Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review
Abstract
1. Introduction
2. Colourism and the Widespread Practices of Skin-Lightening
3. Toxic Ingredients in Skin Lightening Cosmetics
Mercury
4. Hydroquinone
5. Corticosteroids
6. Regulations
7. Notifications on Safety Gate
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pillaiyr, T.; Manickam, M.; Jung, S.-H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in human, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Zhang, W.; Wakamatsu, K.; Ito, S.; Hearing, V.J.; Kraemer, K.H.; Brash, D.E. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc. Natl. Acad. Sci. USA 2004, 101, 15076–15081. [Google Scholar] [CrossRef]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res. 2005, 571, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nordin, F.N.M.; Aziz, A.; Zakaria, Z.; Radzi, C.W.J.W.M. A systematic review on the skin whitening products and their ingredients for safety, health risk and the halal status. J. Cosmet. Dermatol. 2020, 20, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Davids, L.M.; van Wyk, J.; Khumalo, N.P.; Jablonski, N.G. The phenomenon of skin lightening: Is it right to be light? S. Afr. J. Sci. 2016, 112, 11–12. [Google Scholar] [CrossRef]
- Eagle, L.; Dahl, S.; Low, D.R. Ethical issues in the marketing of skin lightening products. In Proceedings of the Australian and New Zealand Marketing Academy Conference ANZMAC, Brisbane, Australia, 1–3 December 2014; pp. 75–81. [Google Scholar]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian herbs in skin whitening; The current development and limitations. Front. Pharmacol. 2020, 11, 982. [Google Scholar] [CrossRef]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and melanogenesis inhibition by indigenous African plants: A review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Naidoo, L.; Khoza, N.; Dlova, N.C. A fairer face, a fairer tomorrow? A review of skin lighteners. Cosmetics 2016, 3, 33. [Google Scholar] [CrossRef]
- Million Insights. Skin Lightening Products Market Analysis Report by Product, by Nature, by Region and Segment Forecasts from 2019 to 2025. 2020. Available online: https://www.millioninsights.com/industry-reports/global-skin-lightening-products-market (accessed on 25 January 2022).
- Benn, E.K.T.; Alexis, A.; Mohamed, N.; Wang, Y.-H.; Khan, I.A.; Liu, B. Skin bleaching and dermatological health of African and Afro-Caribbean populations in the US; new directions for methodologically rigorous, multidisciplinary, and culturally sensitive research. Dermatol. Ther. 2016, 6, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Pollock, S.; Taylor, S.; Oyerinde, O.; Nurmohamed, S.; Dlova, N.; Sarkar, R.; Galadari, H.; Manela-Azulai, M.; Chung, H.S.; Handog, E.; et al. The dark side of skin lightening: An international collaboration and review of a public health issue affecting dermatology. Int. J. Women Dermatol. 2021, 7, 158–164. [Google Scholar] [CrossRef]
- Li, E.P.H.; Min, H.J.; Belk, R.W.; Kimura, J.; Bahl, S. Skin Lightening and Beauty in Four Asian Cultures. In NA—Advances in Consumer Research; Lee, A.Y., Soman, D., Eds.; Association for Consumer Research: Duluth, MN, USA, 2008; Volume 35, pp. 444–449. [Google Scholar]
- Shevde, N. All’s Fair in Love and Cream: A cultural case study of Fair & Lovely in India. Advert. Soc. Rev. 2008, 9, 1–9. [Google Scholar]
- Shroff, H.; Diedrichs, P.C.; Craddock, N. Skin color, cultural capital, and beauty products: An investigation of the use of skin fairness products in Mumbai, India. Front. Public Health 2018, 5, 365. [Google Scholar] [CrossRef] [PubMed]
- Porcheron, A.; Latreille, J.; Jdid, R.; Tschachler, E.; Morizot, F. Influence of skin ageing features on Chinese women’s perception of facial age and attractiveness. Int. J. Cosmet. Sci. 2014, 36, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, O.E.; Petit, A. Skin bleaching: Highlighting the misuse of cutaneous depigmenting agents. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 741–750. [Google Scholar] [CrossRef]
- Gbetoh, M.H.; Amyot, M. Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada. Environ. Res. 2016, 150, 403–410. [Google Scholar] [CrossRef]
- Ricketts, P.; Knight, C.; Gordon, A.; Boischio, A.; Voutchkov, M. Mercury exposure associated with use of skin lightening products in Jamaica. J. Health Pollut. 2020, 10, 200601. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Y.; Ran, M.; Li, Q.; Ruan, Z.; Jin, N. Inhibition of Tyrosinase by Mercury Chloride: Spectroscopic and Docking Studies. Front. Pharmacol. 2020, 11, 81. [Google Scholar] [CrossRef]
- Park, J.-D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.H.; Sakakibara, M.; Sera, K.; Andayanie, E. Mercury exposure and health problems of the students using skin-lightening cosmetic products in Makassar, South Sulawesi, Indonesia. Cosmetics 2020, 7, 58. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin. Toxicol. 2011, 49, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Copan, L.; Fowles, J.; Barreau, T.; McGee, N. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel). Int. J. Environ. Res. Public Health 2015, 12, 10943–10954. [Google Scholar] [CrossRef] [PubMed]
- Loretz, L.J.; Api, A.M.; Barraj, L.M.; Burdickd, J.; Dressler, W.E.; Gettings, S.D.; Han Hsu, H.; Pan, Y.H.L.; Re, T.A.; Renskers, K.J.; et al. Exposure data for cosmetic products: Lipstick, body lotion, and face cream. Food Chem. Toxicol. 2005, 43, 279–291. [Google Scholar] [CrossRef]
- Hamann, C.R.; Boonchai, W.; Wen, L.; Nishijima Sakanashi, E.; Chu, C.-Y.; Hamann, K.; Hamann, C.P.; Sinniah, K.; Hamann, D. Spectrometric analysis of mercury content in 549 skin-lightening products: Is mercury toxicity a hidden global health hazard? J. Am. Acad. Dermatol. 2014, 70, 281–287. [Google Scholar] [CrossRef]
- McRill, C.; Boyer, L.V.; Flood, T.J.; Ortega, L. Mercury toxicity due to the use of a cosmetic cream. J. Occup. Environ. Med. 2000, 42, 4–7. [Google Scholar] [CrossRef]
- Weldon, M.M.; Smolinski, M.S.; Maroufi, A.; Hasty, B.W.; Gilliss, D.; Boulanger, L.; Balluz, L.; Dutton, R.J. Mercury poisoning associated with a Mexican beauty cream. West. J. Med. 2000, 173, 15–18. [Google Scholar] [CrossRef]
- Sastri, M.N.; Kalidas, C. Photochemical estimation of mercuric chloride by Eder’s reaction with ceric ion as sensitizer. Fresenius Z. Anal. Chem. 1955, 148, 3–6. [Google Scholar] [CrossRef]
- Awitor, K.O.; Bernard, L.; Coupat, B.; Fournier, J.P.; Verdier, P. Measurement of mercurous chloride vapor pressure. New J. Chem. 2000, 24, 399–401. [Google Scholar] [CrossRef]
- Ladizinski, B.; Mistry, N.; Kundu, R.V. Widespread use of toxic skin lightening compounds: Medical and psychosocial aspects. Dermatol. Clin. 2011, 29, 111–123. [Google Scholar] [CrossRef]
- Engler, D.E. Mercury “bleaching” creams. J. Am. Acad. Dermatol. 2005, 52, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Murerva, R.; Gwala, F.; Amuti, T.; Muange, M. Childhood effects of pre-natal and post-natal exposure to mercurial skin lightening agents. Literature review. Int. J. Med. Stud. 2022; in press. [Google Scholar] [CrossRef]
- Peregrino, C.P.; Moreno, M.V.; Miranda, S.V.; Rubio, A.D.; Leal, L.O. Mercury levels in locally manufactured Mexican skin-lightening creams. Int. J. Environ. Res. Public Health 2011, 8, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Agorku, E.S.; Kwaansa-Ansah, E.E.; Voegborlo, R.B.; Amegbletor, P.; Opoku, F. Mercury and hydroquinone content of skin toning creams and cosmetic soaps, and the potential risks to the health of Ghanaian women. SpringerPlus 2016, 5, 319. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Kooyers, T.J.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of depigmentation by hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef]
- Aspengren, S.; Norström, E.; Wallin, M. Effects of hydroquinone on cytoskeletal organization and intracellular transport in cultured Xenopous laevis melanophores and fibroblasts. ISRN Cell Biol. 2012, 2012, 524781. [Google Scholar] [CrossRef]
- Rotava, P.A.; Favero, J.S.; Garcia, K.R.; Weiss-Angeli, V. Profile of depigmenting cosmetics and dermatological products on the market. Rev. Ciênc. Farm. Básica Apl. 2020, 41, e643. [Google Scholar] [CrossRef]
- Desmedt, B.; Ates, G.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Hendrickx, B.; Deconinck, E.; De Paepe, K. In vitro dermal absorption of hydroquinone: Protocol validation and applicability on illegal skin-whitening cosmetics. Skin Pharmacol. Physiol. 2016, 29, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Klein-Szanto, A.J.P.; Jaiswal, A.K. Hydroquinones cause specific mutations and lead to cellular transformation and in vivo tumorigenesis. Br. J. Cancer 1998, 78, 312–320. [Google Scholar] [CrossRef][Green Version]
- Nordlund, J.J.; Grimes, P.E.; Ortonne, J.P. The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 781–787. [Google Scholar] [CrossRef]
- Gandhi, V.; Verma, P.; Naik, G. Exogenous ochronosis after prolonged use of topical hydroquinone (2%) in a 50-year-old Indian female. Indian J. Dermatol. 2012, 57, 394–395. [Google Scholar] [CrossRef]
- Bhattar, P.A.; Zawar, V.P.; Godes, K.V.; Patil, S.P.; Nadkarni, N.J.; Gautam, M.M. Exogenous ochronosis. Indian J. Dermatol. 2015, 60, 537–543. [Google Scholar] [CrossRef]
- Olumide, Y.M.; Akinkugbe, A.O.; Altraide, D.; Mohammed, T.; Ahamefule, N.; Ayanlowo, S.; Onyekonwu, C.; Essen, N. Complications of chronic use of skin lightening cosmetics. Int. J. Dermatol. 2008, 47, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Addo, H.A. Squamous cell carcinoma associated with prolonged bleaching. Ghana Med. J. 2000, 34, 144–146. [Google Scholar]
- Ly, F.; Kane, A.; Déme, A.; Ngoh, N.-F.; Niang, S.-O.; Bello, R.; Rethers, L.; Dangou, J.-M.; Dieng, M.-T.-D.; Diousse, P.; et al. First case of squamous cell carcinoma associated with cosmetic use of bleaching compounds. Ann. Dermatol. Venereol. 2010, 137, 128–131. [Google Scholar] [CrossRef]
- Gbandama, K.K.P.; Diabaté, A.; Kouassi, K.A.; Kouassi, Y.I.; Allou, A.-S.; Kaloga, K. Squamous cell carcinoma associated with cosmetic use of bleaching agents: About a case in Ivory Coast. Case Rep. Dermatol. 2019, 11, 322–326. [Google Scholar] [CrossRef]
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1272&from=EN (accessed on 21 January 2022).
- Scientific Committee on Consumer Safety (SCCS). Opinion on α-Arbutin; SCCS/1550/15; Scientific Committee on Consumer Safety: Brussels, Belgium, 2015. [Google Scholar]
- Ahluwalia, A. Topical glucocorticoids and the skin-mechanisms of action: An update. Mediat. Inflamm. 1998, 7, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Dey, V.K. Misuse of topical corticosteroids: A clinical study of adverse effects. Indian Dermatol. Online J. 2014, 5, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Sendrasoa, F.A.; Ranaivo, I.M.; Andrianarison, M.; Raharolahy, O.; Razanakoto, N.H.; Ramarozatovo, L.S.; Rapelanoro Rabenja, F. Misuse of topical corticosteroids for cosmetic purpose in Antananarivo, Madagascar. BioMed Res. Int. 2017, 2017, 9637083. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.; Bondon-Guitton, E.; Montastruc, J.L. Inhaled corticosteroid-induced hair depigmentation in a child. J. Drugs Dermatol. 2013, 12, 119–120. [Google Scholar]
- Petit, A.; Cohen-Ludmann, C.; Clevenbergh, P.; Bergmann, J.-F.; Dubertret, L. Skin lightening and its complications among African people living in Paris. J. Am. Acad. Dermatol. 2006, 55, 873–878. [Google Scholar] [CrossRef]
- Carruthers, J.A.; August, P.J.; Staughton, R.C.D. Observations on the systemic effect of topical clobetasol propionate (Dermovate). BMJ Brit. Med. J. 1975, 4, 203–204. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. Minamata Convention on Mercury. 2019. Available online: https://www.mercuryconvention.org/en/resources/minamata-convention-mercury-text-and-annexes (accessed on 20 September 2021).
- Regulation (EC) 1223/2009, of the European Parliament and of the Council of 30 November 2009, Official Journal of European Union L 342/59 (22 December 2009). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1223&from=EN (accessed on 10 September 2021).
- Regulation (EU) No 344/2013 of 4 April 2013, Amending Annexes II, III and VI to Regulation (EC) No 1223/2009 of the European Parliament and the Council on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0344&from=IT (accessed on 20 January 2022).
- Code of Federal Regulations, Title 21, Chapter I, Part 700, Subpart B, Section 700.13. Use of Mercury Compounds in Cosmetics Including Use as Skinbleaching Agents in Cosmetic Preparations also Regarded as Drugs. Available online: https://www.govinfo.gov/content/pkg/CFR-2012-title21-vol7/pdf/CFR-2012-title21-vol7-sec700-13.pdf (accessed on 3 September 2021).
- CIR Expert Panel. Amended Safety Assessment of Hydroquinone as Used in Cosmetics. 2014. Available online: https://www.cir-safety.org/sites/default/files/hydroquinone.pdf (accessed on 3 February 2022).
- Coronavirus Aid, Relief, and Economic Security Act (CARES Act). 2020. Available online: https://www.congress.gov/116/plaws/publ136/PLAW-116publ136.pdf (accessed on 3 February 2022).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products. Available online: https://ec.europa.eu/safety-gate-alerts/screen/search (accessed on 13 August 2021).
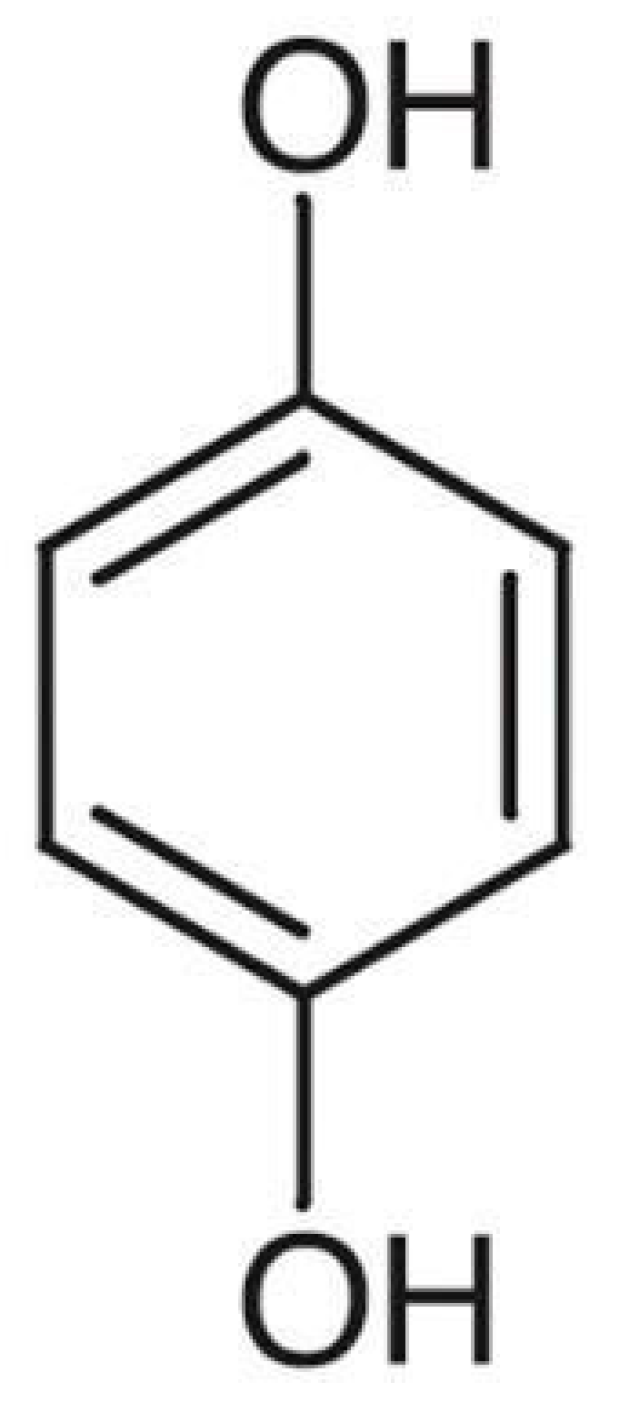
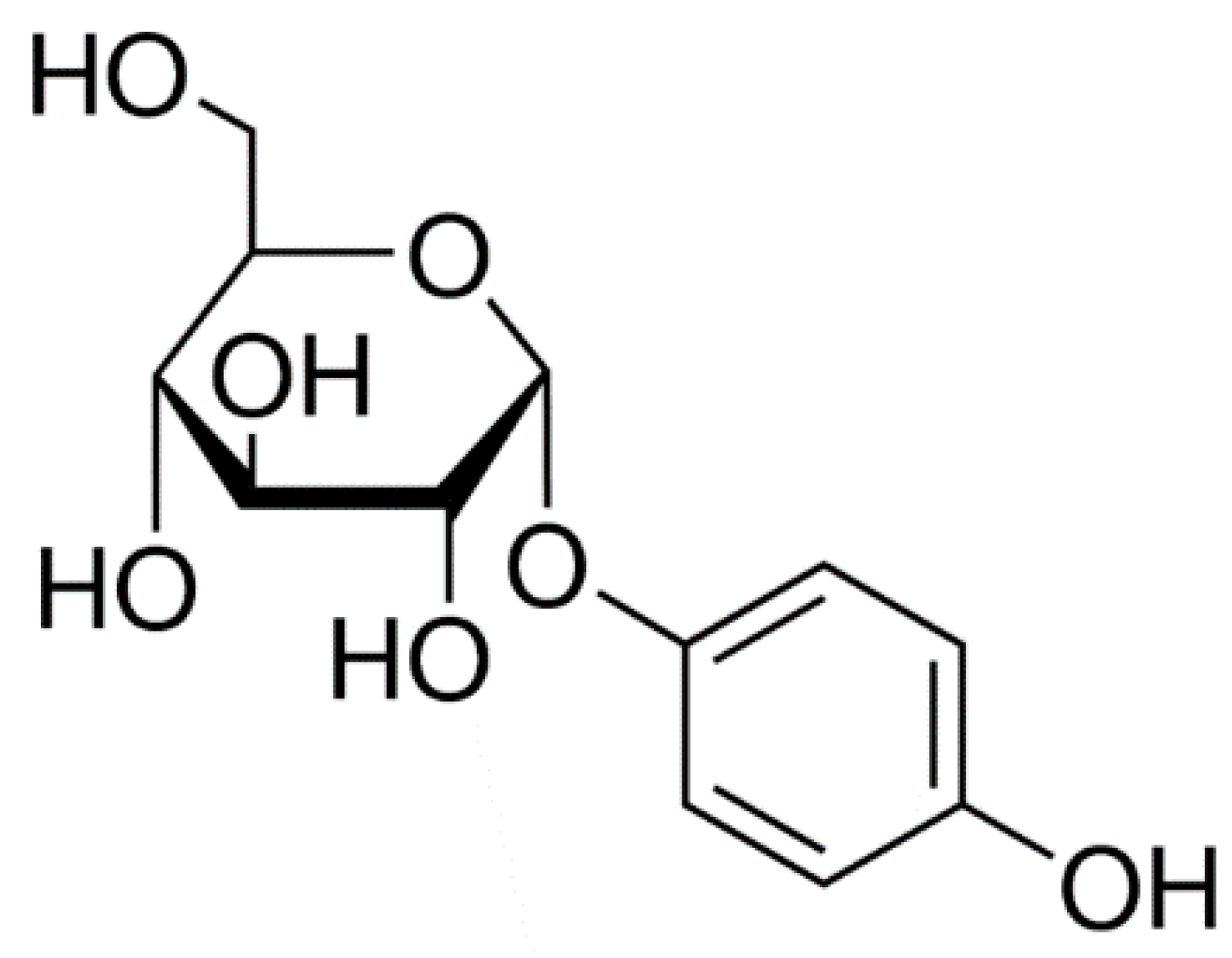
| Skin Lightening Agents | Mechanism of Action |
|---|---|
| Hydroquinone, Azelaic acid, Ellagic acid, Kojic acid, Mequinol, Arbutin, Flavonoids, Resveratrol, N-acetyl glucosamine | Inhibition of tyrosinase activity [9] |
| Alpha-hydroxy acids, salicylic acid | Acceleration of epidermal turnover [9] |
| Retinoids | Inhibition of tyrosinase transcription, epidermal melanin dispersion [8] |
| Vitamin C, Vitamin E | Antioxidant action, acceleration of epidermal turnover [9] |
| Niacinamide, Soy proteins, Linoleic acid | Inhibition of melanosome transfer [8,9] |
| Skin-Lightening Agent | Mechanism of Action | Toxic Effects |
|---|---|---|
| Mercury derivatives | Inhibition of melanin synthesis (competition with copper in tyrosinase’s activity site) | Dermatological, gastrointestinal, neurological and renal effects |
| Hydroquinone | Inhibition of tyrosinase activity | Irritant contact dermatitis, ochronosis; suspected of causing cancer |
| Corticosteroids | Interference with the production of α-MSH | Dermatological (acne, contact dermatitis, skin atrophy) and systemic (Cushing’s syndrome, immunosuppression, hypertension, diabetes mellitus, hypothalamic-pituitary-adrenal axis suppression) effects |
| Year | Mercury | Hydroquinone | Corticosteroids * |
|---|---|---|---|
| 2020 | 9 | 8 | 6 |
| 2019 | 15 | 6 | 2 (1) |
| 2018 | 0 | 8 | 1 |
| 2017 | 0 | 8 | 0 |
| 2016 | 0 | 12 | 2 (1) |
| 2015 | 0 | 19 | 0 |
| 2014 | 2 | 12 | 8 (1) |
| 2013 | 30 | 3 | 2 |
| 2012 | 4 | 22 | 7 (1) |
| 2011 | 1 | 42 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juliano, C.C.A. Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review. Appl. Sci. 2022, 12, 3177. https://doi.org/10.3390/app12063177
Juliano CCA. Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review. Applied Sciences. 2022; 12(6):3177. https://doi.org/10.3390/app12063177
Chicago/Turabian StyleJuliano, Claudia C. A. 2022. "Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review" Applied Sciences 12, no. 6: 3177. https://doi.org/10.3390/app12063177
APA StyleJuliano, C. C. A. (2022). Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review. Applied Sciences, 12(6), 3177. https://doi.org/10.3390/app12063177






