3.1. Changes in Volume
The compatibility of the polymers with the ATFs with respect to volume change was tested initially for the time (168 h) specified in the ASTM D7216-15 standard [
15]. In general, changes in volume depended more on the material type than on the oil used (
Figure 1). The three structural materials (PEEK, PTFE, and PA66) experienced a slight reduction in volume. Although there are no specifications for the compatibility of these materials with ATFs, they seem to be quite reliable when in contact with these oils, according to the measured volume variations, which are around −0.5%.
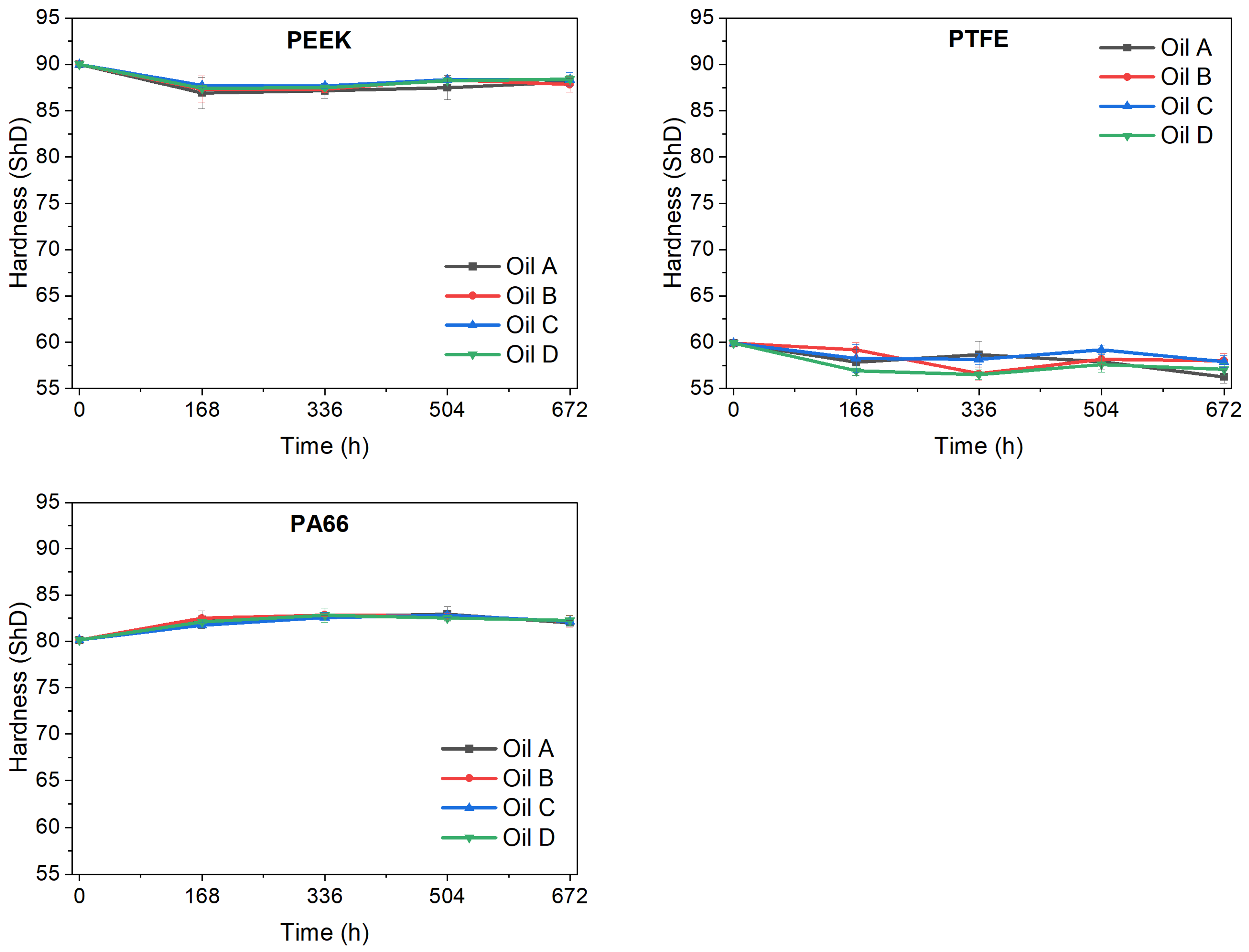
After longer ageing times (from 168 h upwards), PEEK and PA66 hardly changed in volume. The volume variations remained, in general, between 0 and −1%. On the other hand, PTFE showed greater volume variations than its counterparts. Although there are no specifications for these materials when aged in ATFs regarding the admissible changes in volume, it could be concluded that all the materials underwent negligible volume changes. These changes were negligible, not only during the time established in the standardised test, but also for longer time periods.
3.3. Changes in Tensile Strength and Elongation at Break
The stress–strain curves of all materials were obtained before and after their ageing in the ATFs for different periods of time (see
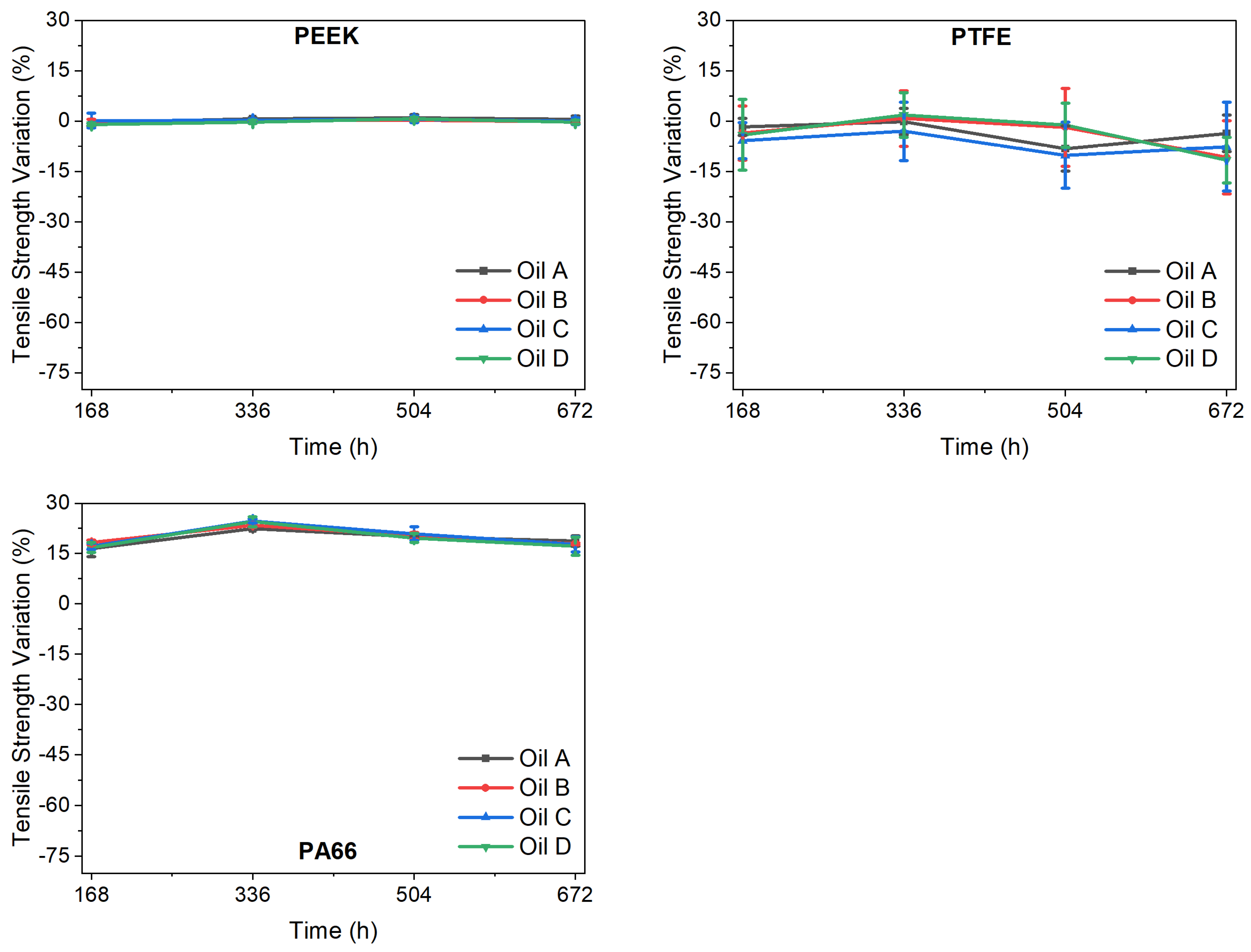
Supplementary Information, Figures S1–S3), and the tensile strength and the elongation at break were obtained from these curves. The polymers had two types of tensile strength behaviour at an aging time of 168 h (
Figure 3), depending on the ATF used. On one hand, the PEEK and PA66 tensile strength variation did not depend on the oil used. On the other hand, PTFE showed mean tensile strength variations of between −1.5% (oil A) and −6% (oil C). Nevertheless, while the tensile strength of PEEK remained constant, regardless of the oil and the ageing time used, this property increased slightly for PA66 and decreased slightly for PTFE.
From 168 h upwards, all materials maintained relatively constant tensile strength values. PEEK and PTFE seem to be compatible with these ATFs, showing low tensile strength variations (null in the case of PEEK), but PA66 should be studied further.
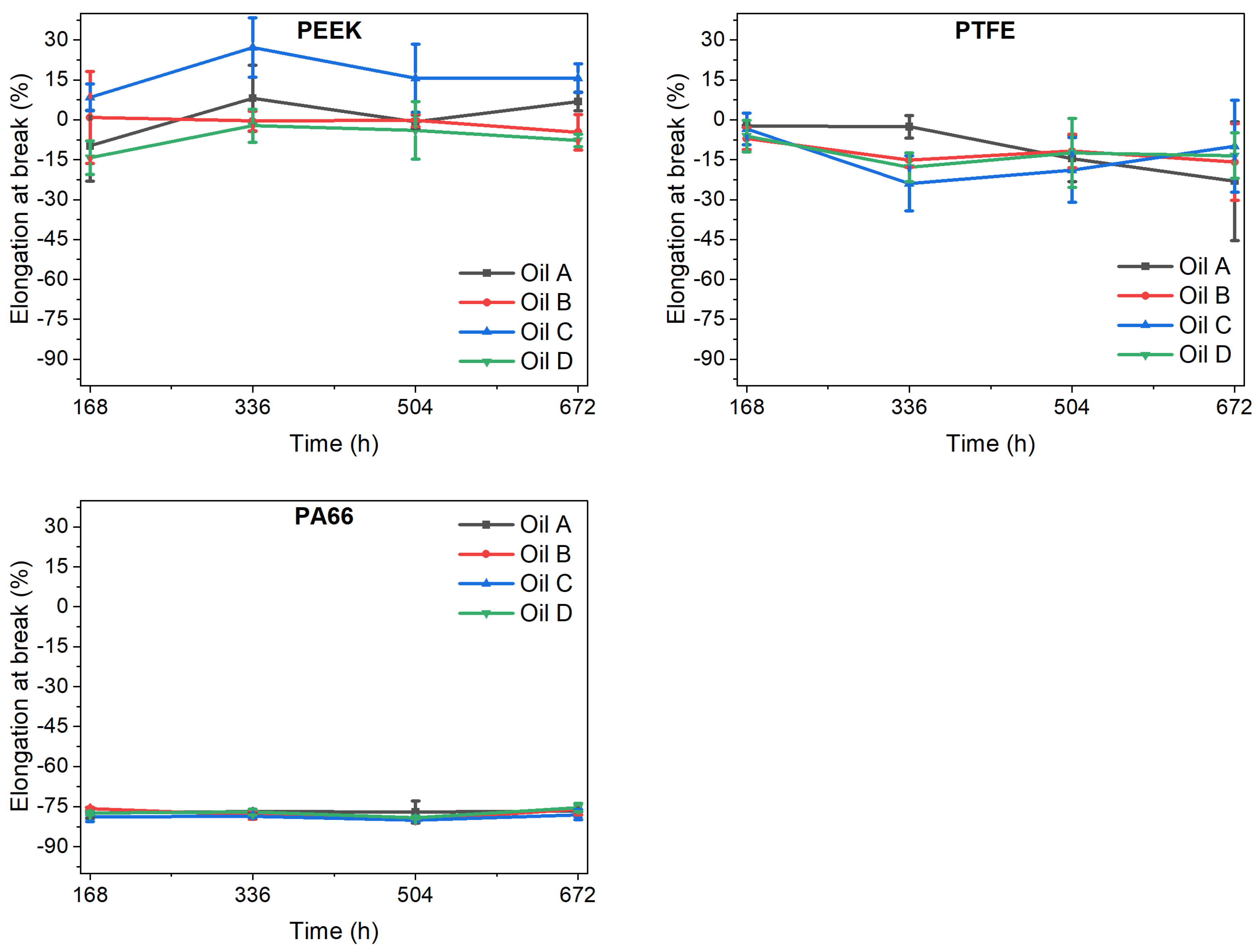
The elongation at break was more affected by the ageing process than was the tensile strength (
Figure 4). In the case of PEEK, variations in elongation at break depended on the ATF used and were between +7% and −15% at 168 h. On the contrary, the PTFE results are aligned with those of tensile strength, with variations in the elongation at break values of between −2 and −7%. Once again, PA66 had contrasting results, with a reduction in elongation at break of around 77%, regardless of the ATF used. This is a clear sign of incompatibility, as the material became less flexible, and thus more fragile.
From 168 h upwards, elongation at break seemed to be more affected by immersion in the ATFs than was the tensile strength, especially in the cases of PEEK and PTFE. PA66 was the only material showing similar elongation at break regardless of the ageing time and oil used, although it showed the greatest reduction in this property. Lann et al. [
13] and Decker et al. [
14] pointed out the tendency of PA66 to crystallize when aged in ATFs, although they concluded that it was the high temperature that caused this effect. According to
Figure 3 and
Figure 4 and
Figure S3, this embrittlement was independent of the type of oil, although in this case the tensile strength increased (15%). On the other hand, PTFE and PEEK showed lower variation in elongation at break than the PA66 and a higher dependency on the oil used.
3.4. Complementary Tests
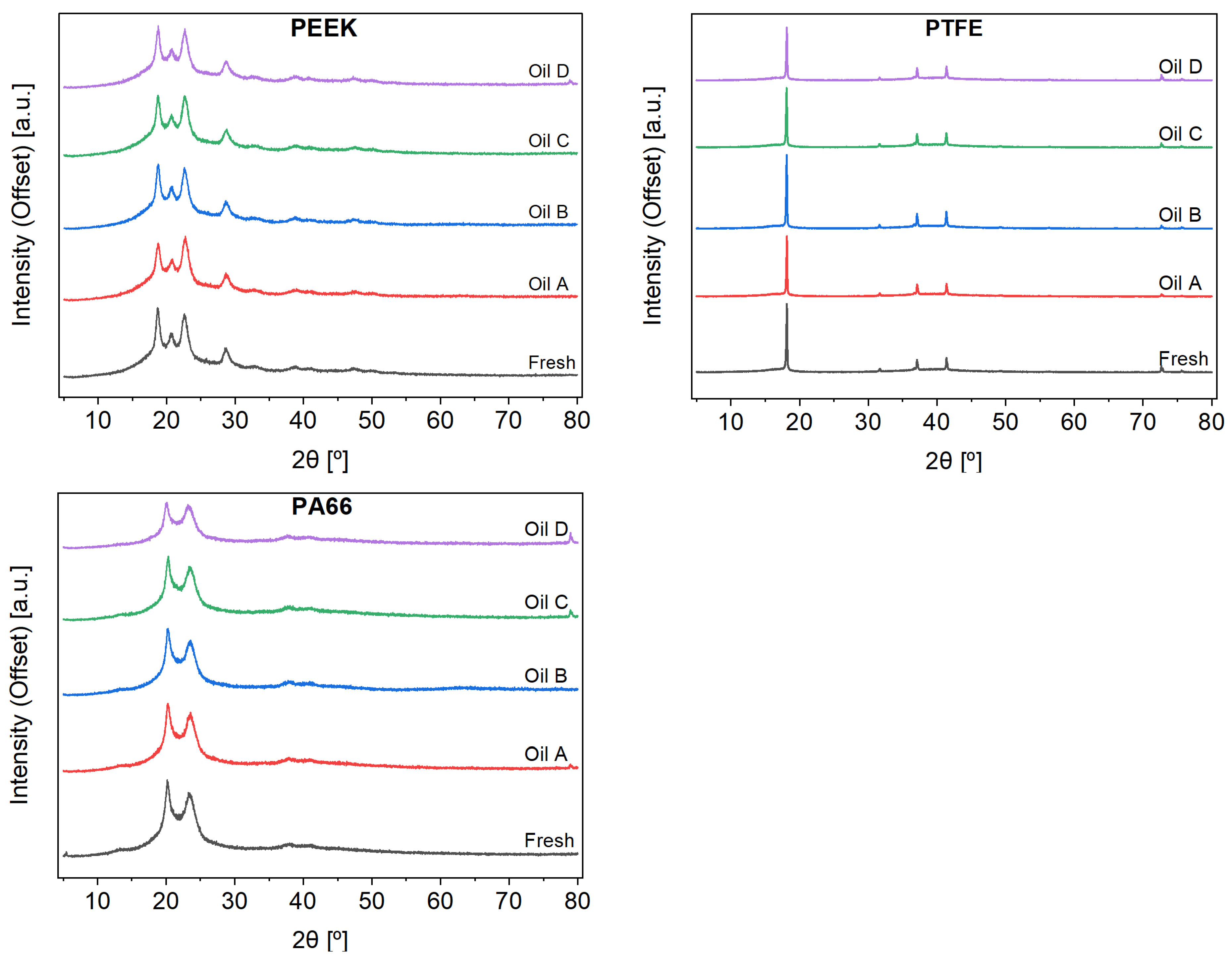
Complementary tests (XRD, FT-IR, and DSC) were performed on the polymers. X-ray diffraction (XRD) experiments were used to search for changes in the crystalline structure of the materials. However, the XRD spectra (
Figure 5) showed no noticeable changes in the crystalline/amorphous areas ratio, which means that it is not possible to correlate these results with the reported changes in the mechanical properties.
Infrared spectroscopy (FTIR) was used to detect possible changes in the polymeric structure of the materials. In the FTIR spectra, the qualitative differences (mainly peak positions) in the spectra of the same material subjected to different ageing conditions are almost negligible. Nevertheless, possible quantitative differences were evaluated by comparing peak areas.
Tables S1–S3 record the peak areas of PEEK, PTFE, and PA66 when fresh and under different ageing conditions. The area ratio of aged material vs. fresh material is calculated for every sample at every peak. In principle, this ratio should be similar for all the peaks within a sample. We considered deviations to be significant in those samples with at least one area acting as outlier according to a two-sided Grubb’s test (
p = 0.05) [
16]. With this criterion, PTFE and PA66 did not show significant differences. However, PEEK in oil C (at both tested times) showed an increase in the absorbances at 2850 cm
−1 and 2915 cm
−1, attributable to –CH– stretching vibrations, suggesting a particular influence of this oil. The sample aged in oil B for 672 h showed a similar trend, whereas the sample aged in oil A for 168 h showed a decrease in absorbance at 2915 cm
−1.
Principal component analysis is a data analysis methodology that allows the conversion of a group of correlated variables (in this case, IR absorbance at different wavenumbers) into a smaller number of uncorrelated variables which, in addition, retain most of the variability of the original data [
17].
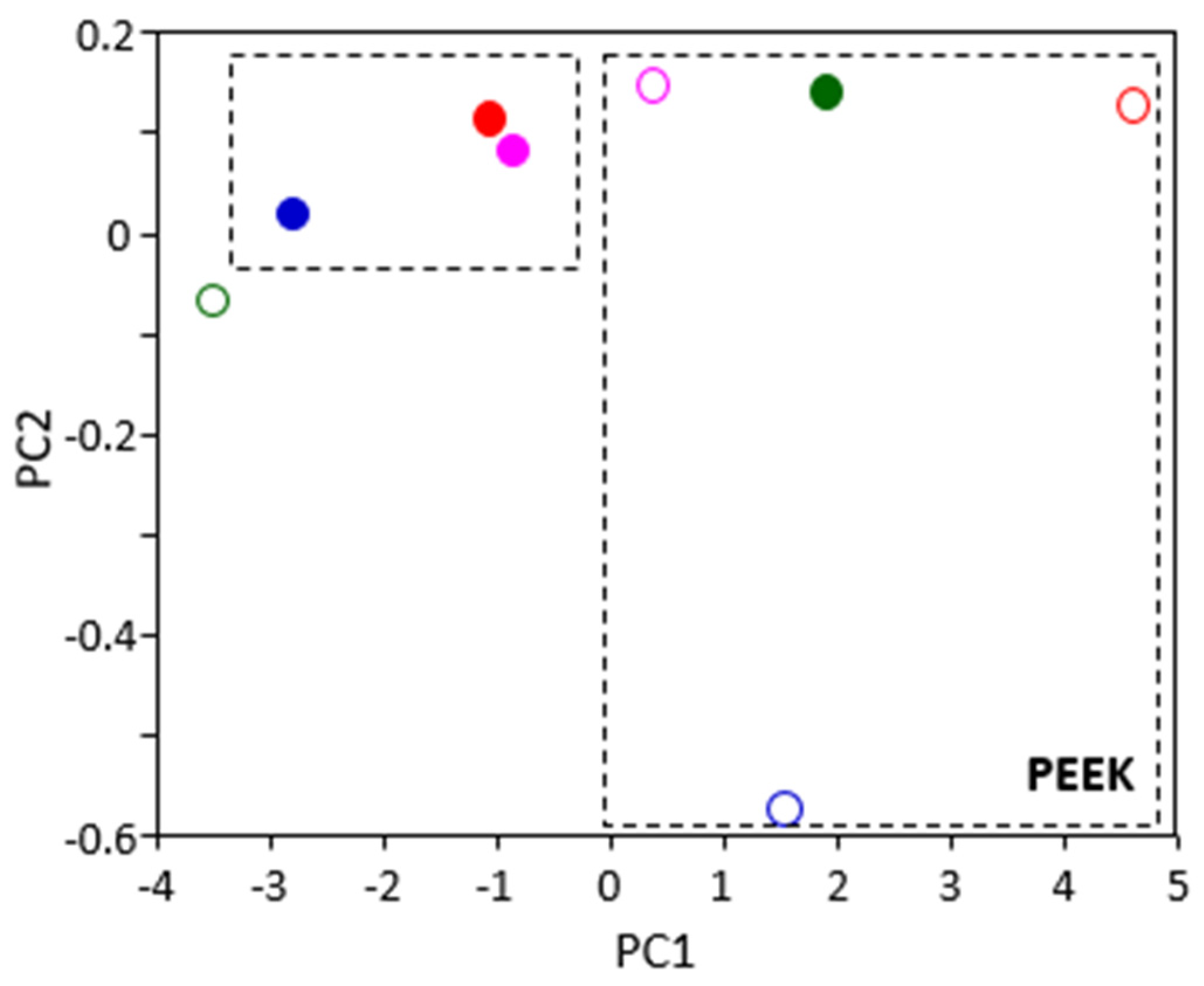
According to PCA analysis of the spectra of the different polymers, the samples of PEEK with 168 h of ageing (solid markers) group together (
Figure 6), as do the samples with 672 h of ageing (empty markers), except for oil B (green markers). These results suggest that, in the case of PEEK, time causes greater effects the oil type does. This would explain, in general, the similarity of results in the PEEK mechanical tests after ageing in the ATFs.
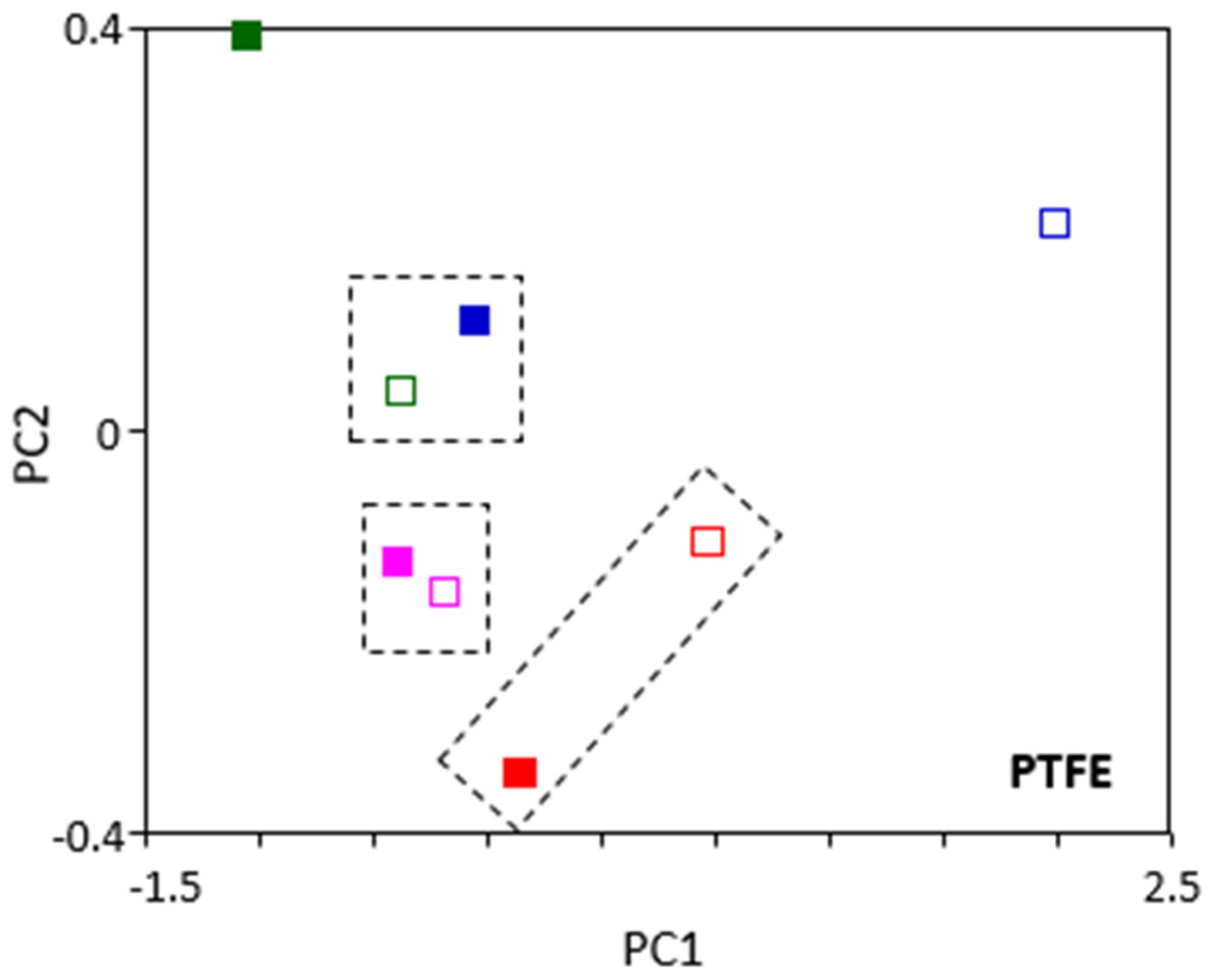
However, the PCA analysis of aged PTFE samples (
Figure 7) revealed the following three groups: oil C (fuchsia markers) after 168 and 672 h, oil D (red markers) after 168 and 672 h, and oil A (blue markers) after 672 h and oil B after 168 h. According to these findings, in the case of oils C and D, the oil type is more important than the ageing time. This is well represented in the dispersion of results for the mechanical properties variation tests, depending on the ATF used in the PTFE ageing.
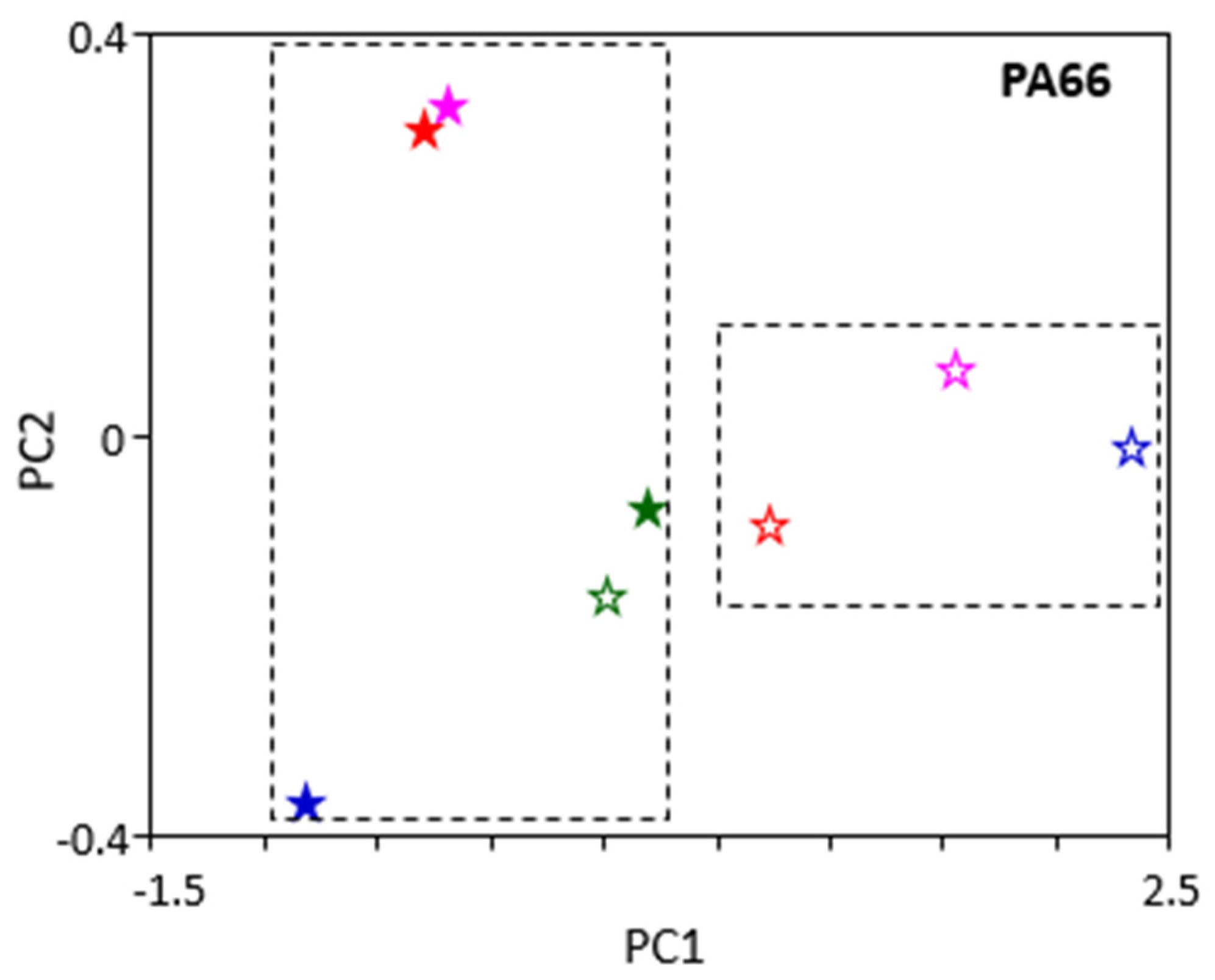
PA66 showed similar behaviour to that of PEEK (
Figure 8). Samples with shorter ageing times grouped together, as did samples with longer ageing times, except with oil B (green markers). This suggests, again, that the ageing time is more important for the PA66 samples than the oil type. These would explain the similarity of results in the PA66 mechanical tests after ageing in the different ATFs.
Differential scanning calorimetry (DSC) was used to check if there were changes in the fusion processes of the materials and, thus, in their crystalline structure. The DSC results for the different material/oil combinations are shown in
Figure S7, and
Table 5,
Table 6 and
Table 7 were obtained from those curves.
The enthalpy of fusion (∆H) for a fully crystalline PEEK would be 164.85 J/g [
18], so the fresh sample, with 31.60 J/g, was not crystalline. When the PEEK was aged in the different ATFs, a rise in the enthalpy associated with an increase in crystallinity was detected (
Table 5), especially for those samples aged in oils B and C. However, this change in the crystalline structure was not large enough to modify the mechanical characteristics of the PEEK. The T
Onset and T
Peak, represent the start of the fusion process and the fusion temperatures, respectively. PEEK is the material that shows the greatest difference between them, and the highest values of all the materials, although very similar to those of PTFE. The difference between T
Onset and T
Peak might be associated with crystallization taking place during the heating process, with the possibility of the peak being double, depending on the heating speed [
18].
In the case of PTFE, the ∆H at fusion for a crystalline sample would be 57.3 J/g [
19,
20]. The results therefore show that, as in the case of PEEK, the fresh sample is not crystalline (
Table 6). The slight increase in enthalpy after ageing does not suggest a notable change in the crystalline structure, confirming the results of the mechanical tests, with very small changes being seen.
Finally,
Table 7 shows the DSC results for PA66. This material showed the greatest changes in mechanical properties (+7 ShD points, +15% tensile strength, and −80% elongation at break) of all the materials in this study. These variations would suggest a certain degree of crystallization, which has been mentioned by Lann et al. [
13] and Decker et al. [
14] after an ageing process of PA66 in ATFs. PA66 is a semi-crystalline polymer [
21,
22,
23], and the variation in the degree of crystallinity can be observed in the evolution of the melting enthalpy [
24].
Table 7 shows that the melting enthalpy nearly doubles after ageing in the different ATFs, meaning that the increase in the degree of crystallinity is proportionately greater than in the other studied polymers. Although the amorphous phase is still more prevalent, according to the XRD results, the change in the structure of PA66 is enough to cause variations in its mechanical properties.
Finally, according to the changes in the different mechanical properties tested for the materials after being aged in the four ATFs, PEEK and PTFE may be considered to be compatible in all cases, as the changes in volume, hardness and tensile strength are negligible. Although they presented higher variations in their elongation at break, we consider these variations to be acceptable. That is not the case for PA66, which showed a very large decrease (~75%) in elongation at break. This, together with the increase in tensile strength (15%) and a slight increase in hardness (the hardness of the other polymers decreased), denotes a certain embrittlement of the PA66. These results allow the degree of compatibility of the ATFs tested with these polymers to be established, as shown in
Table 8.