directed chemistry.
As a method for site-specific protein caging, another genetic approach using unnatural caged amino acids has been widely employed [5,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Using the genetic code extension technology such as notably amber mutation and four-base codon mutation [43], in which an unnatural amino acid is coded to a specific codon, the desired position of the protein of interest can be replaced with a caged amino acid (Figure 2B). Until now, caged aspartic acid [35,39], caged serine [36,51], caged glycine [37,43], caged tyrosine [5,38,40,48,50], caged cysteine [42,47], caged lysine [44,49,52,53], caged phosphoserine [46] and caged phosphotyrosine [46] have been utilized for photo-activation of various proteins including enzymes [35,39,42,43,44,45], intein proteins [36,47], ion channels [37,38,40], receptors [46], phosphorylation cascade proteins [50,51], and so on [5,48,49,52,53]. The site-specific incorporation of unnatural caged amino acid into proteins was first achieved in E. coli [35,36,42,43,44] and a cell-free protein expression system [46], and subsequently their photoactivation was examined in vitro. Furthermore, the recombinant caged proteins were transduced into mammalian cells with a transfection reagent to utilize their photoactivatable functions for clarifying the spatial and temporal molecular mechanism of the living system [5,48]. Next, to photo-regulate ion channels on the plasma membrane of living cells, in situ expression of site-specifically caged proteins was performed in Xenopus oocytes by injecting the caged amino acids-combined tRNA through microinjection [37,38,40]. Recently, the genetic code extension technology has become applicable to eukaryotic cells by using the genetic expression system for the orthogonal pair of pyrrolysyl-tRNA synthetase and the corresponding tRNA, enabling the genetic incorporation of caged amino acids into proteins in yeast cells [42,51] and mammalian cells [5,49,50,51,52,53]. By using such a genetic encoding system, a pioneer group, Chin et al. demonstrated light-induced intracellular localization change of caged nuclear localization proteins in human cells [49] and achieved photoactivation of receptor-mediated signal transduction by caging a phosphorylation protein, STAT1 [50]. Furthermore, to understand the role of cancer-specific mutation, they reported to photo-activate the synthesis of the oncometabolite (R)-2-hydroxyglutarate through caging an isocitrate dehydrogenase mutant in normal cells and exposing those engineered cells to light [52]. Such genetic caging with unnatural amino acid introduction was also reported to achieve light-induced gene editing through caging of Cas9 [53], photo-activation of immune response through caging of MEK1 [5], and screening of caspase substrate proteins through caging of caspase-3 [5] in human cells. Thus, in this approach, a variety of wild-type proteins could be site-specifically caged by utilizing various caged amino acids. Moreover, this approach is advantageous in introducing a photodegradable protection into the active site inside the closed structure of proteins because the caged amino acid can be incorporated on translation before protein folding. However, it requires advanced and specialized gene engineering techniques, and therefore is not easily available.
Other strategies in protein caging have also been reported. In semisynthetic approaches, a small part of the protein was chemically synthesized by incorporating caged amino acid through peptide synthesis, and then linked with a major part of the protein to produce a site-specifically caged protein [54,55,56]. First, as a pioneering approach, site-specific caged ribonuclease S (RNase S) was prepared by mixing a synthesized caged S-peptide and S-protein [54]. RNase S is consisting of two peptide fragments, S-peptide (1-20 residues) and S-protein (21-124 residues), and these fragments bind in a self-assembly manner to exert the enzymatic activity. In this report, by optimizing the replacement site of caged amino acids in S-peptide, the caged RNase S was activated by light in an off-on manner [54]. This approach was greatly developed by the expressed protein ligation (EPL) method (Figure 2C) [55,56]. In EPL-based caging, the fusion protein of a C-terminally-truncated target protein with a self-processing intein domain is overexpressed in E. coli, and through the intein-mediated processing reaction [57], the C-terminal is converted to a reactive thioester moiety. In parallel, the C-terminal domain peptide including a cysteine residue at the N-terminal end and caged amino acids at the desired positions is chemically synthesized. Then, the caged C-terminal peptide is linked to the C-terminal end of the truncated protein through native chemical ligation [58]. A signal transduction protein with multiple caged phosphorylated serine residues was reported to be prepared by this approach [55,56]. Based on the high degree of certainty and freedom of peptide synthesis, the semisynthetic approaches allow for the precise introduction of multiple photodegradable protecting groups and the expansion of the range of amino acids that can be protected. However, the caging position is limited to the terminal sequence of proteins, leading to limitation of applicable proteins. Recently, a total chemical synthesis-based approach was also reported to overcome this limitation of the semisynthetic protein caging [59], but it requires chemical reactions that are too specialized for anyone to immediately utilize. In ligand-directed caging approaches, a ligand molecule that selectively binds to the active site of a protein assists site-specific modification with the caging group [60,61]. In a pioneering study of this approach, the substrate peptide with a thiol-reactive moiety through a photocleavable linker was utilized for active-site specific caging [60]. This synthesized peptide was recognized with enzyme and reacted with the cysteine residue selectively at the active site. Based on this approach, a protein kinase was caged in vitro and introduced into living cells by microinjection, leading to light-induced activation of phosphorylation for cellular morphological change [60]. Similarly, a small substrate analogue with photodegradable ability was reported to specifically incorporated into a serine residue at the active site of enzyme, and the protected activity of enzyme was regenerated by light [61]. Hamachi et al. have presented a number of sophisticated reports in ligand-directed chemistry for in situ protein labeling [62,63,64]. For site-specific caging, a photocleavable linker was inserted between the ligand for proteins of interest and a special reactive group, and after mixing with proteins, this caging reagent could be selectively attached to amino acid residue surrounding the active site through a ligand-directed proximity effect on the reaction (Figure 2D) [65]. By employing this method, an enzyme and a receptor protein were efficiently caged with the reagents including specific ligand, respectively, and demonstrated to be uncaged by light exposure. Thus, ligand-directed approaches can achieve site-specific caging of intact proteins of interest simply by mixing with the specific caging reagents. However, the application is limited to enzymes and binding proteins. Moreover, ligands with an appropriate dissociation constant, which must meet both the requirements for the selective binding in the caging step and the rapid releasing in the photoactivation step, are needed. Such demands of this approach may be issues in terms of versatility.
4. Sterically Bulky Caging of Proteins
In order to solve the problems of caged proteins, we developed a sterically bulky caging method [66]. Among conventional protein caging methods, the approach of randomly modifying amino groups on the protein surface has great advantages in terms of simplicity and versatility, as long as the inhibitory effect on the function can be improved. In order to improve the suppression of any protein functions before photodegradation, we aimed to increase the steric hinderance of each protecting group. If most of the protein surface can be hidden with the randomly modified protecting groups, the interaction at the functional site of the protein will surely be suppressed. This was our strategy at the beginning of this study. At that time, in another project, we had been studying the introduction of photodegradable protecting groups neighboring to promoter regions as a site-specific caging technique for DNA plasmids. In that project, we had developed a simple method of bulking up the protecting group to protect the adjacent promoter [67]. A biotin-modified photodegradable protecting group was developed and introduced neighboring to the promoter, and then, the caged plasmid was mixed with streptavidin (SA), a bulky biotin-binding protein consisting of tetramers. In this study, the bulky SA molecule was confirmed to attach on the introduced biotin moiety on the plasmid and efficiently suppress transcription, probably due to the steric hinderance against the binding of transcription factors onto the neighboring promoter region [67]. Therefore, we hypothesized that this sterically bulky caging approach using biotinylated caging reagent (BCR) and SA could be applied to random caging of proteins (Figure 3A).
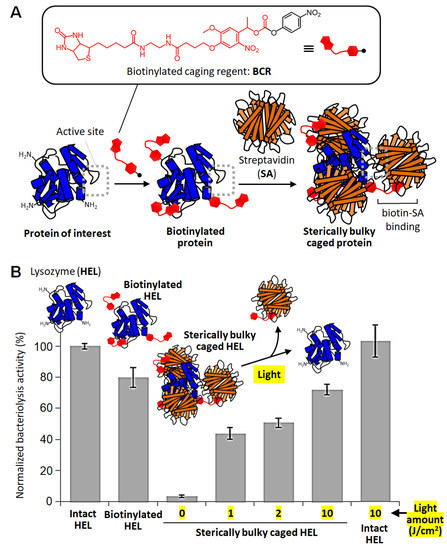
Figure 3.
Sterically bulky caging of proteins. (A) Schematic illustration of the sterically bulky caging method and the chemical structure of biotinylated caging reagent (BCR). (B) Bacteriolytic activity of caged lysozyme before and after caging and light exposure [66].
First, we used hen egg lysozyme (HEL), a bacteriolytic enzyme, as a model protein and randomly introduced biotinylated photodegradable protecting groups with BCR [66]. Mass spectrometry analysis showed that one to three protecting groups were introduced per HEL molecule. When the biotinylated protected HEL was mixed with SA (more than eight equivalents), electrophoretic analysis confirmed the preparation of a bulky caged HEL consisting of one HEL and one to three SA bound. The bacteriolytic activity of the caged HELs was found to be 80% of that of the untreated HELs when only the small biotinylated protective groups were modified. On the other hand, after SA binding, the activity decreased to 3.6% (Figure 3B) [66]. This result indicates that the sterically bulky caging approach using the steric hindrance of SA is very effective in inhibiting the activity. When the bulky caged HELs were exposed to light, the protective groups were decomposed, and electrophoretic analysis confirmed that the unmodified HELs were generated according to the amount of exposed light. The activity after light exposure was recovered to more than 70% (Figure 3B). Thus, it was shown that photoactivatable proteins can be easily prepared by sterically bulky caging using BCR and SA [66].
To demonstrate the versatility of the sterically bulky caging method, we applied it to transferrin (Tf), a human iron transport protein [68]. When the bulky caged Tf was applied to cultured cells, its binding to the Tf receptor on the cell surface was inhibited and it was not taken up into the cells. After light irradiation, it was taken up into the cells in response to light, as confirmed by confocal laser microscopy (Figure 4A) [68]. Thus, this method was applicable to the light-induced control of a transporter protein on living cells without any significant damage onto the cells. Here, Tf has been employed as a carrier for intracellular drug delivery, and when Tf modified with a drug via a linker that cleaves intracellularly is applied to cells, this leads to accumulation of drugs in the cells [69]. Therefore, an apoptosis-inducing drug, doxorubicin (Dox), was installed onto Tf as a model cargo molecule, and the Tf–Dox conjugate was served to sterically bulky caging. By applying the caged conjugate to cell culture, it was confirmed that Dox was introduced in response to light exposure, inducing cell death [68]. These results provided proof of principle that the sterically bulky caged Tf can be employed as a photoactivatable molecular device for the intracellular delivery of cargos (Figure 4B). In this way, we were able to convert other proteins with different activities and structures into photo-responsive proteins simply by sequentially treating with BCR and SA. It is noteworthy that the functions of any proteins we tested were severely suppressed to a non-detectable level by the steric hinderance of SA. In addition, it requires only a small number of the biotinylated photodegradable protection group per a protein molecule for functional suppression, leading to the high efficiency of photoactivation, because the required amount of light for fully uncaging is small. Accordingly, the present sterically bulky caging method is expected to be applied to life science and biotechnology as a useful tool for photoactivation of proteins.
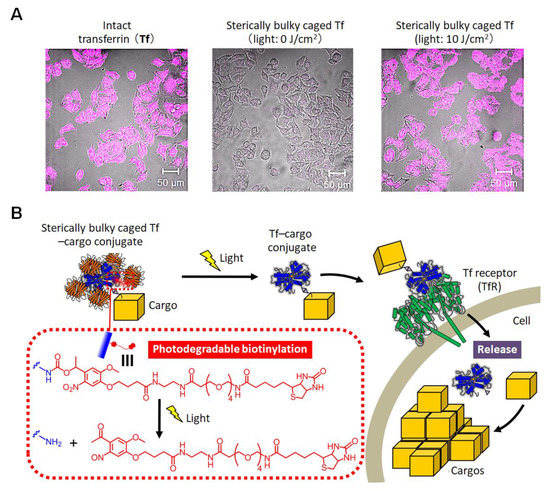
Figure 4.
Sterically bulky caging of transferrin (Tf). (A) Fluorescence microscopy images of colon cancer DLD1 cells treated with intact Tf or sterically bulky caged Tf after exposure to light at 0 and 10 J/cm2 [68]. (B) Schematic illustration of light-induced intracellular delivery of cargos by using sterically bulky caged Tf [68].
5. Photolytic Protein Aggregates
As another strategy to make proteins photo-responsive, a method for the preparation of photolytic protein aggregates is introduced. Protein crystals and aggregates have been reported to be applied to solid-state enzymes and protein stabilization for thirty years [70,71], because they are highly stable against proteolysis and denaturation, and can accumulate an active protein inside them at a high concentration. Utilizing these advantages, protein aggregates, which are reversibly dissolved by stimuli, have been studied to be applied to protein delivery [72]. However, photolytic protein aggregates have not been reported. In the development of the sterically bulky caging method described above, we accidentally noticed that photolytic protein aggregates were generated when the number of SA equivalents to the biotinylated protein was reduced. We hypothesized that the aggregate formation was due to the polymerization of the multiply biotinylated protein through cross-linking with SA, which has four biotin-binding sites. Furthermore, we thought that it would be interesting to use the photolytic protein aggregates as a new tool for light-induced release of active proteins (Figure 5A) [73]. First, the procedures for the preparation of the aggregates were optimized by using biotinylated protected HEL and SA. When high concentrations of both components were mixed in a 1:1 ratio in aqueous solution, the aqueous solution started to become cloudy. By adding a large excess of free biotin molecules to stop the growth of aggregates via blocking the biotin-binding sites of SA, micrometer-sized aggregates were obtained (Figure 5B). No residual proteins were detected in the supernatant after centrifugation, indicating that almost all of HEL and SA were incorporated into the aggregates. When the aggregates were exposed to light, they dissolved according to the amount of exposed light (Figure 5C), and SA and HEL were released into the aqueous solution, as shown by electrophoretic analysis [73]. At maximum, 90% of the HEL used for aggregate formation was released without any modification. The bacteriolytic activity was also recovered up to 90% in the wild-type HEL used for aggregate formation. Thus, the specific activity of the photo-dissolved HEL from aggregates was the same as that of the wild type, suggesting that aggregate formation and photo-irradiation do not cause irreversible denaturation. Such photolytic aggregate formation and light-dependent dissolution were also observed with biotinylated protected Tf [73]. Similar to the sterically bulky caging described above, we were also able to form aggregates using Dox-modified Tf and confirm the induction of cell death in response to light exposure (Figure 5D). Thus, this method is also considered to be highly versatile.
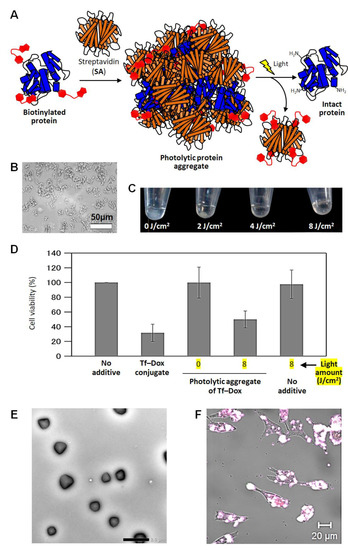
Figure 5.
Photolytic protein aggregates using biotinylated caging reagent and streptavidin (SA). (A) Schematic illustration of photolytic protein aggregates. (B) Microscopic image of photolytic aggregates of lysozyme (HEL). (C) Photographs of photolytic HEL aggregates collected at the bottom of the tube by centrifugation after light irradiation (0–8 J/cm2). (D) Cell viability of colon cancer DLD1 cells treated with photolytic aggregates of transferrin–doxorubicin conjugate (Tf–Dox) before and after light irradiation. (E) Electron microscopy images of photolytic HEL nanoaggregates prepared in a w/o emulsion. (F) Confocal microscopy images of DLD1 cells treated with cell-permeable peptide-modified photolytic HEL nanoaggregates. All figures are modified from the published article [73].
Next, we examined the processing method of the photolabile protein aggregates. When we simply mixed biotinylated protected proteins with SA as described above, micro-sized aggregates with non-uniform shape and size were produced. Furthermore, this size is too large to be intravenously administered in vivo, nor can it enter cells. To down-size the aggregates to the submicron rang, we mixed biotinylated HEL and SA in water/oil (w/o) emulsion droplets with submicron sizes. As a result, it was confirmed by electron microscopy that aggregates with a relatively uniform shape of several hundred nanometers in diameter could be prepared (Figure 5E) [73]. When such protein aggregates are utilized in living cells, they must have cell permeability. Here, the biotinylated functional molecules can be easily modified onto the surface of the present aggregates by adding them instead of biotin for blocking the biotin-binding sites on the surfaces. We modified the submicron-size aggregates with a biotinylated cell-permeable peptide, and applied them to colon cancer cells, resulting in accumulation of the aggregates in the cells (Figure 5F) [73]. Thus, the miniaturized protein aggregates can be easily functionalized through modification with biotinylated functional molecules, and therefore, in principle, they are decollated with tumor-targeting ligands and antibodies for the delivery of therapeutic proteins. At the same time, by depositing the present protein aggregates on the substrate without stopping their growth, it was also possible to form the micro-sheet of protein aggregates with several hundred micro-meter thickness [73]. On the aggregate sheets, the protein of interest was eluted by light exposure selectively from the exposed area. This technology is promisingly applied as a smart material surface that can release the protein of interest at the desired timing and position in a wide range of applications, from fundamental studies to tissue engineering.
6. Summary
Through the recent progress of high-throughput genome analysis, it is becoming increasingly important to analyze the spatiotemporal functions of the protein corresponding to the critical gene for the difference in cellular phenotypes. In this context, the photoactivation tool for proteins are attractive because it can clearly demonstrate the spatiotemporal functions of the protein of interest in living systems. As briefly introduced above, a recent excellent article by Wang et al. demonstrated the promising application of protein photoactivation in proteomics [5]. In this high-impact study, photoactivation of caged caspase-3 enabled the temporal profiling of the substrate proteins of caspase-3 just after light exposure, leading to identification of new proteolytic substrates in early apoptotic processes. As this study was able to minimize the effects of other late-activated caspases, light-induced temporal activation realizes the elimination of the noises from other reactions of sequential and parallel cascades in complex biochemical reactions. Such temporal proteomics based on protein photoactivation is expected to give new insights into the biological systems and to reveal a series of new target substrates for drug discovery and diagnostics in future. However, there are often significant barriers in using the conventional protein photoactivation system. To date, many methods have been developed to improve the certainty of light-induced control of protein functions. Most of the developed methods require specialized techniques of gene engineering and tremendous try-and-error approaches in optimization for each target protein [5]. In this review, our two approaches for protein photoactivation were briefly introduced as one of the solutions for possibly overcoming the barriers. Both of our approaches can be easily converted into photo-responsive proteins by simply treating the protein with the biotinylated caging reagent and SA, and are expected to be applicable to a wide range of proteins in principle. Compared with optogenetics and in situ caging systems based on the genetic code extension technology, chemical modification approaches currently have bottlenecks in the way that caged proteins are introduced into the cells. In recent years, a variety of protein-transfection reagents have been developed and are commercially available, and this problem is being solved. Therefore, research teams can simply cage any proteins of interests through synthetic or semi-synthetic methods, and after transduction into the cells, perform intracellular photoactivation for spatio-temporal function analysis, temporal proteomics and so on in the near future. As another application, the therapeutic potential of protein photoactivation and photo-release has been reported [5,6,7,8,9]. Actually, Wang et al. demonstrated photoactivation of a cytotoxic protein as protein pro-drugs, achieving therapeutic effects in tumor-loaned mice [5]. In such applications, the stability of caged proteins and the penetration of light deep into the living body are challenges. From the viewpoint of stability, our two approaches are superior to other caged proteins because they are protected by bulky protection and aggregation structures. Moreover, the combination of the ligand to be modified with the photodegradable protecting group and its binding protein is not limited to biotin and SA. It is also possible to employ a combination of reactive moieties that bioorthogonally form a covalent bond. Such modification may further improve in vivo stability of caged proteins. In addition, the photoresponsive wavelength can also be altered to be longer for increasing bio-permeability by using different photodegradable linkers. Furthermore, for activation in the depths where light cannot reach, we can develop a protein that responds to other external stimuli or specific environments in addition to light by changing the chemical properties of the linker, though the subject is changed slightly from photoactivation. In this way, by hybridizing synthetic molecular tools with proteins, it will be possible to rationally provide functions that are difficult to create with the genetic code alone, and to create molecular technologies that can remotely control any proteins of interest for bioscientific, biotechnological and biomedical purposes in the future.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT), Grant-in-Aid for Young Scientists (A) 24686094, Scientific Research (C) 15K06575 and Scientific Research (B) 21H01723, and by the Japan Science and Technology Agency (JST), PRESTO 16815021 and MIRAI program 19217334.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data presented in this review are all referred from our previously-reported paper.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Gautier, A.; Gauron, C.; Volovitch, M.; Bensimon, D.; Jullien, L.; Vriz, S. How to control proteins with light in living systems. Nat. Chem. Biol. 2014, 10, 533–541. [Google Scholar] [CrossRef]
- Wu, Y.I.; Frey, D.; Lungu, O.I.; Jaehrig, A.; Schlichting, I.; Kuhlman, B.; Hahn, K.M. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 2009, 461, 104–108. [Google Scholar] [CrossRef]
- Inagaki, H.K.; Jung, Y.; Hoopfer, E.D.; Wong, A.M.; Mishra, N.; Lin, J.Y.; Tsien, R.Y.; Anderson, D.J. Optogenetic control of Drosophila using a red-shifted channel rhodopsin reveals experience-dependent influences on courtship. Nat. Methods 2014, 11, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Morri, M.; Sanchez-Romero, I.; Tichy, A.M.; Kainrath, S.; Gerrard, E.J.; Hirschfeld, P.P.; Schwarz, J.; Janovjak, H. Optical functionalization of human Class A orphanG-protein-coupled receptors. Nat. Commun. 2018, 9, 1950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Liu, Y.; Zheng, S.; Wang, X.; Zhao, J.; Yang, F.; Zhang, G.; Wang, C.; Chen, P.R. Time-resolved protein activation by proximal decaging in living systems. Nature 2019, 569, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Høgset, A.; Prasmickaite, L.; Selbo, P.K.; Hellum, M.; Engesæter, B.Ø.; Bonsted, A.; Berg, K. Photochemical internalisation in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 95–115. [Google Scholar] [CrossRef]
- Gu, Z.; Biswas, A.; Zhao, M.; Tang, Y. Tailoring nanocarriers for intracellular protein delivery. Chem. Soc. Rev. 2011, 40, 3638–3655. [Google Scholar] [CrossRef]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef]
- Brieke, C.; Rohrbach, F.; Gottschalk, A.; Mayer, G.; Heckel, A. Light-controlled tools. Angew. Chem. Int. Ed. 2012, 51, 8446–8476. [Google Scholar] [CrossRef]
- Ghosh, M.; Song, X.; Mouneimne, G.; Sidani, M.; Lawrence, D.S.; Condeelis, J.S. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 2004, 304, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Deiters, A.; Groff, D.; Ryu, Y.; Xie, J.; Schultz, P.G. A genetically encoded photocaged tyrosine. Angew. Chem. Int. Ed. 2006, 45, 2728–2731. [Google Scholar] [CrossRef] [PubMed]
- Selbo, P.K.; Sandvig, K.; Kirveliene, V.; Berg, K. Release of gelonin from endosomes and lysosomes to cytosol by photochemical internalization. Biochim. Biophys. Acta 2000, 1475, 307–313. [Google Scholar] [CrossRef]
- Peng, K.; Tomatsu, I.; Kros, A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef]
- Levskaya, A.; Weiner, O.D.; Lim, W.A.; Voigt, C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 2009, 461, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.M.; Lee, M.; Kim, C.Y.; Chang, K.Y.; Heo, W.D. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem. Biol. 2014, 21, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Nihongaki, Y.; Kawano, F.; Nakajima, T.; Sato, M. Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nat. Biotechnol. 2015, 33, 755–760. [Google Scholar] [CrossRef]
- Gil, A.A.; Carrasco-López, C.; Zhu, L.; Zhao, E.M.; Ravindran, P.T.; Wilson, M.Z.; Goglia, A.G.; Avalos, J.L.; Toettcher, J.E. Optogenetic control of protein binding using light-switchable nanobodies. Nat. Commun. 2020, 11, 4044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lohman, A.W.; Zhuravlova, Y.; Lu, X.; Wiens, M.D.; Hoi, H.; Yaganoglu, S.; Mohr, M.A.; Kitova, E.N.; Klassen, J.S.; et al. Optogenetic control with a photocleavable protein, Phocl. Nat. Methods 2017, 14, 391–394. [Google Scholar] [CrossRef]
- Lu, X.; Wen, Y.; Zhang, S.; Zhang, W.; Chen, Y.; Shen, Y.; Lemieux, M.J.; Campbell, R.E. Photocleavable proteins that undergo fast and efficient dissociation. Chem. Sci. 2021, 12, 9658–9672. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C.R. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat. Methods 2007, 4, 619–6289. [Google Scholar] [CrossRef] [PubMed]
- Curley, K.; Lawrence, D.S. Light-activated proteins. Curr. Opin. Chem. Biol. 1999, 3, 84–88. [Google Scholar] [CrossRef]
- Lawrence, D.S. The preparation and in vivo applications of caged peptides and proteins. Curr. Opin. Chem. Biol. 2005, 9, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Ellis-Davies, G.C.; Nemoto, T.; Miyashita, Y.; Iino, M.; Kasai, H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001, 4, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Marriott, G. Caged protein conjugates and light-directed generation of protein activity: Preparation, photoactivation, and spectroscopic characterization of caged G-actin conjugates. Biochemistry 1994, 33, 9092–9097. [Google Scholar] [CrossRef]
- Thompson, S.; Spoors, J.A.; Fawcett, M.-C.; Self, C.H. Photocleavable nitrobenzyl–protein conjugates. Biochem. Biophys. Res. Commun. 1994, 201, 1213–1219. [Google Scholar] [CrossRef]
- Self, C.H.; Thompson, S. Light activatable antibodies: Models for remotely activatable proteins. Nat. Med. 1996, 2, 817–820. [Google Scholar] [CrossRef]
- Ottl, J.; Gabriel, D.; Marriott, G. Preparation and photoactivation of caged fluorophores and caged proteins using a new class of heterobifunctional, photocleavable cross-linking reagents. Bioconj. Chem. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- Bédouet, L.; Adenier, H.; Pulvin, S.; Bedel-Cloutour, C.; Thomas, D. Recovery of the oxidative activity of caged bovine haemoglobin after UV photolysis. Biochem. Biophys. Res. Commun. 2004, 320, 939–944. [Google Scholar] [CrossRef]
- Marriot, G.; Heidecker, M. Light-directed generation of the actin activated ATPase activity of caged heavy meromysin. Biochemistry 1996, 35, 3170–3174. [Google Scholar] [CrossRef]
- Golan, R.; Zehavi, U.; Naim, M.; Patchornik, A.; Smirnoff, P.; Inhibition of, E. coli β-galactosidase by 2-nitro-1-(4,5-dimethoxy-2-nitrophenyl)ethyl, a photo-reversible thiol label. Biochim Biophys Acta 1996, 1293, 238–242. [Google Scholar] [CrossRef]
- Zou, K.; Cheley, S.; Givens, R.S.; Bayley, H. Catalytic subunit of protein kinase A caged at the activating phosphothreonine. J. Am. Chem. Soc. 2002, 124, 8220–8229. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Ichetovkin, I.; Song, X.; Condeelis, J.S.; Lawrence, D.S. A new strategy for caging proteins regulated by kinases. J. Am. Chem. Soc. 2002, 124, 2440–2441. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Niblack, B.; Walker, B.; Bayley, H. A photogenerated pore-forming protein. Chem. Biol. 1995, 2, 391–400. [Google Scholar] [CrossRef]
- Mendel, D.; Eilman, J.A.; Schultz, P.G. Construction of a light-activated protein by unnatural amino acid mutagenesis. J. Am. Chem. Soc. 1991, 113, 2758–2760. [Google Scholar] [CrossRef]
- Cook, S.N.; Jack, W.E.; Xiong, X.; Danley, L.E.; Ellman, J.A.; Schultz, P.G.; Noren, C.J. Photochemically lnitiated Protein Splicing. Angew. Chem. Int. Ed. 1995, 34, 1629–1630. [Google Scholar] [CrossRef]
- England, P.M.; Lester, H.A.; Davidson, N.; Dougherty, D.A. Site-specific, photochemical proteolysis applied to ion channels in vivo. Proc. Natl. Acad. Soc. USA 1997, 94, 11025–11030. [Google Scholar] [CrossRef]
- Miller, J.C.; Silverman, S.K.; England, P.M.; Dougherty, D.A.; Lester, H.A. Flash decaging of tyrosine sidechains in an ion channel. Neuron 1998, 20, 619–624. [Google Scholar] [CrossRef][Green Version]
- Short, G.F.; Lodder, M.; Laikhter, A.L.; Arslan, T.; Hecht, S.M. Caged HIV-1 protease: Dimerization is independent of the ionization state of the active site aspartates. J. Am. Chem. Soc. 1999, 121, 478–479. [Google Scholar] [CrossRef]
- Tong, Y.; Brandt, G.S.; Li, M.; Shapovalov, G.; Slimko, E.; Karschin, A.; Dougherty, D.A.; Lester, H.A. Tyrosine decaging leads to substantial membrane trafficking during modulation of an inward rectifier potassium channel. J. Gen. Physiol. 2001, 117, 103–118. [Google Scholar] [CrossRef]
- Petersson, E.J.; Brandt, G.S.; Zacharias, N.M.; Dougherty, D.A.; Lester, H.A. Caging proteins through unnatural amino acid mutagenesis. Methods Enzymol. 2003, 360, 258–273. [Google Scholar]
- Wu, N.; Deiters, A.; Cropp, T.A.; King, D.; Schultz, P.G. A genetically encoded photocaged amino acid. J. Am. Chem. Soc. 2004, 126, 14306–14307. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Nakayama, K.; Kaida, Y.; Majima, T. Design and synthesis of photochemically controllable caspase-3. Angew. Chem. Int. Ed. 2004, 116, 5761–5763. [Google Scholar] [CrossRef]
- Endo, M.; Nakayama, K.; Majima, T. Design and synthesis of photochemically controllable restriction endonuclease BamHI by manipulating the salt-bridge network in the dimer interface. J. Org. Chem. 2004, 69, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Cornish, V.W.; Mendel, D.; Schultz, P.G. Probing protein structure and function with an expanded genetic code. Angew Chem Int Ed 1995, 34, 621–633. [Google Scholar] [CrossRef]
- Rothman, D.M.; Petersson, E.J.; Vázquez, M.E.; Brandt, G.S.; Dougherty, D.A.; Imperiali, B. Caged phosphoproteins. J. Am. Chem. Soc. 2005, 127, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ji, A.; Ai, H. Caging proteins through unnatural amino acid mutagenesis. J. Am. Chem. Soc. 2015, 137, 2155–2158. [Google Scholar] [CrossRef]
- Edwards, W.F.; Young, D.D.; Deiters, A. Light-activated cre recombinase as a tool for the spatial and temporal control of gene function in mammalian cells. ACS. Chem. Biol. 2009, 4, 441–445. [Google Scholar] [CrossRef]
- Gautier, A.; Nguyen, D.P.; Lusic, H.; An, W.; Deiters, A.; Chin, J.W. Genetically encoded photocontrol of protein localization in mammalian cells. J. Am. Chem. Soc. 2010, 132, 4086–4088. [Google Scholar] [CrossRef]
- Arbely, E.; Torres-Kolbus, J.; Deiters, A.; Chin, J.W. Photocontrol of tyrosine phosphorylation in mammalian cells via genetic encoding of photocaged tyrosine. J. Am. Chem. Soc. 2012, 134, 11912–11915. [Google Scholar] [CrossRef]
- Lemke, E.A.; Summerer, D.; Geierstanger, B.H.; Brittain, S.M.; Schultz, P.G. Control of protein phosphorylation with a genetically encoded photocaged amino acid. Nat. Chem. Biol. 2007, 3, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.S.; Elsässer, S.J.; Mahesh, M.; Bachman, M.; Balasubramanian, S.; Chin, J.W. Photoactivation of mutant isocitrate dehydrogenase 2 reveals rapid cancer-associated metabolic and epigenetic changes. J. Am. Chem. Soc. 2016, 138, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.; Borchardt, E.K.; Brown, K.; Asokan, A.; Deiters, A. Optical Control of CRISPR/Cas9 Gene Editing. J. Am. Chem. Soc. 2015, 137, 5642–5645. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, T.; Hamachi, I. Caged RNase: Photoactivation of the enzyme from perfect off-state by site-specific incorporation of 2-nitrobenzyl moiety. Bioorg. Med. Chem. Lett. 2003, 13, 13–15. [Google Scholar] [CrossRef]
- Pellois, J.P.; Hahn, M.E.; Muir, T.W. Simultaneous triggering of protein activity and fluorescence. J. Am. Chem. Soc. 2004, 126, 7170–7171. [Google Scholar] [CrossRef]
- Hahn, M.E.; Muir, T.W. Photocontrol of Smad2, a multiphosphorylated cell-signaling protein, through caging of activating phosphoserines. Angew. Chem. Int. Ed. 2004, 43, 5800–5803. [Google Scholar] [CrossRef]
- Cotton, G.J.; Muir, T.W. Peptide ligation and its application to protein engineering. Chem. Biol. 1999, 6, R247–R256. [Google Scholar] [CrossRef]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B. Synthesis of proteins by native chemical ligation. Science 1999, 266, 776–778. [Google Scholar] [CrossRef]
- Tang, S.; Wan, Z.; Gao, Y.; Zheng, J.S.; Wang, J.; Si, Y.Y.; Chen, X.; Qi, H.; Liu, L.; Liu, W. Total chemical synthesis of photoactivatable proteins for light-controlled manipulation of antigen–antibody interactions. Chem. Sci. 2016, 7, 1891–1895. [Google Scholar] [CrossRef]
- Curley, K.; Lawrence, D.S. Photoactivation of a signal transduction pathway in living cells. J. Am. Chem. Soc. 1998, 120, 8573–8574. [Google Scholar] [CrossRef]
- Loudwig, S.; Nicolet, Y.; Masson, P.; Fontecilla-Camps, J.C.; Bon, S.; Nachon, F.; Goeldner, M. Photoreversible inhibition of cholinesterases: Catalytic serine-labeled caged butyrylcholinesterase. ChemBioChem 2003, 4, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, S.; Miyagawa, M.; Takaoka, Y.; Tamura, T.; Hamachi, I. Ligand-directed tosyl chemistry for protein labeling in vivo. Nat. Commun. 2008, 5, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, S.; Yasui, R.; Miki, T.; Ojida, A.; Hamachi, I. Ligand-directed acyl imidazole chemistry for labeling of membrane-bound proteins on live cells. J. Am. Chem. Soc. 2012, 134, 3961–3964. [Google Scholar] [CrossRef]
- Tamura, T.; Ueda, T.; Goto, T.; Tsukidate, T.; Shapira, Y.; Nishikawa, Y.; Fujisawa, A.; Hamachi, I. Rapid labelling and covalent inhibition of intracellular native proteins using ligand-directed N-acyl-N-alkyl sulfonamide. Nat. Commun. 2018, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Kioi, Y.; Yasui, R.; Takaoka, Y.; Miki, T.; Fujishima, S.; Hamachi, I. One-step construction of caged carbonic anhydrase I using a ligand-directed acyl imidazole-based protein labeling method. Chem. Sci. 2013, 4, 2573–2580. [Google Scholar] [CrossRef]
- Takamori, S.; Yamaguchi, S.; Ohashi, N.; Nagamune, T. Sterically bulky caging for light- inducible protein activation. Chem. Commun. 2013, 49, 3013–3015. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Cheng, Y.; Nakajima, S.; Furuta, T.; Nagamune, T. Light-activated gene expression from site-specific caged DNA with a biotinylated photolabile protection group. Chem. Commun. 2010, 46, 2244–2246. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Takamori, S.; Yamamoto, K.; Ishiwatari, A.; Minamihata, K.; Yamada, E.; Okamoto, A.; Nagamune, T. Sterically bulky caging of transferrin for photoactivatable intracellular delivery. Bioconjugate Chem. 2021, 32, 1535–1540. [Google Scholar] [CrossRef]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef]
- Clair, N.L.; Navia, M.A. Cross-linked enzyme crystals as robust biocatalysts. J. Am. Chem. Soc. 1992, 114, 7314–7316. [Google Scholar] [CrossRef]
- Schmidt, S.R. Protein bodies in nature and biotechnology. Mol. Biotechnol. 2013, 54, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Luft, J.C.; Tian, S.; Owens, G., Jr.; Pandya, A.A.; Berglund, P.; Pohlhaus, P.; Maynor, B.W.; Smith, J.; et al. Rendering protein-based particles transiently insoluble for therapeutic applications. J. Am. Chem. Soc. 2012, 134, 8774–8777. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, A.; Yamaguchi, S.; Takamori, S.; Yamahira, S.; Minamihata, K.; Nagamune, T. Photolytic protein aggregates: Versatile materials for controlled release of active proteins. Adv. Health Mater. 2016, 5, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).