Performance Assessment of Low-Temperature Solar Collector with Fullerenes C60 Manufactured at Low Cost in an Emerging Country
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling and Simulating of the Solar Collector
2.1.1. Modeling
2.1.2. Simulation
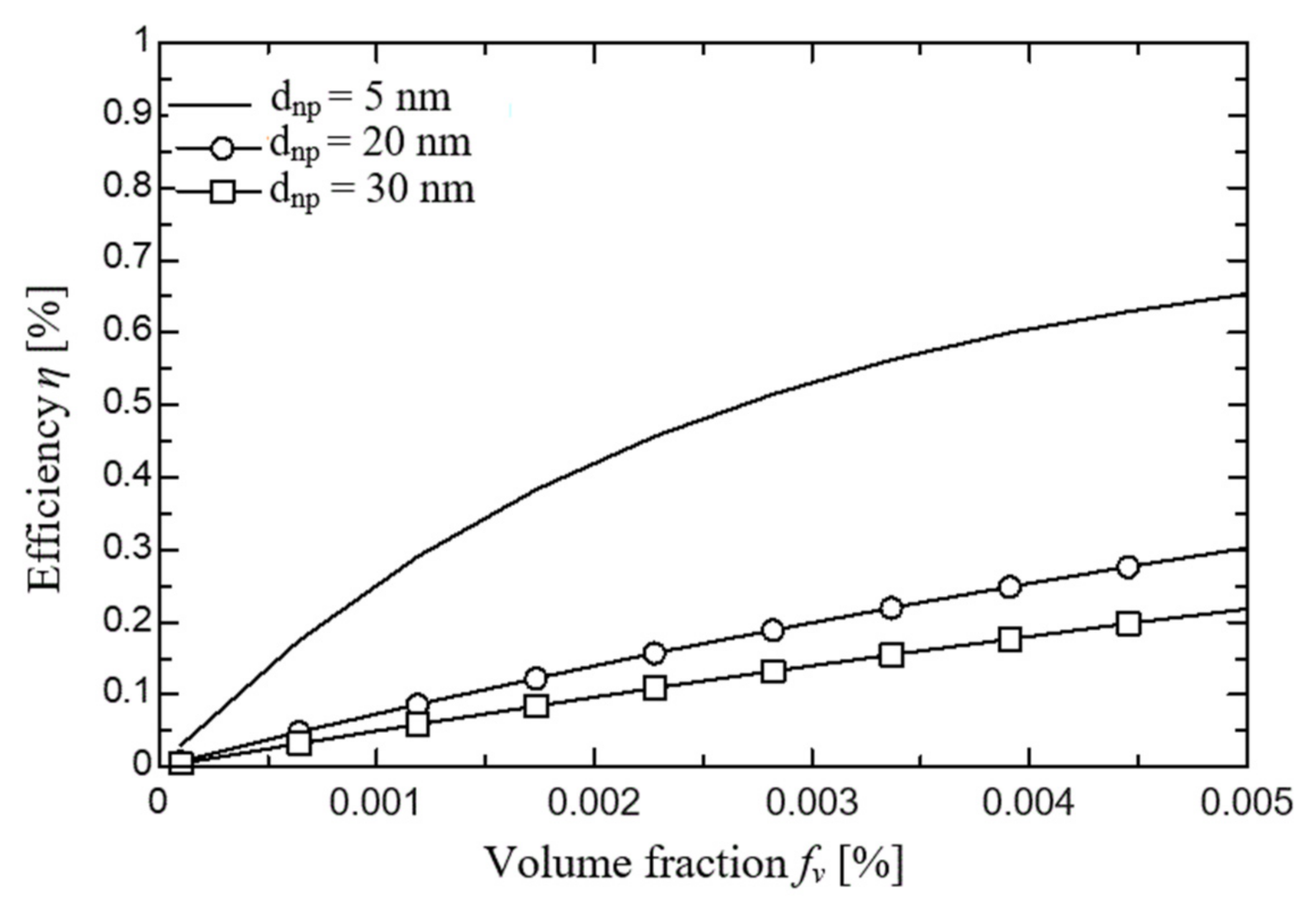
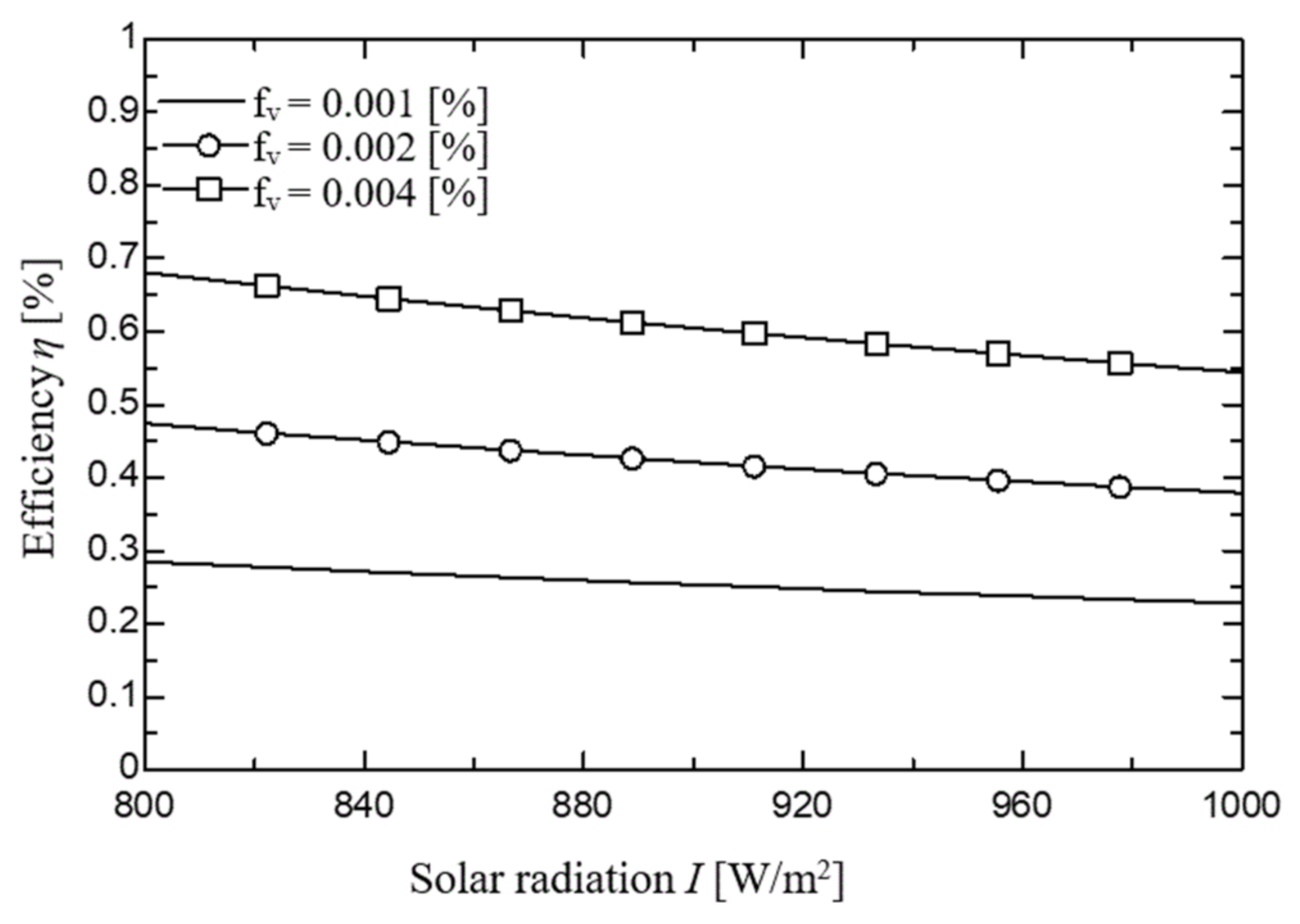
2.1.3. Simulation Results
2.2. Low-Cost Fabrication and Characterization of Nanoparticles
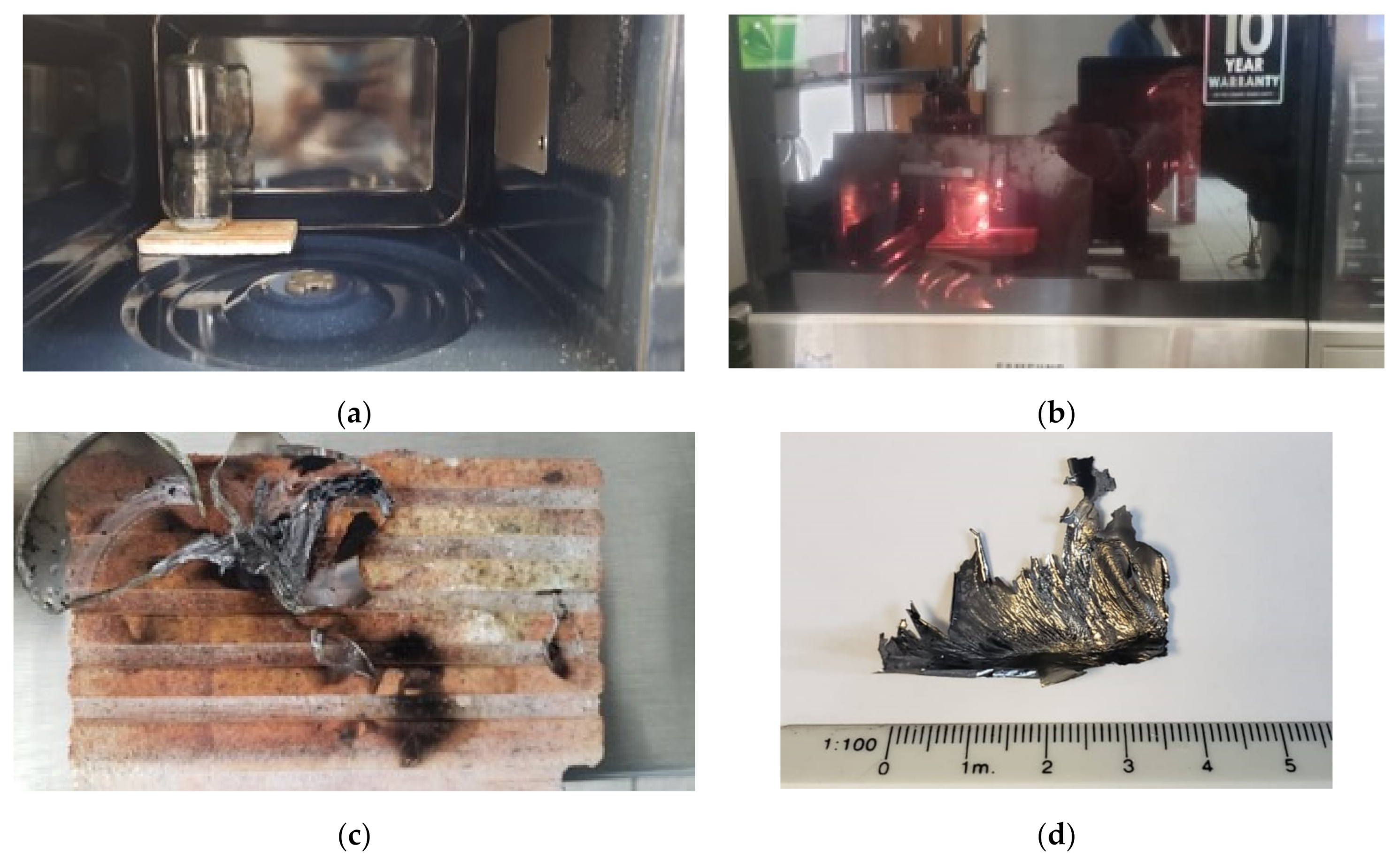
2.2.1. Production of Fullerene C60 Thin Films
2.2.2. Production of Fullerene C60 Nanoparticles
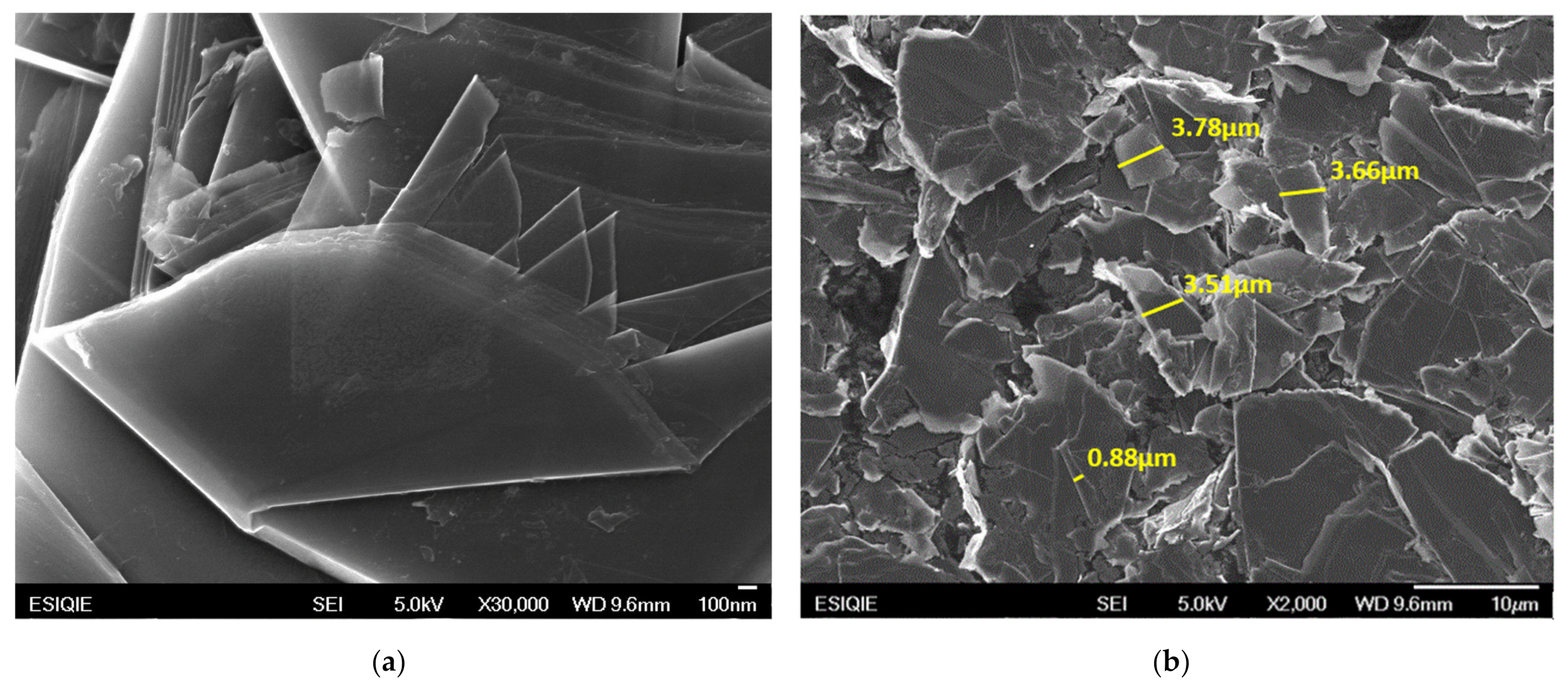
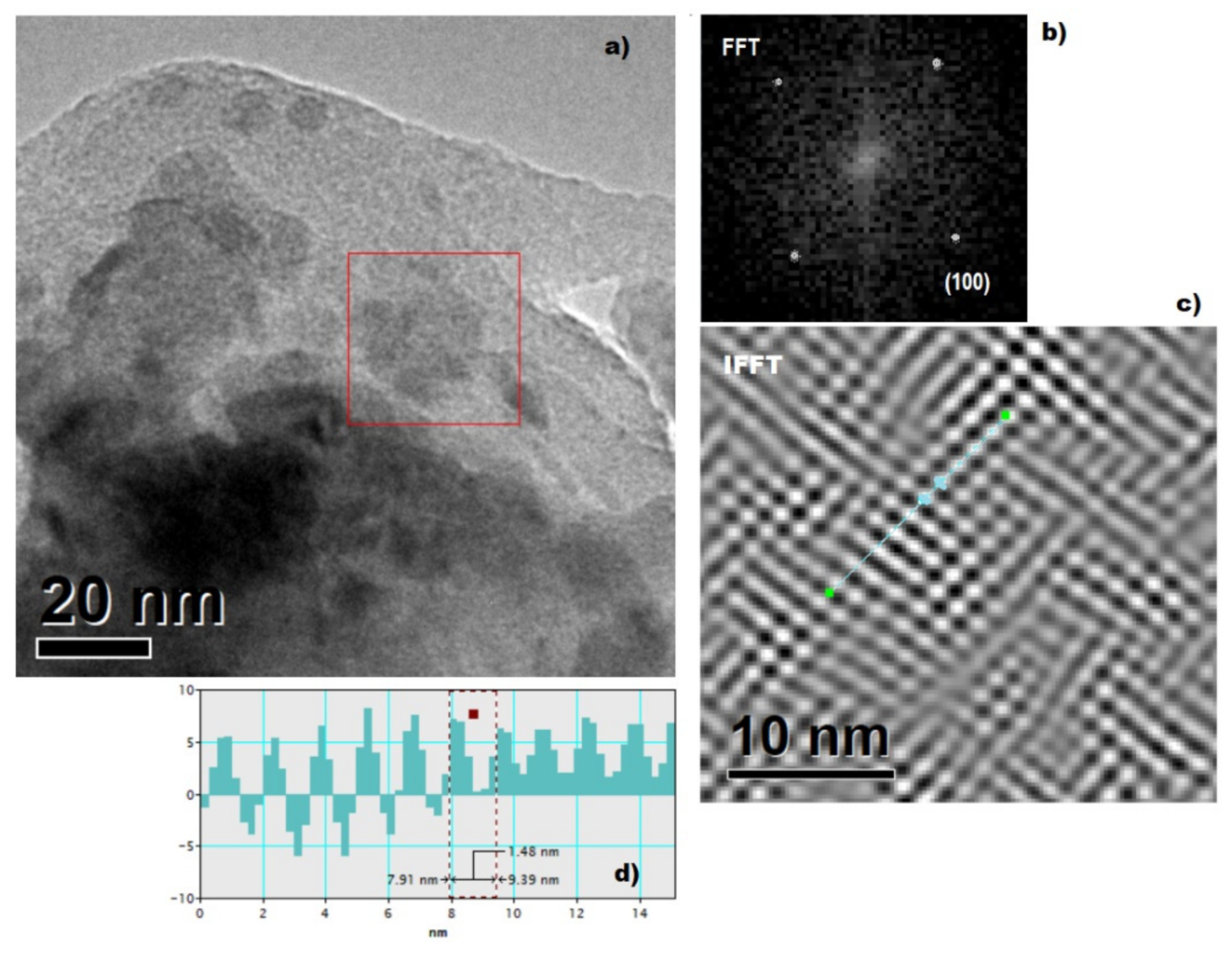
2.2.3. Characterization of the Nanoparticles
2.2.4. Preparation of the Nanofluid
2.3. Experimental Procedure of the Solar Collector
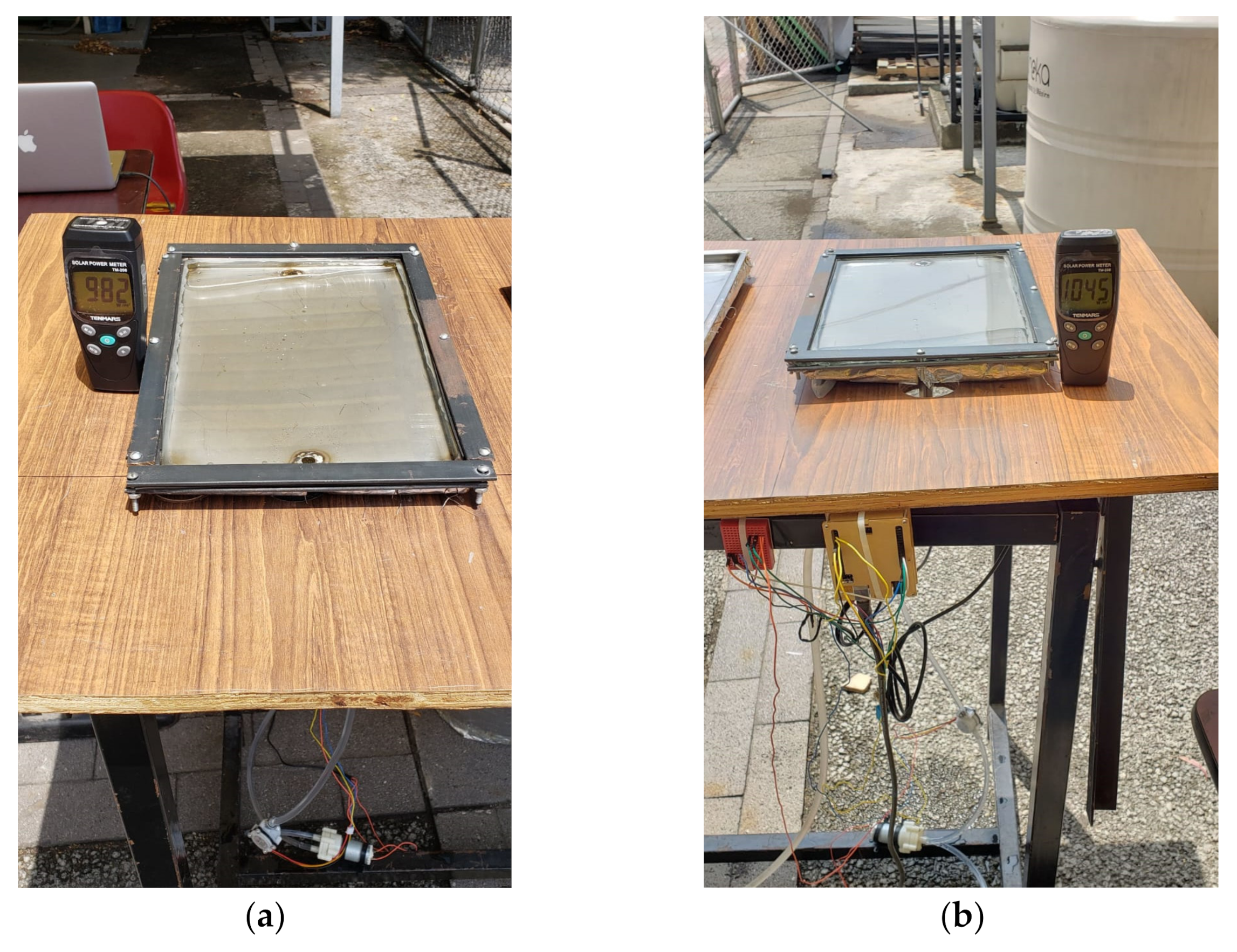
2.3.1. Test Rig of the Solar Collector
2.3.2. Data Reduction
2.3.3. Uncertainty of Measuring Instruments
3. Results and Discussion
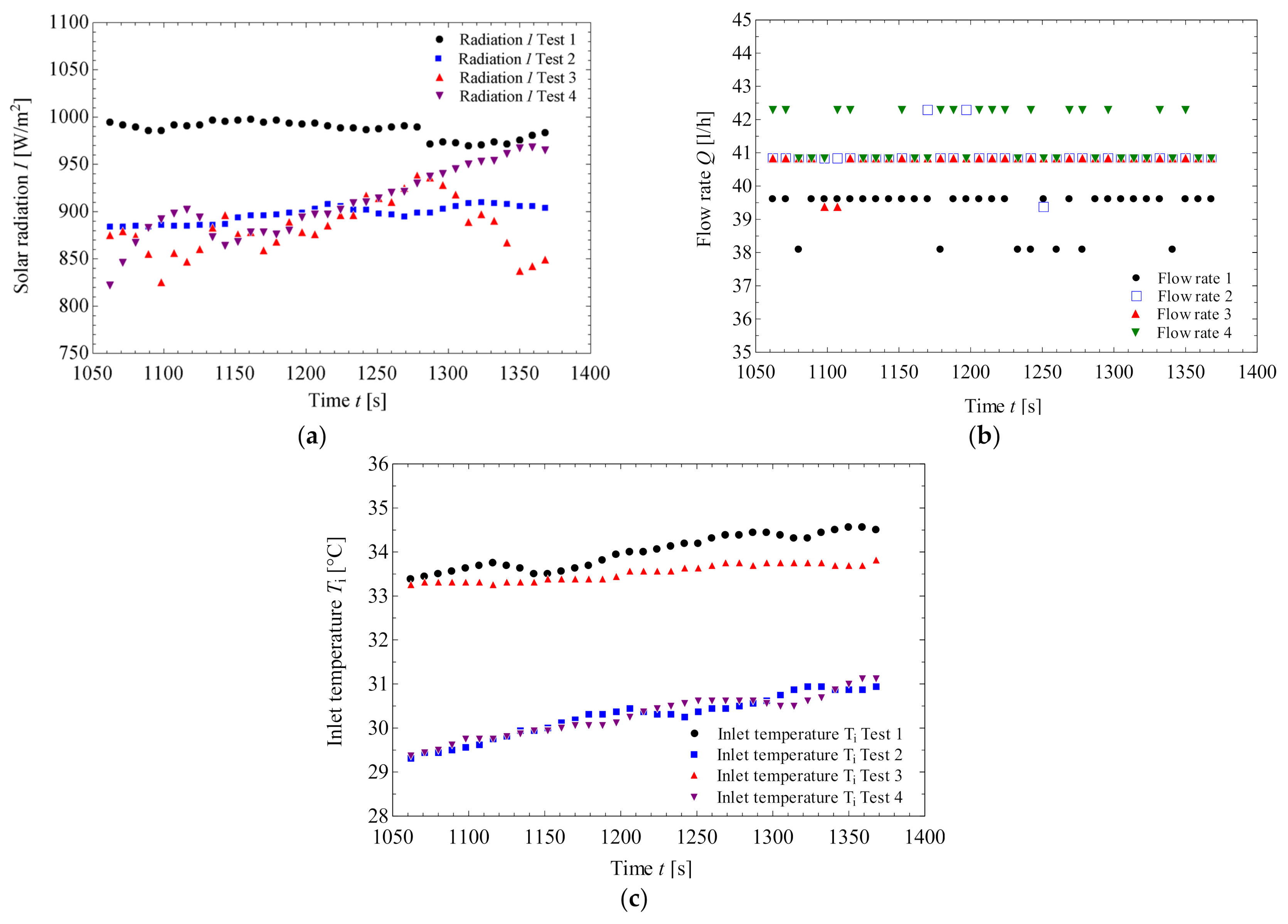
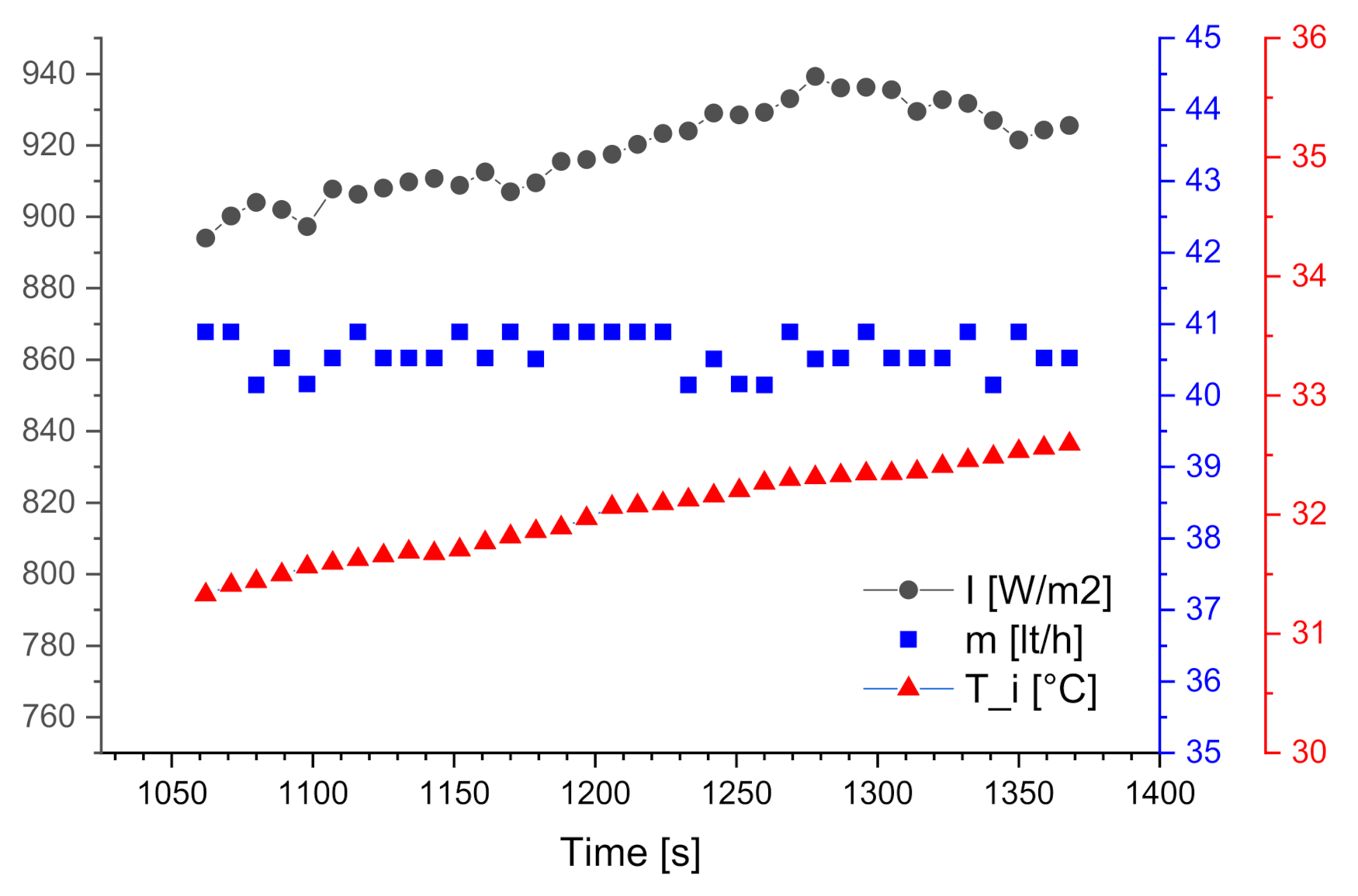
3.1. Steady State Results
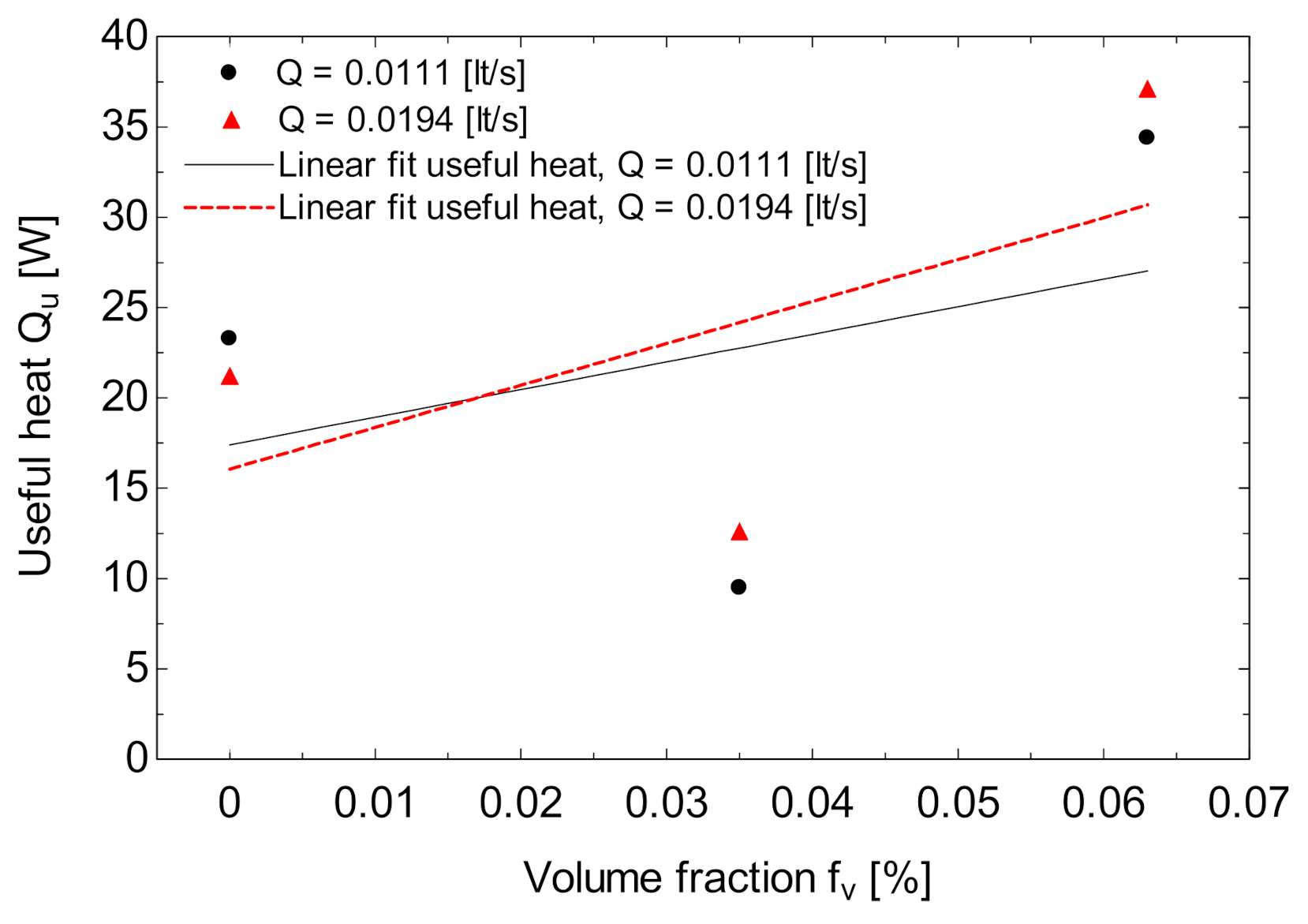
3.2. Solar Collector Performance Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Justo, J.J.; Mwasilu, F.; Lee, J.; Jung, J.W. AC-microgrids versus DC-microgrids with distributed energy resources: A review. Renew. Sustain. Energy Rev. 2013, 24, 387–405. [Google Scholar] [CrossRef]
- Venegas, M.; García-Hernando, N.; Zacarías, A.; de Vega, M. Performance of a solar absorption cooling system using nanofluids and a membrane-based microchannel desorber. Appl. Sci. 2020, 10, 2761. [Google Scholar] [CrossRef] [Green Version]
- Romage, G.; Jiménez, C.; de Jesús, R.J.; Zacarías, A.; Carvajal, I.; Jiménez, J.A.; Pineda, J.; Venegas, M. Modeling and simulation of a hybrid compression/absorption chiller driven by stirling engine and solar dish collector. Appl. Sci. 2020, 10, 9018. [Google Scholar] [CrossRef]
- Hewakuruppu, Y.L.; Taylor, R.A.; Tyagi, H.; Khullar, V.; Otanicar, T.; Coulombe, S.; Hordy, N. Limits of selectivity of direct volumetric solar absorption. Sol. Energy 2015, 114, 206–216. [Google Scholar] [CrossRef]
- Minardi, J.E.; Chuang, H.N. Performance of a “black” liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Muhammad, M.J.; Muhammad, I.A.; Sidik, N.A.C.; Yazid, M.N.A.W.M.; Mamat, R.; Najafi, G. The use of nanofluids for enhancing the thermal performance of stationary solar collectors: A review. Renew. Sustain. Energy Rev. 2016, 63, 226–236. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A. A review on optical properties and application of nanofluids in direct absorption solar collectors (DASCs). Renew. Sustain. Energy Rev. 2017, 72, 10–32. [Google Scholar] [CrossRef]
- Delfani, S.; Esmaeili, M.; Karami, M. Application of artificial neural network for performance prediction of a nanofluid-based direct absorption solar collector. Sustain. Energy Technol. Assess. 2019, 36, 100559. [Google Scholar] [CrossRef]
- Guo, C.; Liu, C.; Jiao, S.; Wang, R.; Rao, Z. Introducing optical fiber as internal light source into direct absorption solar collector for enhancing photo-thermal conversion performance of MWCNT-H2O nanofluids. Appl. Therm. Eng. 2020, 173, 115207. [Google Scholar] [CrossRef]
- Struchalin, P.G.; Yunin, V.S.; Kutsenko, K.V.; Nikolaev, O.V.; Vologzhannikova, A.A.; Shevelyova, M.P.; Gorbacheva, O.S.; Balakin, B.V. Performance of a tubular direct absorption solar collector with a carbon-based nanofluid. Int. J. Heat Mass Transf. 2021, 179, 121717. [Google Scholar] [CrossRef]
- Arai, N.; Itaya, Y.; Hasatani, M. Development of a “volume heat-trap” type solar collector using a fine-particle semitransparent liquid suspension (FPSS) as a heat vehicle and heat storage medium Unsteady, one-dimensional heat transfer in a horizontal FPSS layer heated by thermal radiatio. Sol. Energy 1984, 32, 49–56. [Google Scholar] [CrossRef]
- Webb, B.W.; Viskanta, R. Analysis of heat transfer and solar radiation absorption in an irradiated thin, falling molten salt film. J. Sol. Energy Eng. Trans. ASME 1985, 107, 113–119. [Google Scholar] [CrossRef]
- Bueke, A.R.; Etter, D.E.; Hudgens, C.R.; Wiedenheft, C.J.; Wittenberg, L.J. Thermal and Photochemical Studies of Solar Energy. Sol. Energy Mater. 1982, 6, 481–490. [Google Scholar]
- Das, S.K.; Choi, S.U.S.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Martínez, V. Estudio del Tratamiento a Altas Presiones en la Estabilidad de un Nanofluido. Master’s Thesis, Universidad de Santiago de Chile, Santiago, Chile, 2016. [Google Scholar]
- Wu, D.; Zhu, H.; Wang, L.; Liu, L. Critical Issues in Nanofluids Preparation, Characterization and Thermal Conductivity. Curr. Nanosci. 2009, 5, 103–112. [Google Scholar] [CrossRef]
- Hosseini, S.M.S.; Shafiey Dehaj, M. Assessment of TiO2 water-based nanofluids with two distinct morphologies in a U type evacuated tube solar collector. Appl. Therm. Eng. 2021, 182, 116086. [Google Scholar] [CrossRef]
- Rubbi, F.; Das, L.; Habib, K.; Aslfattahi, N.; Saidur, R.; Rahman, M.T. State-of-the-art review on water-based nanofluids for low temperature solar thermal collector application. Sol. Energy Mater. Sol. Cells 2021, 230, 111220. [Google Scholar] [CrossRef]
- Ahbabi Saray, J.; Heyhat, M.M. Modeling of a direct absorption parabolic trough collector based on using nanofluid: 4E assessment and water-energy nexus analysis. Energy 2022, 244, 123170. [Google Scholar] [CrossRef]
- Behura, A.K.; Gupta, H.K. Efficient Direct Absorption Solar Collector Using Nanomaterial Suspended Heat Transfer Fluid. Mater. Today Proc. 2019, 22, 1664–1668. [Google Scholar] [CrossRef]
- Vakili, M.; Yahyaei, M.; Ramsay, J.; Aghajannezhad, P.; Paknezhad, B. Adaptive neuro-fuzzy inference system modeling to predict the performance of graphene nanoplatelets nanofluid-based direct absorption solar collector based on experimental study. Renew. Energy 2021, 163, 807–824. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted efficiency of a Low-temperature Nanofluid-based direct absorption solar collector. J. Sol. Energy Eng. Trans. ASME 2009, 131, 0410041–0410047. [Google Scholar] [CrossRef]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Small particles, big impacts: A review of the diverse applications of nanofluids. J. Appl. Phys. 2013, 113, 011301. [Google Scholar] [CrossRef]
- Gupta, H.K.; Agrawal, G.D.; Mathur, J. An experimental investigation of a low temperature Al2O3-H2O nanofluid based direct absorption solar collector. Sol. Energy 2015, 118, 390–396. [Google Scholar] [CrossRef]
- Karami, M.; Akhavan-Bahabadi, M.A.; Delfani, S.; Raisee, M. Experimental investigation of CuO nanofluid-based Direct Absorption Solar Collector for residential applications. Renew. Sustain. Energy Rev. 2015, 52, 793–801. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Z.; Du, Z.; Xu, X.; Zhang, Z.; Fang, X. A highly stable hydroxylated graphene/ethylene glycol-water nanofluid with excellent extinction property at a low loading for direct absorption solar collectors. Thermochim. Acta 2020, 684, 178487. [Google Scholar] [CrossRef]
- Joseph, A.; Thomas, S. Energy, exergy and corrosion analysis of direct absorption solar collector employed with ultra-high stable carbon quantum dot nanofluid. Renew. Energy 2022, 181, 725–737. [Google Scholar] [CrossRef]
- Sreehari, S.; Joseph, A.; Thomas, S. Development of a low cost nanofluid based direct absorption solar collector. Mater. Today Proc. 2019, 22, 2424–2430. [Google Scholar] [CrossRef]
- Dall’Asén, A.G.; Verdier, M.; Huck, H.; Halac, E.B.; Reinoso, M. Nanoindentation on carbon thin films obtained from a C 60 ion beam. Appl. Surf. Sci. 2006, 252, 8005–8009. [Google Scholar] [CrossRef]
- Rusop, M.; Tian, X.M.; Kinugawa, T.; Soga, T.; Jimbo, T.; Umeno, M. Preparation and characterization of boron-incorporated amorphous carbon films from a natural source of camphoric carbon as a precursor material. Appl. Surf. Sci. 2005, 252, 1693–1703. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Kumar, M.; Okazaki, T.; Hiramatsu, M.; Ando, Y. The use of camphor-grown carbon nanotube array as an efficient field emitter. Carbon N. Y. 2007, 45, 1899–1904. [Google Scholar] [CrossRef]
- Kar, S.; Chaudhuri, S. Optical properties of diamond films deposited by low temperature microwave plasma CVD from camphor. Mater. Lett. 2004, 58, 3029–3033. [Google Scholar] [CrossRef]
- Kalita, G.; Masahiro, M.; Uchida, H.; Wakita, K.; Umeno, M. Few layers of graphene as transparent electrode from botanical derivative camphor. Mater. Lett. 2010, 64, 2180–2183. [Google Scholar] [CrossRef]
- Lew, A.; Krutzik, P.O.; Hart, M.E.; Chamberlin, A.R. Increasing rates of reaction: Microwave-assisted organic synthesis for combinatorial chemistry. J. Comb. Chem. 2002, 4, 95–105. [Google Scholar] [CrossRef]
- Martínez-Palou, R. Microwave-assisted synthesis using ionic liquids. Mol. Divers. 2010, 14, 3–25. [Google Scholar] [CrossRef]
- Valencia, A. Evaluación numérica del rendimiento de un colector solar de absorción directa usando nanopartículas de grafito. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2018. [Google Scholar]
- Valencia, A.; Zacarías, A.; Eduardo, E.; De Jesús, J.; Jiménez, C.; Gustavo, J. Tema A4 Termofluidos “ Evaluación numérica del rendimiento de un colector solar de absorción directa usando nanopartículas de grafito”. Congr. SOMIM 2021, 194–200. [Google Scholar]
- Deceased, J.A.D.; Beckman, W.A. Solar Engineering of Thermal Processes; Wiley: Hoboken, NJ, USA, 1982; Volume 3, ISBN 9780470873663. [Google Scholar]
- Gorji, T.B.; Ranjbar, A.A. A numerical and experimental investigation on the performance of a low-flux direct absorption solar collector (DASC) using graphite, magnetite and silver nanofluids. Sol. Energy 2016, 135, 493–505. [Google Scholar] [CrossRef]
- Klein, S.A. Engineering Equation Solver, 1992–2015, f-Chart Software. 2018. Available online: https://fchartsoftware.com/ees/ (accessed on 10 March 2022).
- Zacarías, A.; Quiroz, J.A.; Gutiérrez-Urueta, G.L.; Venegas, M.; Carvajal, I.; Rubio, J. Comparison between adiabatic and non-adiabatic absorption chillers using ammonia-lithium nitrate and water-lithium bromide solutions. Heat Transf. Res. 2020, 51, 609–621. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Ghajar, A.J. Transferencia de Calor y Masa, 4th ed.; Mc Graw Hill: Mexico City, Mexico, 2011; ISBN 978-607-15-0540-8. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Academic Press: New York, NY, USA, 1996; ISBN 9780080540771. [Google Scholar]
- Olson, J.R.; Topp, K.A.; Pohl, R.O. Specific heat and thermal conductivity of of solid Fullerenes. Science 1993, 259, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Diky, V.V.; Zhura, L.S.; Kabo, A.G.; Markov, V.Y.; Kabo, G.J.; Zhelezny, V.P.; Khanchych, K.Y.; Motovoy, I.V.; Nikulina, A.S. High-temperature heat capacity of C60 fullerene. J. Mol. Liq. 2001, 328, 115416. [Google Scholar] [CrossRef]
- Zhelezny, V.P.; Khanchych, K.Y.; Motovoy, I.V.; Nikulina, A.S. Viscous behaviour of o-xylene/fullerene C60 solutions. J. Mol. Liq. 2021, 328, 115416. [Google Scholar] [CrossRef]
- Martínez, J. Caracterización de Película Delgada de Carbono Obtenida vía Radiación de Microondas. Ph.D. Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2013. [Google Scholar]
- Martínez-Reyes, J.; Barriga-Arceo, L.G.D.; Rendón-Vazquez, L.; Martínez-Guerrero, R.; Romero-Partida, N.; Palacios-González, E.; Garibay-Febles, V.; Ortiz-López, J. Microstructural Characterization of Large Area C60 Films Obtained by Conventional Microwave Oven Irradiation. World J. Nano Sci. Eng. 2012, 2, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Serebryanayaa, N.R.; Blanka, V.D.; Ivdenkoa, V.A.; Chernozatonskiib, L.A. Pressure-induced superhard phase of C60. Solid State Commun. 2001, 118, 183–187. [Google Scholar] [CrossRef]
- Serebryanaya, N.R. X-ray Reference Patterns with PDF Number 49-1717 and 49-1718 database PDF-2 (2003) Produced for Superhard Pressure-Induced Phases of Fullerite C60. Available online: https://www.icdd.com/pdf-2/ (accessed on 10 March 2022).
- Chavda, V.P. Nanobased Nano Drug Delivery; Elsevier Inc.: Rajkot, India, 2019; ISBN 9780128140291. [Google Scholar]
- Bock Choon Pak, Y.I.C. Hydrodynamic and Heat Transfer Study of Dispersed Fluids With Submicron Metallic Oxide. Exp. Heat Transf. A J. Therm. Energy Transp. Storage Convers. 2013, 37–41. Available online: http://www.tandfonline.com/loi/ueht20 (accessed on 10 March 2022).
- Barrera, E.E. Análisis Experimental de un Colector Solar de Absorción Directa Utilizando Nanopartículas de Grafito. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2020. [Google Scholar]





| Thermophysical Property | Water [45] | Fullerene |
|---|---|---|
| Density, kg/m3 | 997 | 1720 [46] |
| Specific heat, J/kg K | 4180 | 506.1 [47,48] |
| Thermal conductivity, W/m K | 0.607 | 0.4 [46] |
| Viscosity, kg/m s | 0.00798 | 0.133231 [49] |
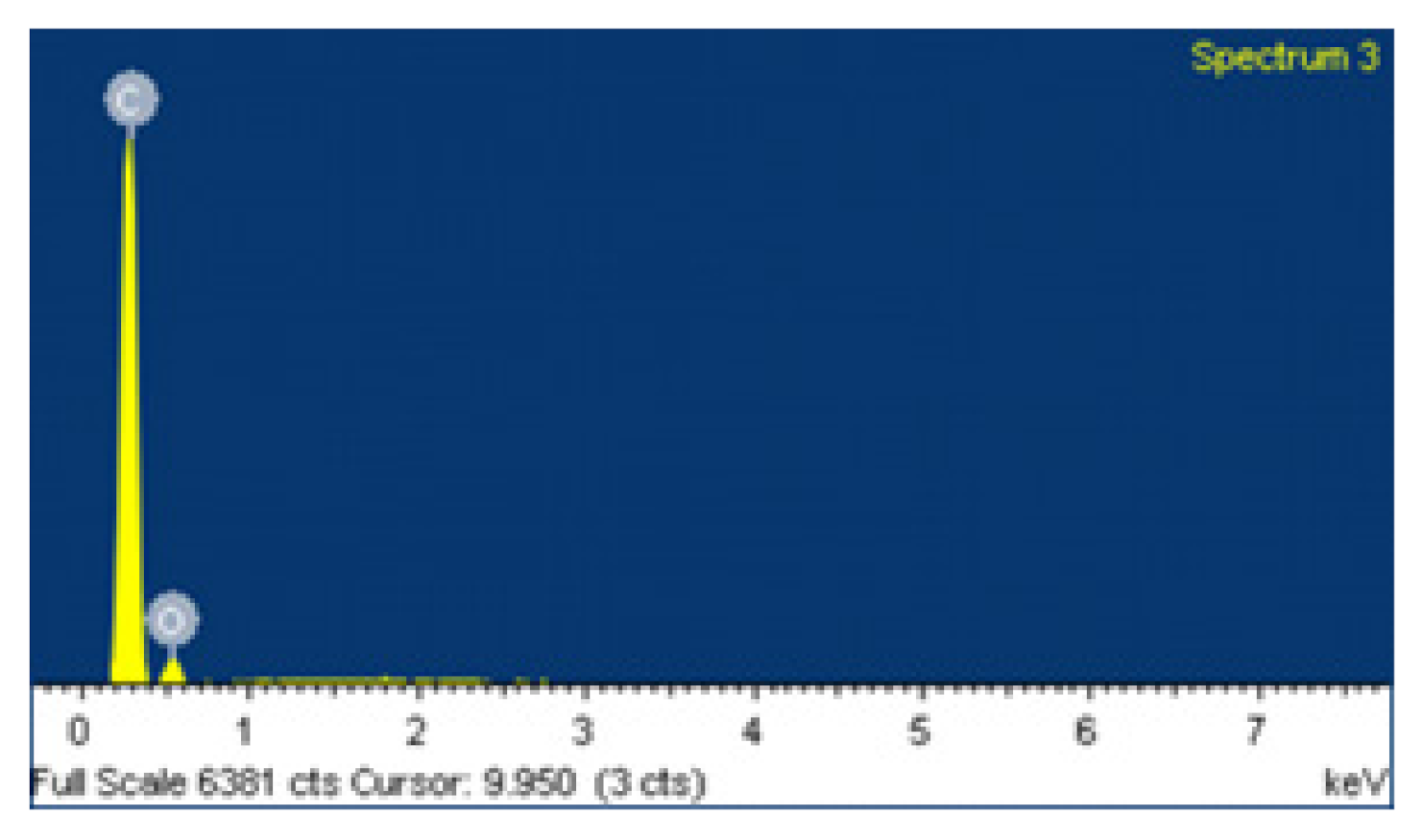
| Standard | Elements | Weight [%] | Atomic [%] |
|---|---|---|---|
| C | C K | 84.05 | 87.53 |
| O | O K | 15.95 | 12.47 |
| Totals | 100 | 100 |
| Thermophysical Properties | Range | Precision |
|---|---|---|
| Flowmeter, LT/min | 0.3–6 | ±5% FS |
| Pyranometer, W/m2 | 1–1999 | ±5% FS |
| Inlet temperature, Ti, °C | −20–100 | 1 |
| Outlet temperature, To, °C | −20–150 | 1.5 |
| Environment temperature, Ta, °C | −20–100 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera, E.E.; Medina, A.; Díaz-Barriga, L.G.; Zacarías, A.; Rubio, J.d.J.; Gutiérrez, G.L.; Cruz, J.M.; De Vega, M.; García, N.; Venegas, M. Performance Assessment of Low-Temperature Solar Collector with Fullerenes C60 Manufactured at Low Cost in an Emerging Country. Appl. Sci. 2022, 12, 3161. https://doi.org/10.3390/app12063161
Barrera EE, Medina A, Díaz-Barriga LG, Zacarías A, Rubio JdJ, Gutiérrez GL, Cruz JM, De Vega M, García N, Venegas M. Performance Assessment of Low-Temperature Solar Collector with Fullerenes C60 Manufactured at Low Cost in an Emerging Country. Applied Sciences. 2022; 12(6):3161. https://doi.org/10.3390/app12063161
Chicago/Turabian StyleBarrera, Esteban Eduardo, Abraham Medina, Lucía Graciela Díaz-Barriga, Alejandro Zacarías, José de Jesús Rubio, Geydy Luz Gutiérrez, José Michael Cruz, Mercedes De Vega, Néstor García, and María Venegas. 2022. "Performance Assessment of Low-Temperature Solar Collector with Fullerenes C60 Manufactured at Low Cost in an Emerging Country" Applied Sciences 12, no. 6: 3161. https://doi.org/10.3390/app12063161
APA StyleBarrera, E. E., Medina, A., Díaz-Barriga, L. G., Zacarías, A., Rubio, J. d. J., Gutiérrez, G. L., Cruz, J. M., De Vega, M., García, N., & Venegas, M. (2022). Performance Assessment of Low-Temperature Solar Collector with Fullerenes C60 Manufactured at Low Cost in an Emerging Country. Applied Sciences, 12(6), 3161. https://doi.org/10.3390/app12063161










