Abstract
The formation of ice can be very detrimental to flight safety, since the ice accumulated on the surfaces of the aircraft can alter both the aerodynamics and the weight, leading in some cases to catastrophic stall situations. To date, only active Ice Protection Systems (IPS), which require energy to work, are being employed. The use of passive coatings able to prevent, delay, or reduce ice accretion in real flight icing conditions can be viewed as a valuable instrument to reduce the environmental footprint of aircraft. The majority of work in the literature focuses on testing superhydrophobic coatings at a speed equal to or lower than 50 m/s or rather in combination with an active system. The present study was aimed at understanding the effectiveness of two superhydrophobic coatings applied on two NACA0015 wing profiles in reducing the ice formation in relevant flight icing conditions, through tests carried out in an Icing Wind Tunnel at 50 and 95 m/s and at temperatures ranging between −3 and −23 °C. Results demonstrated that at temperatures higher than −12 °C, at both 50 and 95 m/s, with exposure time ranging between 72 and 137 s, the developed coatings can be helpful in reducing the ice accretion by 12 to 100%.
1. Introduction
Aircraft icing has been widely recognized as a severe weather hazard to flight safety in cold climates [1], since the ice accretion on aircraft surfaces alters the flight aerodynamics, reducing lift and increasing weight and drag, thus leading to dangerous stall conditions with a temporary or permanent loss of control of the aircraft.
Currently, to hinder the ice accretion dangers, active Ice Protection Systems (IPS) requiring energy are being employed, either to prevent icing (anti-icing) or to remove ice once it has been formed (de-icing). The use of active IPS, which can be thermal, electro-mechanic [2], electro-thermal [3], pneumatic, or a glycol-based fluid type [4], implicates an increase in construction complexity, weight, manufacturing, and management costs, an increase of onboard power consumption, and then an increase of CO2 emissions [5].
It would be highly desirable and advantageous if surfaces could passively reduce or delay the ice formation and facilitate ice removal. While active methods rely on energy input from an external system for the anti-/de-icing operation, passive methods take advantage of the physical properties of the airframe surfaces (e.g., surface wettability) to prevent, delay, or reduce ice formation and accretion [1], and avoid/reduce the run-back of ice [6].
In this contest, superhydrophobic coatings owing to their extraordinary water repellency and no requirement for additional energy consumption can be viewed as excellent candidates for icephobicity in this area [7,8].
However, whether or not superhydrophobicity implies icephobicity, is a debated topic [9,10], and, generally, it is a misunderstanding to believe that to design a surface with high contact angles should consequently lead to an icephobic surface [9].
Cao et al. [11] found that the anti-icing capability of surfaces depends not only on their superhydrophobicity but also on the surface morphology; Varanasi et al. [12] found that icephobic properties of superhydrophobic surfaces can be compromised in the case of frost formation, so under high humidity, the surface turns into a hydrophilic surface; Chen et al. [13] found that the superhydrophobic surfaces cannot reduce the ice adhesion, and the ice adhesion strength on superhydrophobic surfaces is comparable to that observed for superhydrophilic surfaces, because of the mechanical interlocking between the ice and the surface texture.
During a flight, ice accretion is usually due to supercooled water droplets impinging on aircraft surfaces, and the icephobicity of a surface not only depends on intrinsic surface properties but also on the ice formation conditions [5].
Therefore, it would be highly recommended and helpful to assess the icephobicity of these coatings through a test conducted at aircraft cruise velocities and in relevant icing conditions. However, most studies use droplets’ impact velocities below 10 m/s [14,15] or freezing conditions not in line with typical aircraft icing conditions [13,16].
To the best of our knowledge, only in a few studies of superhydrophobic surfaces were tested in an Icing Wind Tunnel (IWT) at relevant speed, i.e., 50 m/s or higher, and by generating both rime and glaze ice [17,18,19]. Other studies were focused on the effect of hydrophobic/superhydrophobic coatings on the reduction of power supply needed to keep the aircraft surfaces ice-free when combined with active Ice Protection Systems (IPS) [6,9,20,21,22]. Table 1 represents a short review of these studies, performed to the best of our knowledge.

Table 1.
Review of Icing Wind Tunnel (IWT) test conditions performed on hydrophobic and superhydrophobic coatings and the main results.
Table 1 highlights that IWT tests at a speed higher than 50 m/s were carried out only by combining coatings with active IPS [20].
In this scenario, the present work aims to test at 50 and 95 m/s and at temperatures ranging between −3 and −23 °C, two superhydrophobic coatings applied on two NACA0015 wing profiles, without any active IPS. Selected IWT test conditions take into account common performances of General Aviation, CS-23, and CS-25 categories [23]. Ice accretion was assessed by measuring the ice thickness at the stagnation region and the extension length of the compact ice accreted along the airfoils.
The wetting properties of surfaces were studied by static contact angles, surface free energy, and work of adhesion measurements. The surface morphology of the coating’s surfaces was observed by optical and scanning electron microscopy, and the roughness was opportunely measured. Cutting and a tape test and adhesion test at 23 and −30 °C were performed in order to assess the durability of these coatings, especially at low temperatures.
2. Materials and Methods
Two nanostructured coatings (hereafter labeled as 1 and 2) [24] were applied with an aerograph with a layer-by-layer process, as a usual paint, on three different substrates: (1) 5 × 5 cm × cm flat composite samples made of carbon fiber and epoxy resin painted with a commercial aeronautical paint purchased from Akzo-Nobel (labeled as C); (2) 5 × 5 cm × cm flat samples in stainless steel (hereafter labeled as S) pretreated with a sandpaper P40 to increase the roughness surface and improve the coating’s adhesion, and; (3) two wing profiles NACA0015 manufactured with a 3D printing machine, having a length of 135 mm and a chord of 100 mm made of acrylonitrile butadiene styrene (hereafter labeled as A). Samples were named as: X-i with X = Substrate, which is C, S, or A, and I = Surface, namely, R for Reference, and 1 or 2 for coatings. Hence AR, CR, and SR are the references, whereas A1, A2, C1, C2, S1, and S2 are the coated samples.
Unlike samples C and S, sample A had one-half painted with coatings, leaving the other half uncoated as a reference to better compare the coating and reference.
The roughness of substrates before and after the application of the coating was measured by employing a SAMA SA6260 surface roughness meter. Roughness measurements, performed according to the ISO 4288 [25], were reported as Ra, which represents the arithmetic average of the absolute values of the profile height deviations from the mean line.
The optical images were acquired with a microscope USB Dino-Lite AM4815ZTL at 140×.
The contact angle (CA) measurements were performed at 23 °C in compliance with the ASTM D7490–13 [26] standard, with 3 µL of water (H2O) and diiodomethane (CH2I2), by acquiring at least six measurements. Contact angles were rapidly acquired, within 30 s of depositing the drop, to avoid changes in angles. The mean values and standard deviations of CA were the input data of a Matlab tool (a CIRA home-made tool) which solves the two equations (1), derived by the application of the Owens-Wendt-Kaelble method [27,28] for the assessment of the solid surface free energy (SFE). Due to the stochastic nature of the CA experimental measurements, a third liquid was introduced, i.e., formamide (HCONH2) [29,30]. The software uses the third liquid to minimize the error on the third Equations (2) and (3), giving as output the optimized values of the CA, along with an assessment of polar and dispersive components of SFE, which is useful to calculate the SFE through Equation (4), the work of adhesion (WA) through Equation (5), and the surface polarity (SP) through Equation (6).
Here are the polar and dispersive components of the liquid i, and are the polar and dispersive components of the solid, respectively, and θi is the CAs measured with the liquid i.
Sample morphology and coatings’ thickness were acquired using a field emission scanning electron microscope (SEM) Tescan Mira3 (Tescan Orsay Holding, Brno-Kohoutovice, Czech). Samples were observed in the top section after metallization.
Cutting and tape tests were carried out according to the ASTM D 3359-09 standard [31] by making a grid incision with a specific cutter on the coated samples and then applying adhesive tape to cover the cut area. The test adhesive was vigorously removed and the involved area was inspected. According to the standard, the test passed if the area involved had detached flakes of coating at intersections less than 15%.
The adhesion tests were carried out according to the ASTM C633 [32]. The test consisted of coating one face of a substrate fixture, bonding this coating to the face of a loading fixture, and subjecting this assembly of coating and fixtures to a tensile load normal to the plane of the coating. The tensile load to the assembly of the coating and fixtures should not permit eccentric load or bending moment to the specimen. A cyanoacrylate was employed as adhesive. Adhesion or cohesion strength was calculated as Maximum load/cross-sectional area. The adhesion strength of the coating was given a failure if rupture was entirely over the coating-substrate interface. The cohesion strength of the coating was validated if rupture was only within the coating. This test was performed at room temperature and at −30 °C.
Icing wind tunnel (IWT) tests were performed in the CIRA IWT facility, which is a closed-loop circuit, refrigerated wind tunnel with three interchangeable test sections and one open jet configuration, whose main mission is to perform icing tests. The cloud generation for icing conditions simulation is carried out by the spray bar system, which is able to generate water droplets with diameters (median volumetric diameter—MVD) and concentrations (liquid water content—LWC) covering nearly all the envelope as prescribed by the CS-25 Appendix C for both continuous and intermittent cloud conditions. Two samples A and two coatings, i.e., 1 and 2, were contemporary tested by dividing each sample into two parts, one of which was coated with a coating of A1 and A2, and the other left uncoated as a reference (AR) (see Figure 1). The two samples were mounted with the two reference sides in opposite positions, as shown in Figure 1b, in order to minimize the effect of a possible cloud inhomogeneity.
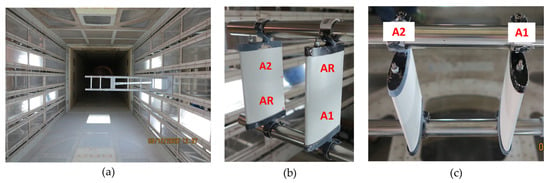
Figure 1.
Different views of samples A mounted in the IWT facility; (a) front view, (b) lateral view, and (c) top view.
To have a three-dimensional shape of the accumulated ice overlapped with the neat airfoil, 3D laser scan reconstructions were performed (Figure 2). Three-dimensional ice measurements and reconstruction were performed using an all-purpose portable measuring arm system integrated with a laser scanner for fast 3D surface data capture (ROMER 8525-7). Innovmetric PolyWorks was employed to get data points, align the ice point clouds to the 3D model of the corresponding “clean” sample, and, finally, generate a polygon mesh.

Figure 2.
3D laser scan reconstructions performed before and after IWT test 6.
3. Results
3.1. Coatings’ Characterization
3.1.1. Roughness
Figure 3 shows the mean values of roughness measured on C and S samples before and after the application of the coatings. The roughness of the SR sample before the sandpaper treatment was 0.39 µm which increased to 1.3 µm after the P40 treatment, as reported in Figure 3. After the application of coating 1 (S1), the roughness did not change significantly, but it did after the application of coating 2 (S2) and achieved a Ra = 3.1 µm. Whereas sample CR had a roughness of 3.7 µm, which did not appreciably change after the application of both coatings since only a slight increase of the roughness could be measured, and only for C1 (from 3.7 to 4.1 µm).
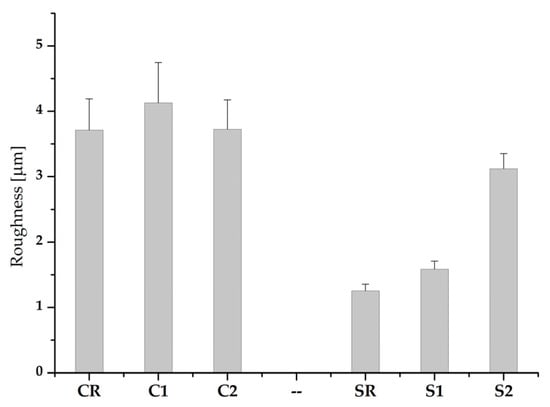
Figure 3.
Roughness mean values Ra for C and S samples, before and after the application of the coatings.
3.1.2. Optical Microscopy
Figure 4 displays the optical images acquired at 140× of magnification of all flat samples’ surfaces, along with the corresponding roughness profiles. It highlights that the surface morphology of the CR sample was homogeneous and it became slightly denser and more compact after the application of the coatings (C1 and C2 in Figure 4b,c). The shape of the roughness profiles confirmed the homogenous succession of peaks and valleys in all C samples. Contrary, the treatment of SR with the sandpaper P40 gave the coarse morphology shown in Figure 4g. As a consequence, in the SR roughness profile (Figure 4l), the valleys appeared larger than the peaks as a footprint of the scratches. Although at this magnification the scratches were still visible and after the application of the coatings (Figure 4h,i), the roughness profiles of coated samples displayed that both coatings partially filled the valleys and created their own roughness with a homogeneous distribution of peaks and valleys (Figure 4m,n).
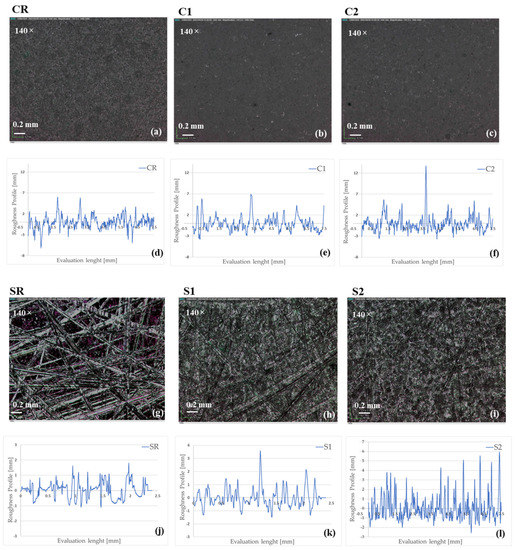
Figure 4.
Optical microscopy images acquired at 140× of CR (a), C1 (b), C2 (c), SR (g), S1 (h), and S2 (i) samples’ surfaces, and the corresponding roughness profiles for CR (d), C1 (e), C2 (f), SR (j), S1 (k), and S2 (l).
3.1.3. Scanning Electron Microscopy
In order to further investigate the morphology at the micro- and nano-scale, SEM images in the top view were acquired at different magnifications (see Figure 5).
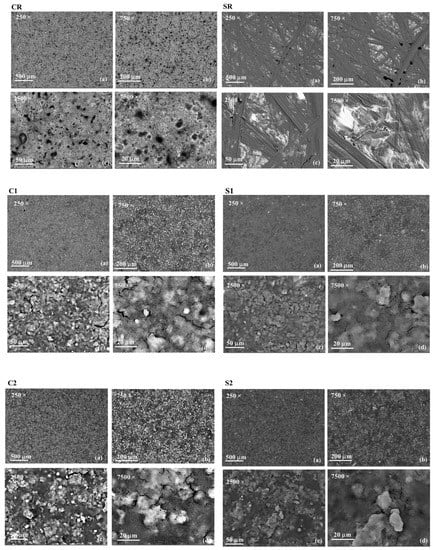
Figure 5.
Top View of SEM images at 250× (a), 750× (b), 2500× (c), and 7500× (d) of CR, SR, C1, S1, C2, and S2 samples.
By comparing the SEM images of C1 and C2 and S1 and S2 samples with CR and SR, respectively, it highlighted that in both cases, the coatings were able to completely cover the substrate morphology, since neither the sponge-like morphology of the commercial paint, characterized by the presence of micropores, nor the scratches of the SR surface were visible once the coatings were applied. Generally speaking, it seemed that the same coating, applied on different substrates generated the same morphology, with the presence of micro-sized aggregates which emerged from the matrix. However, some little differences could be detected. In fact, by comparing C1 and S1, and C2 and S2 samples, it seemed that for both coatings, the aggregation of nanoparticles emerged in the C samples more than it occurred for the S samples. This result could be ascribed to the different surface nature and/or morphology of the substrates. The same results were observed by Yilgor et al. [33] by applying the same layers number of the same coating to different polymeric substrates. In fact, they found different nanoparticles aggregation at the surface depending on the substrate nature (i.e., TPSU, PMMA, PC, and TPU), which could probably be responsible for the slight differences in CA. In Figure 5, it should also be noticed that at the highest magnification, small microcracks and fractures appear, most of all on C1 and C2 surfaces. As reported in [19], during the IWT tests, these fractures on the samples’ surfaces could become ice nucleation sites.
3.1.4. Wettability
Due to the superhydrophobicity of coatings, the CA measurements on 3D samples (A1 and A2) have been impossible to be perform; the water droplets, in fact, do not retain on the surfaces but quickly glide away. Therefore, for A1 and A2 samples, the success of the coatings’ application and then the superhydrophobicity of surfaces was assessed by observing that the water droplets slipped rapidly away from the A1 surface (right side of Figure 6), whereas they adhered to the AR surface (left side of Figure 6). The same behavior was observed for the A2 sample (data not shown for brevity). Instead, Figure 7 shows a representative picture of 3 µL of water droplets on all flat surfaces, i.e., CR, C1, C2, SR, S1, and S2 samples. The CA measurements and the corresponding optimized values are listed in Table 2, whereas the SFE, WA, and SP of coated and reference surfaces are displayed in Figure 8.
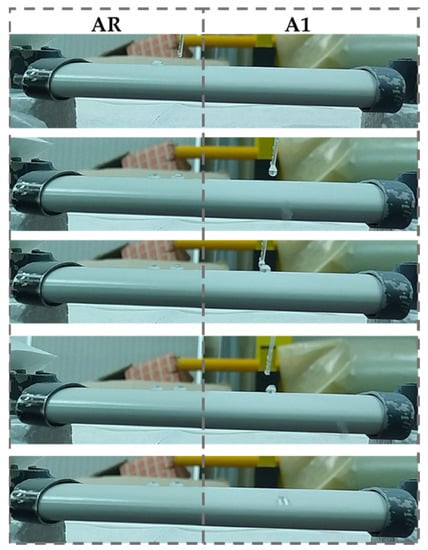
Figure 6.
Images sequences of water droplets dropping on AR (left) and A1 (right) surfaces.

Figure 7.
Water CA measurements on CR, C1, and C2 (top) and SR, S1, and S2 samples (bottom).

Table 2.
Experimentally measured CA; the optimized values are also reported.
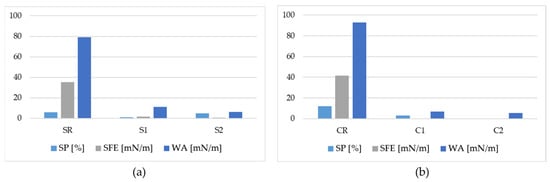
Figure 8.
SFE, WA, and SP of SR, S1, and S2 (a), and of CR, C1, and C2 (b) samples.
As reported in Table 2, all coated surfaces were superhydrophobic. However, some little difference in the wettability of the samples prepared by applying the same coating on different substrates could be noticed. In fact, the CA of S1 and S2 were slightly lower than those measured for C1 and C2, respectively. This could be ascribed to slightly different morphologies observed for C and S samples, also responsible for the different measured roughness (Figure 3). In fact, as reported in Section 3.1.3 and in Figure 5, in the C samples, the micro-aggregates emerge more than occurred for the S samples, thereby improving the roughness (Figure 3) and the Cassie-Baxter state, and reducing the wettability as a consequence. This is in line with that observed by other authors who found such increased contact angles with the increased surface roughness [34].
The SFE of reference samples SR and CR were 35 mN/m (γsP = 2 mN/m and γsD = 33 mN/m) and 42 mN/m (γsP = 5 mN/m and γsD = 36.5 mN/m), respectively, whereas the values of 79 and 93 mN/m were found for the corresponding WA. After the application of the coating, we observed a reduction of 99% in SFE in the C samples (0.3 mN/m for both C1 and C2), and 95–98% for the S samples (1.6 mN/m for S1 and 0.8 mN/m for S2). Accordingly, the WA was reduced by 93–94% for the C samples (6.8 mN/m for C1 and 5.5 mN/m for C2) and by 86–92% for the S samples (11.1 M/m for S1 and 6.3 mN/m for S2). With respect to CR, SP decreased by 75% and 100% in C1 and C2, from 12 to 3 and 0, respectively, whereas it was reduced by 83% and 17% for S1 and S2, from 6 to 1 and 5, respectively, with respect to SR.
3.2. Durability Tests
3.2.1. Cutting and Tape Test
An inspection of surfaces after the cutting and tape test revealed that both coatings passed the test since the area involved in detached flakes of coating at intersections was less than 15% (Figure 9-top). Moreover, after the test, the surfaces remained superhydrophobic as highlighted by the residual low wettability of the surfaces after the test (see pictures of water droplets at the bottom of Figure 9).
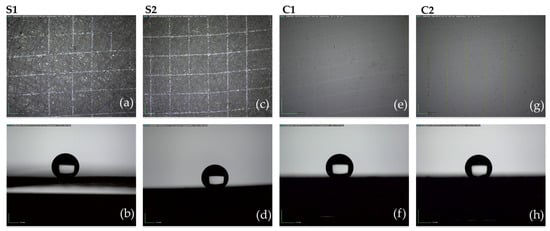
Figure 9.
Cutting and tape test carried out on S1 (a,b), S2 (c,d), C1 (e,f), and C2 (g,h) samples. On the top are the images of surfaces after the test; on the bottom, water droplets on tested surfaces to measure the residual wettability.
3.2.2. Adhesion Tests
Figure 10a shows the Zwick Roll Z010 machine employed to perform the adhesion test. Some pictures of samples before and after the test are also displayed (Figure 10b,c, respectively). Tests performed at room temperature revealed that failure at adhesive-coating interfaces occurred for SR and S1 samples, at an adhesion strength of 8.1 and 9.9 MPa, respectively; whereas, failures at adhesive-substrate interfaces (see the schematic drawing in Figure 11b) occurred for S2 (10.7 MPa), CR (19.9 MPa), C1 (7.2 MPa), and C2 (10.2 MPa), meaning that the adhesion strength of the interface adhesive-coating was higher than the reported strength. At −30 °C, only C samples were tested. It was found that also at low temperatures for CR, C1, and C2 sample failures occurred at the adhesive-substrate interface at adhesion strengths of 2.9 MPa per CR, 2.2 MPa for C1, and 1.8 for C2. So, the adhesion strength of the adhesive-coating interfaces was higher than the measured values. The measured adhesion strength decreased as the temperature decreased, as shown in Figure 11a, which may be due to the more brittleness of adhesive at low temperatures [35]. In Figure 11b, a schematic drawing of the failure at the adhesive-substrate interface is shown for clarity.
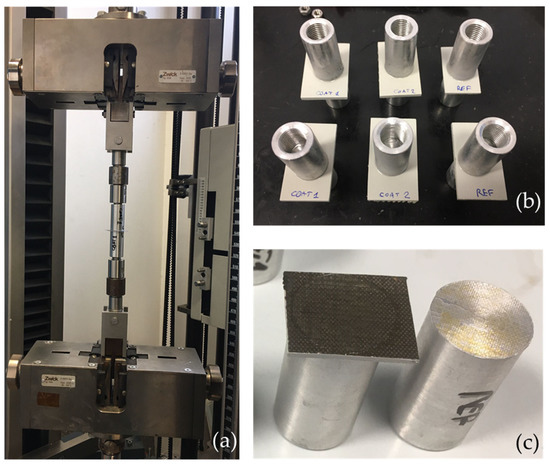
Figure 10.
The Zwick Roll Z010 machine employed to perform the adhesion test (a), C samples before (b) and after the test (c).
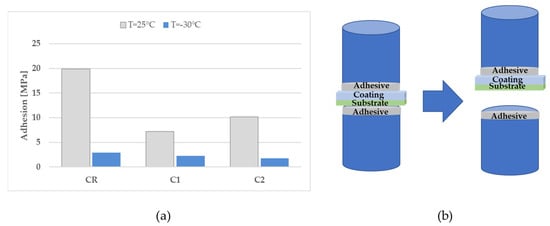
Figure 11.
Adhesion test results performed on C samples at 25 °C and −30 °C (a); schematic draw of most adhesion failures occurred during the testing of C samples (b).
3.3. IWT Tests
Table 3 details the IWT test conditions selected by taking into account common performances of General Aviation, CS-25 (Test 1,2), and CS-23 (Test 4–6) categories [23]. The temperatures investigated ranged from −3 to −23 °C, at an altitude of 3000 m and an LWC of 0.3 g/m3. Two different speeds were considered, i.e., 50 and 95 m/s, so the covered flight path (in clouds) ranged between about 7 and 13 km. Tests 1 referred to the CS-25 vehicles category and investigated the lowest temperatures (−23 °C), the highest speed (95m/s), and the highest LWC (0.5–0.6 g/m3). Test 2 replicated the test 1 conditions, but at sea level (altitude 83 m). Test 3–6 referred to the CS-23 vehicles category, investigating the temperature ranging between −3 and −12 °C, LWC was fixed to 0.3 g/m3 and MVD at 20 µm, and altitude at 3000 m, corresponding to severe icing cloud conditions which can occur at altitudes above 600 m [36]. In tests 3–6, along with the temperature, also the effect of speed was investigated (50 and 95 m/s).

Table 3.
IWT test matrix.
Figure 12 shows the pictures of both samples after each test. The surface of each sample was one-half superhydrophobic (A1 and A2 sites), whereas the other half was left as a reference (AR). First of all, it must be noted that the rime ice generated in tests 1, 2, and 5 (Figure 12a,b,e) covered quite uniformly the two samples, without taking into account the different wettability of the two half-sites of the samples. A deeper analysis of images revealed the presence of isolated frozen droplets along the airfoil, especially for coated surfaces which were evident in test 2 and test 5 (Figure 12b,e).
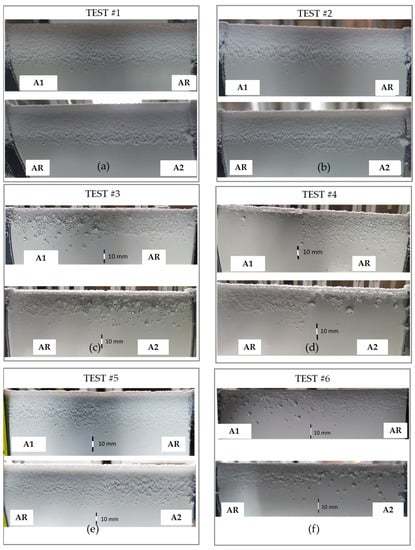
Figure 12.
Pictures of ice accreted on A1, A2, and AR samples during the IWT test 1 (a), test 2 (b), test 3 (c), test 4 (d), test 5 (e), test 6 (f).
On the other hand, in glaze ice conditions, the low wettability of coated surfaces caused a reduction of the accreted ice, as highlighted in tests 3, 4, and 6 (Figure 12c,d,f), with a larger amount of isolated frozen droplets. This result seemed to provide evidence that, especially in glaze ice conditions, the high degree of surfaces’ hydrophobicity caused the droplets to easily roll on the surface, reducing contact duration and thus ice accumulation. Additionally, in both rime and glaze ice, the isolated frozen droplets had a spherical shape, meaning that they preserved the low wettability at low temperatures and in dynamic conditions. The same behavior was observed at −5 °C by Fortin et al. [22] on hydrophobic and superhydrophobic surfaces (CA of 118–152°).
Most of the droplets frozen as isolated ice had dimensions larger than the frozen droplets near the denser ice (Lci in Figure 13a), probably because the droplets slipped on the superhydrophobic surface until they met an isolated iced droplet on which they could grow.
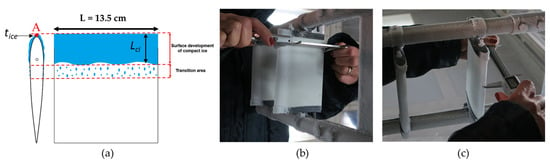
Figure 13.
Schematic drawing of the accreted ice shape. With an angle of attack equal to zero, A represents the stagnation point (a). Measurement of the samples’ chord length after each test (b,c).
Measurements of the accreted ice thickness, tice, and the extension length of the compact ice, Lci, accreted along the airfoils (continuous blue zone in the schematic drawing in Figure 13a), were performed. In detail, for the measurements of tice, seven marks were drawn on each airfoil at a 2 cm distance, and tice was measured as the difference in the chord length before and after the IWT tests (see pictures in Figure 13b,c).
Results, displayed in Figure 14a,b, show that in rime ice conditions, the tice measured on A1 and A2 was similar to or higher than those measured on AR; whereas in glaze ice conditions, in test 3 and test 6, the tice measured on A1 was reduced by −12 and −34%, respectively, with respect to the AR site; lower differences were observed in all other cases. This was because, at the stagnation point (“A” in Figure 13a for an attack angle equal to zero), the ice accretes in spite of the surface properties since the local velocity of the airflow there is zero. For the A1 surface in test 4, in fact, the ice accreted only at the stagnation point.
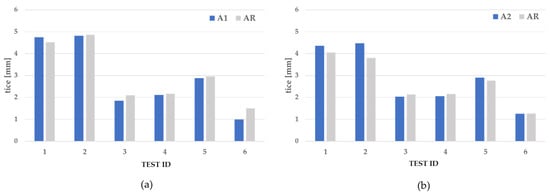
Figure 14.
Accreted ice thickness tice on A1 and AR (a), and on A2, and AR (b), during the IWT tests.
The extension length of the compact ice Lci was measured after each test and compared to the corresponding reference. Values were subtracted of the corresponding tice and reported in Figure 15.
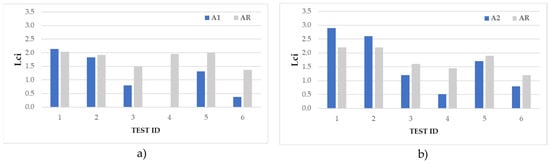
Figure 15.
Length of the compact ice Lci accreted from the leading edge alongside the airfoils A1 and AR (a), and A2 and AR (b) during the IWT tests.
Results show that in rime ice conditions (test 1 and test 2), the ice accreted along the coated airfoil generally increased with respect to the reference. In detail, Lci measured on the A1 site in test 1 and test 2 was +5% and −5%, respectively, compared to those measured on the AR. In both tests, Lci on the A2 site was higher than the AR (+39 and +22%, respectively).
A different scenario could be observed in glaze ice conditions (tests 3, 4, and 6) and in test 5, for which Lci measured on A1 and A2 was considerably lower than those measured on the AR (from −12 to −100%). By supposing that in all cases the high hydrophobicity of the surface caused the droplets to easily roll on the surface, these results seemed to give evidence that the lower the temperature, the faster the freezing of droplets. As a consequence, in test 1 and test 2, the droplets froze before rolling away, increasing Lci. Whereas in tests 3–6, the droplets slipped away, froze as isolated droplets, or did not freeze at all.
Hence, as the temperature increased, the effect of the superhydrophobicity increased and the difference of Lci (ΔLci) measured on coated and reference surfaces became larger (Figure 16).
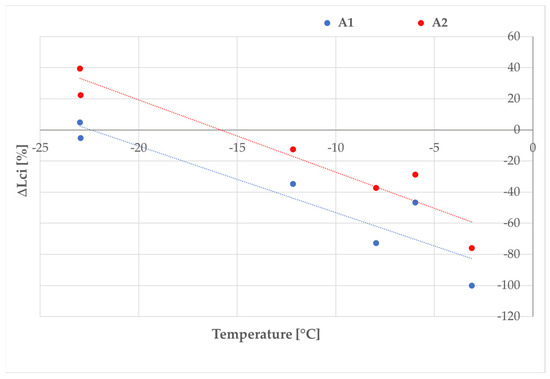
Figure 16.
Percentage variation in Lci of coated surfaces with respect to the references as a function of the tests’ temperature.
The superhydrophobicity of A1 and A2 was preserved after the IWT test campaign.
The 3D scan acquired after test 6 was overlapped with the neat airfoil 3D reconstruction (Figure 17). In the inset (Figure 17d,e), a magnification of the airfoils with an assessment of the lengths of the compact ice Lci accumulated starting from the leading edge was also reported. These values were in good agreement with those measured and reported in Figure 15, and listed in Table 4. The comparison was made by adding the thickness of the ice accumulated at the stagnation point.
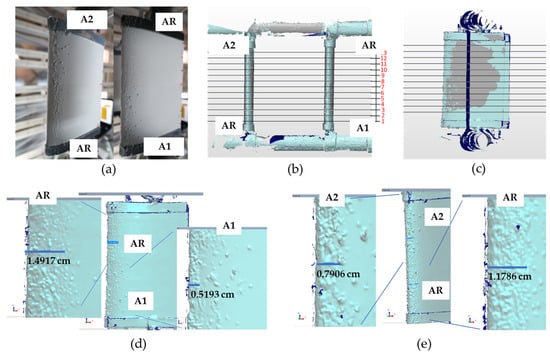
Figure 17.
(a) Pictures of samples after test 6; (b,c) front and side view of slices where the ice contour was extracted; (d,e) 3D scan of A1 and A2 samples after test 6.

Table 4.
Comparison between Lci values measured experimentally and through 3D scan (test 6).
4. Discussion and Conclusions
To be an anti-icing material, the hydrophobic/superhydrophobic coatings must significantly prevent, delay, or reduce the ice accretion in real flight icing conditions. The majority of work in the literature (see Table 1) focuses on testing superhydrophobic coatings at a speed equal to or lower than 50 m/s or rather in combination with an active system.
The present study was aimed at understanding the effectiveness of superhydrophobic coatings to reduce ice formation in relevant flight icing conditions, at 50 and 95 m/s and at temperatures ranging between −3 and −23 °C. Two superhydrophobic coatings generated as a combination of roughness and low SFE were applied on two NACA0015 wing profiles and tested in an IWT without any active IPS.
First, we observed the morphology of surfaces and measured their roughness and wettability; then, we evaluated the durability of these surfaces; finally, we tested them in an IWT. We found that at −23 °C and 95 m/s, with an exposure time of 77–79 s, the application of superhydrophobic coatings seems even detrimental, causing an increase of accreted ice on both the stagnation region (−1–+17%) and along the airfoils (−5–+39%). Whereas, at 95 m/s, but in glaze conditions, i.e., −6 and −8 °C, both superhydrophobic coatings achieved lower ice accretion at both the stagnation region (−2–−34%) and along the airfoils (−29–−73%) for an exposure time of 72–137 s. At 50 m/s for a longer exposure time (227–228 s) and at temperatures of −3 and −12 °C, the accreted ice was reduced mainly along the airfoils (by −12–−100%), whereas no differences can be observed at the stagnation region (−5–+5%), probably due to the higher exposure time.
These findings suggest that the effectiveness of these coatings in reducing the ice accretion largely depends on the tested conditions, so they can be highly helpful for a short exposure time, also at 95 m/s, and mainly in glaze ice conditions. Instead, for use in a wide range of flight icing conditions, both rime and glaze, and long exposure time, they must be considered as a support to an active system.
5. Patents
Data reported in this manuscript refer to coatings of a formulation that is protected by the patent submission: Piscitelli, F. Italian Patent Application N. IT102021000032444, “Rivestimento superidrofobico e ghiacciofobico di un substrato, metodo per il suo ottenimento e substrato così rivestito”, 23 December 2021.
Funding
This research was funded by The Italian Ministry for Education, University and Research (MIUR) through the National Aerospace Research Program (PRORA) D.M. 305/98 art. 4 comma 1 as SMOS (SMart On-board Systems) project.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
The author would like to thank Lorenzo Notarnicola for his support, and Floriana Albano, Antonio Auletta, Antonio Ragni, Marco Schettino and all other CIRA IWT colleagues for the IWT test execution and the interesting discussions about IWT results. The author would like to thank especially Gianpaolo Elia for the 3D reconstruction of accreted ice and Francesco Marra for the execution of the adhesion test and for SEM acquisitions.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ma, L.; Zhang, Z.; Gao, L.; Liu, Y.; Hui, H. Bio-Inspired Icephobic Coatings for Aircraft Icing Mitigation: A Critical Review. Rev. Adhes. Adhes. 2020, 8, 168–198. [Google Scholar] [CrossRef]
- Schulz, M.; Sinapius, M. Evaluation of Different Ice Adhesion Tests for Mechanical Deicing Systems; SAE Technical Paper; SAE: Warrendale, PA, USA, 2015. [Google Scholar]
- Zhao, Z.; Chen, H.; Liu, X.; Liu, H.; Zhang, D. Development of high-efficient synthetic electric heating coating for anti-icing/de-icing. Surf. Coat. Technol. 2018, 349, 340–346. [Google Scholar] [CrossRef]
- Yao, X.; Chen, B.; Morelle, X.P.; Suo, Z. Anti-icing propylene-glycol materials. Extrem. Mech. Lett. 2021, 44, 101225. [Google Scholar] [CrossRef]
- Huang, X.; Tepylo, N.; Pommier-Budinger, V.; Budinger, M.; Bonaccurso, E.; Villedieu, P.; Bennani, L. A survey of icephobic coatings and their potential use in a hybrid coating/active ice protection system for aerospace applications. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef] [Green Version]
- Antonini, C.; Innocenti, M.; Horn, T.; Marengo, M.; Amirfazli, A. Understanding the effect of superhydrophobic coatings on energy reduction in anti-icing systems. Cold Reg. Sci. Technol. 2011, 67, 58–67. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-Inspired Strategies for Anti-Icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- Hejazi, V.; Sobolev, K.; Nosonovsky, M. From superhydrophobicity to icephobicity: Forces and interaction analysis. Sci. Rep. 2013, 3, 2194. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing Superhydrophobic Coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; He, M.; Li, K.; Cui, D.; Zhang, Q.; Zeng, X.; Zhang, Y.; Wang, J.; Song, Y. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Kulinich, S.; Farzaneh, M. On ice-releasing properties of rough hydrophobic coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic Surfaces: Are They Really Ice-Repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Yeong, Y.H.; Sokhey, J.; Loth, E. Ice Adhesion on Superhydrophobic Coatings in an Icing Wind Tunnel. In Contamination Mitigating Polymeric Coatings for Extreme Environments; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Mora, J.; García, P.; Muelas, R.; Agüero, A. Hard Quasicrystalline Coatings Deposited by HVOF Thermal Spray to Reduce Ice Accretion in Aero-Structures Components. Coatings 2020, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- Rivero, P.J.; Rodriguez, R.J.; Larumbe, S.; Monteserín, M.; Martín, F.; García, A.; Acosta, C.; Clemente, M.J.; García, P.; Mora, J.; et al. Evaluation of Functionalized Coatings for the Prevention of Ice Accretion by Using Icing Wind Tunnel Tests. Coatings 2020, 10, 636. [Google Scholar] [CrossRef]
- Morita, K.; Kimura, S.; Sakaue, H. Hybrid System Combining Ice-Phobic Coating and Electrothermal Heating for Wing Ice Protection. Aerospace 2020, 7, 102. [Google Scholar] [CrossRef]
- Villeneuve, E.; Blackburn, C.; Volat, C. Design and Development of an Experimental Setup of Electrically Powered Spinning Rotor Blades in Icing Wind Tunnel and Preliminary Testing with Surface Coatings as Hybrid Protection Solution. Aerospace 2021, 8, 98. [Google Scholar] [CrossRef]
- Fortin, G.; Adomou, M.; Perron, J. Experimental Study of Hybrid Anti-Icing Systems Combining Thermoelectric and Hydro-Phobic Coatings; SAE International: Montreal, QC, Canada, 2011. [Google Scholar]
- EASA. Available online: https://www.easa.europa.eu/document-library/certification-specifications/cs-25-amendment-26-0 (accessed on 22 December 2020).
- Piscitelli, F. Rivestimento Superidrofobico e Ghiacciofobico di un Substrato, Metodo per il Suo Ottenimento e Substrato Così Rivestito. Italian Patent Application N. IT102021000032444, 23 December 2021. [Google Scholar]
- ISO 4288:1996; Geometrical Product Specifications (GPS)–Surface Texture: Profile Method–Rules and Procedures for the As-sessment of Surface Texture. International Organization for Standardization: Geneva, Switzerland, 1996.
- D7490-13; Standard Test Method for Measurement of the Surface Tension of Solid Coatings, Substrates and Pigments using Contact Angle Measurements. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Piscitelli, F.; Chiariello, A.; Dabkowski, D.; Corraro, G.; Marra, F.; Di Palma, L. Superhydrophobic Coatings as Anti-Icing Systems for Small Aircraft. Aerospace 2020, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Piscitelli, F.; Tescione, F.; Mazzola, L.; Bruno, G.; Lavorgna, M. On a simplified method to produce hydrophobic coatings for aeronautical applications. Appl. Surf. Sci. 2019, 472, 71–81. [Google Scholar] [CrossRef]
- ASTM D3359–09; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM: West Conshohocken, PA, USA, 2009.
- ASTM C633-13; Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings. ASTM: West Conshohocken, PA, USA, 2017.
- Yilgor, I.; Bilgin, S.; Isik, M.; Yilgor, E. Facile preparation of superhydrophobic polymer surfaces. Polymer 2012, 53, 1180–1188. [Google Scholar] [CrossRef]
- Shang, H.M.; Wang, Y.; Takahashi, K.; Cao, G.Z.; Li, D.; Xia, Y.N. Nanostructured superhydrophobic surfaces. J. Mater. Sci. 2005, 40, 3587–3591. [Google Scholar] [CrossRef]
- Adams, R.D.; Mallick, V. The Effect of Temperature on the Strength of Adhesively-Bonded Composite-Aluminium Joints. J. Adhes. 1993, 43, 17–33. [Google Scholar] [CrossRef]
- Fortin, G.; Ilinca, A.; Perron, J. A study of Icing Events at Murdochville: Conclusions for the Wind Power Industry. In Proceedings of the Quebec Premier Wind Energy Event, International Conference, Wind Energy Remote Regions, Magdalene Islands, QC, Canada, October 2005. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).