Modification of Hand Muscular Synergies in Stroke Patients after Robot-Aided Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
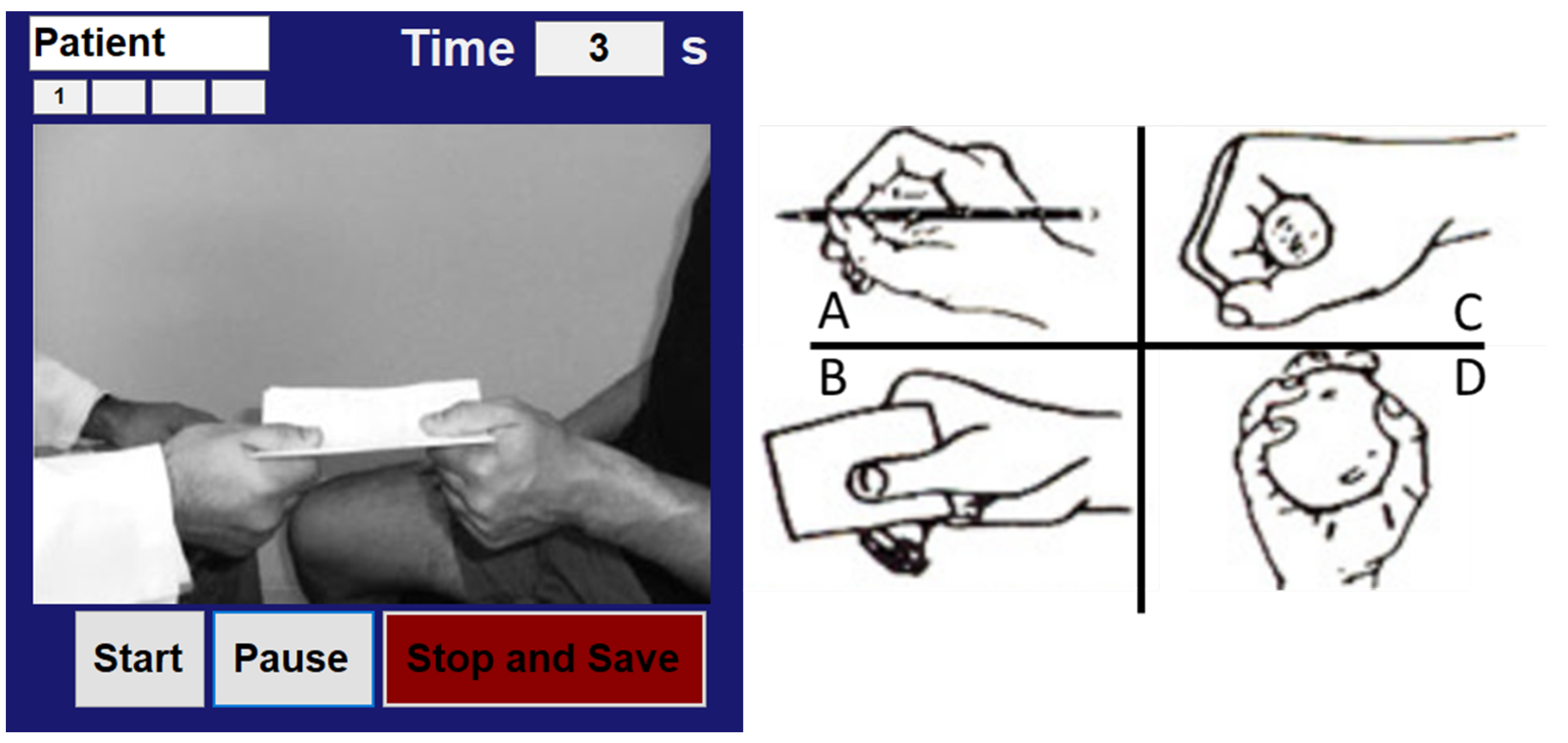
2.2. Experimental Protocol
2.3. Gloreha Sinfonia
2.4. EMG Acquisition and Muscle Synergies Extraction
2.5. Performance Indices
- Cosine similarity (CS): it is a measure of the degree of similarity between two different vectors, described by Equation (3). It is the measure of the cosine of the angle between the two non-zero vectors and , and is a value ranging from −1 to 1. The CS is equal to 0, when the angle measured between the two vectors is equal to . In our analysis, a CS close to 1 indicates equal synergies, and values close to −1 indicate vectors of equal but opposite synergies. It can be computed aswhere is the angle between the two synergy vectors and , is the norm of the synergy vector, and ∗ denotes the scalar product.
- Similarity index (SI): it is computed as the weighted sum of the difference between two synergy vectors, as described by Equation (4). SI represents a similarity index between two synergy vectors, able to take into account the Euclidean distance between the two vectors of synergies. Hence, two vectors with a high SI are in the same region of the vector space. It can be computed aswhere S is the number of muscular synergies, and and are the two synergy vectors.
2.6. Statistical Analysis
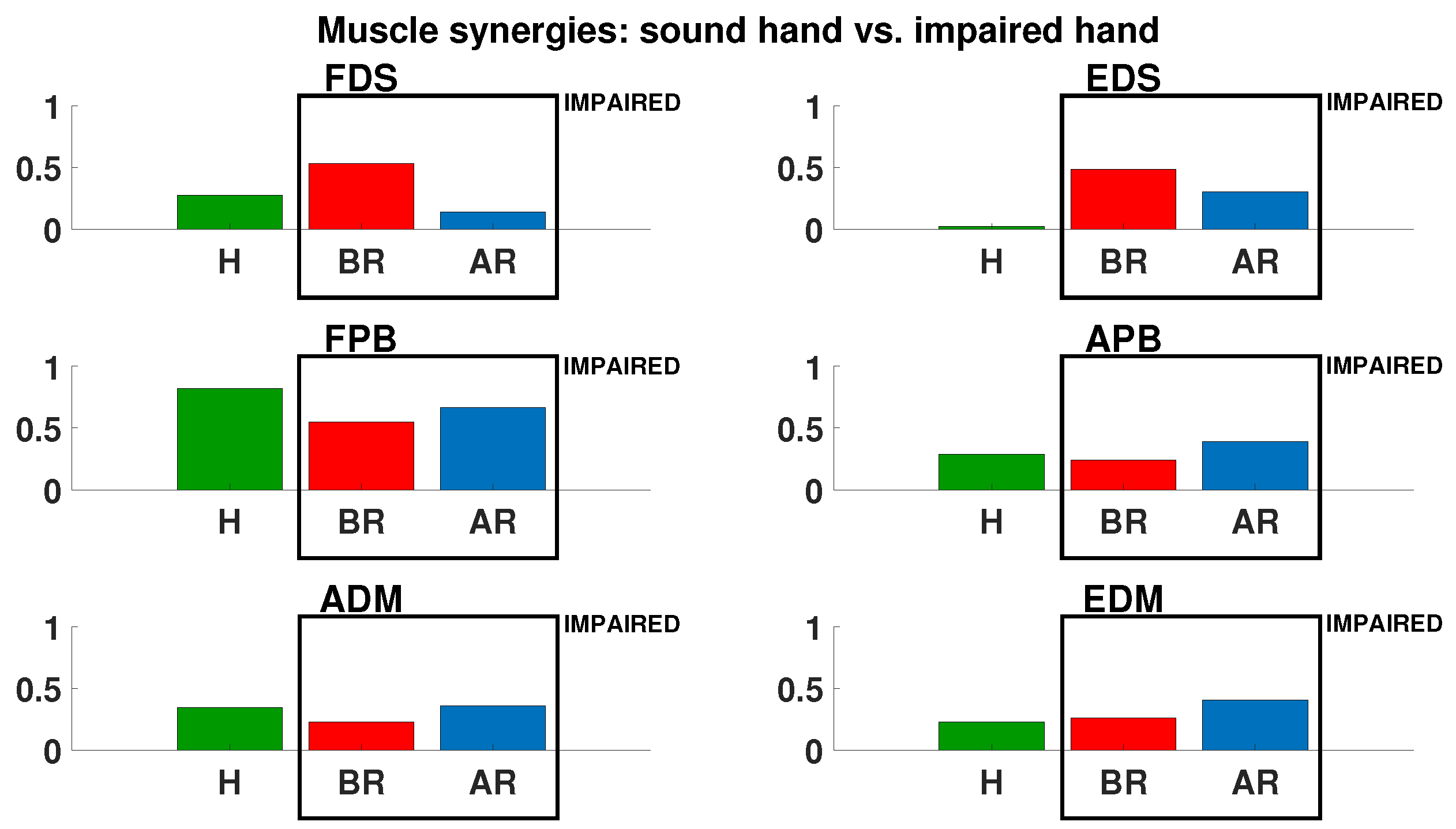
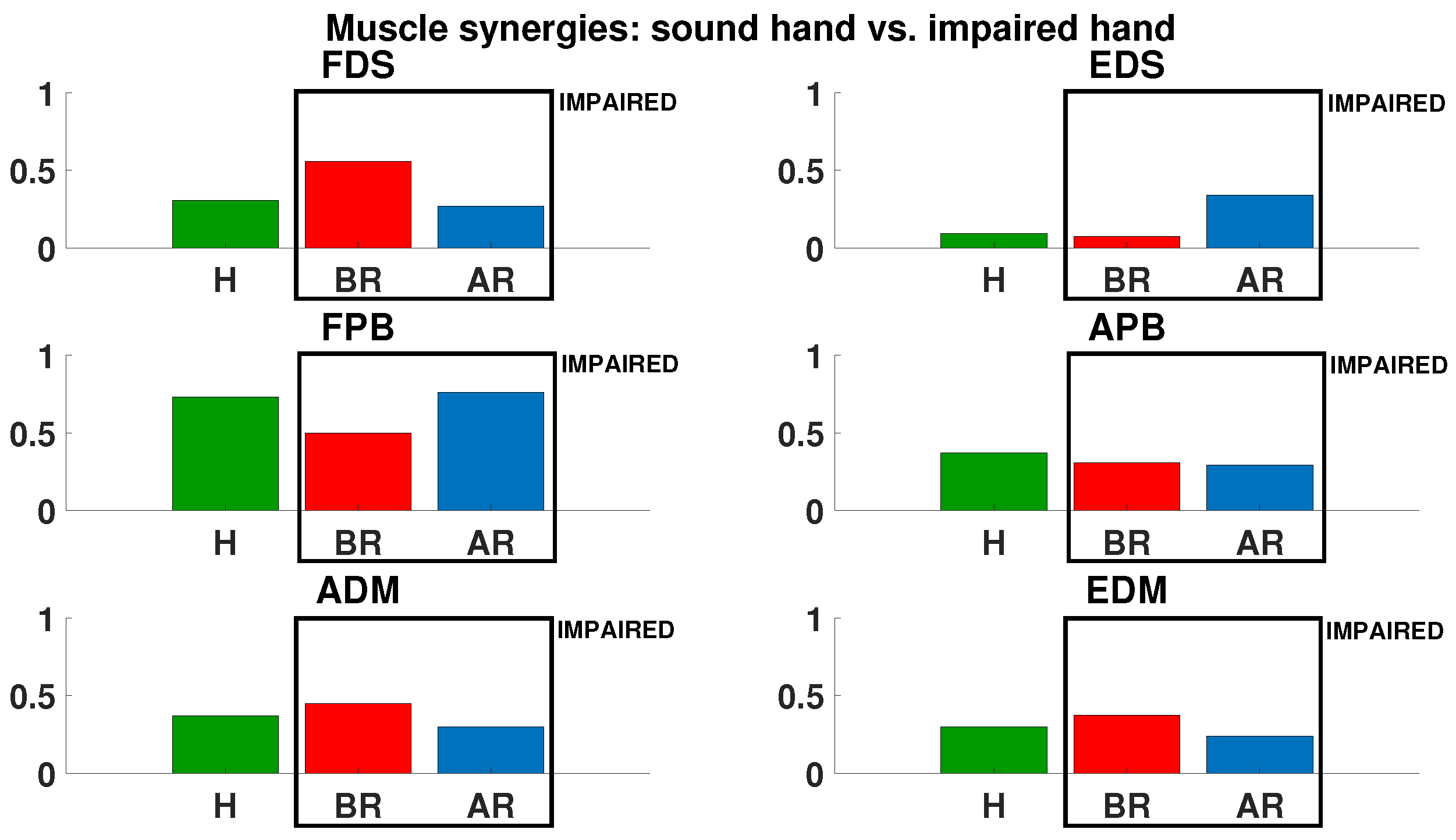
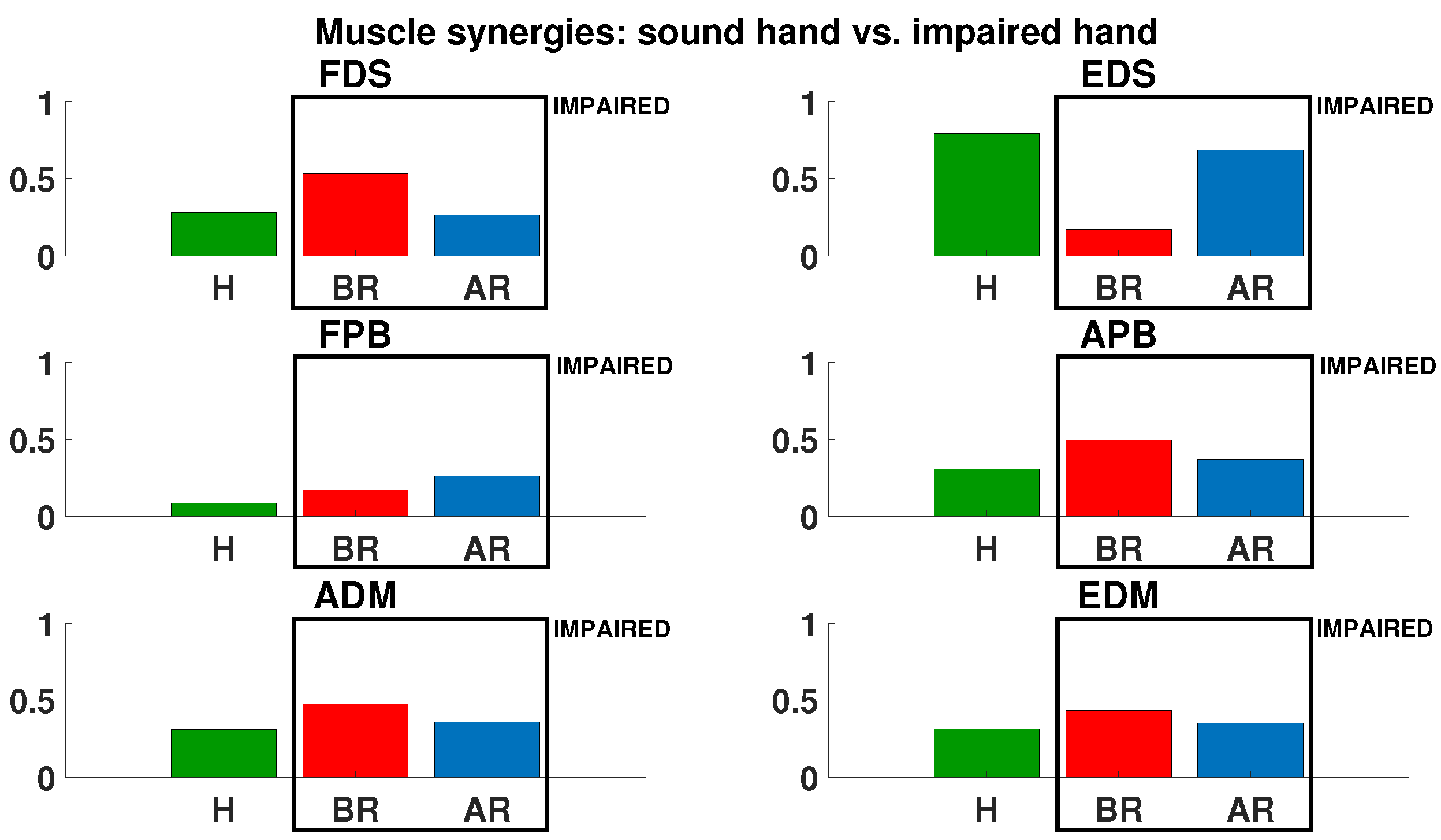
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santello, M.; Baud-Bovy, G.; Jörntell, H. Neural bases of hand synergies. Front. Comput. Neurosci. 2013, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, A.B.; Weir, R. Muscle synergies as a predictive framework for the EMG patterns of new hand postures. J. Neural Eng. 2009, 6, 036004. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.E.; Iqbal, K.; White, G.; Hutchinson, T.E. A systematic review on muscle synergies: From building blocks of motor behavior to a neurorehabilitation tool. Appl. Bionics Biomech. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N. The Co-Ordination and Regulation of Movements; Pergamon Press: New York, NY, USA, 1966. [Google Scholar]
- Bernstein, N.A. On the Construction of Movements; Routledge: New York, NY, USA, 1947. [Google Scholar]
- Bizzi, E.; Cheung, V.C. The neural origin of muscle synergies. Front. Comput. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. On the methodological implications of extracting muscle synergies from human locomotion. Int. J. Neural Syst. 2017, 27, 1750007. [Google Scholar] [CrossRef]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of muscle synergy outcomes in clinics, robotics, and sports: A systematic review. Appl. Bionics Biomech. 2018, 2018. [Google Scholar] [CrossRef]
- Tresch, M.C.; Cheung, V.C.; d’Avella, A. Matrix factorization algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef]
- Liew, B.X.; Del Vecchio, A.; Falla, D. The influence of musculoskeletal pain disorders on muscle synergies—A systematic review. PLoS ONE 2018, 13, e0206885. [Google Scholar] [CrossRef]
- Santello, M.; Lang, C.E. Are movement disorders and sensorimotor injuries pathologic synergies? When normal multi-joint movement synergies become pathologic. Front. Hum. Neurosci. 2015, 8, 1050. [Google Scholar] [CrossRef]
- Safavynia, S.; Torres-Oviedo, G.; Ting, L. Muscle synergies: Implications for clinical evaluation and rehabilitation of movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.E.; Iqbal, K.; White, G.; Holtz, J.K. A review of EMG techniques for detection of gait disorders. In Artificial Intelligence-Applications in Medicine and Biology; InTechOpen: London, UK, 2019. [Google Scholar]
- Cheung, V.C.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef]
- McMorland, A.J.; Runnalls, K.D.; Byblow, W.D. A neuroanatomical framework for upper limb synergies after stroke. Front. Hum. Neurosci. 2015, 9, 82. [Google Scholar] [CrossRef]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front. Hum. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Dipietro, L.; Krebs, H.I.; Fasoli, S.E.; Volpe, B.T.; Stein, J.; Bever, C.; Hogan, N. Changing motor synergies in chronic stroke. J. Neurophysiol. 2007, 98, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Plasticity of the human motor cortex and recovery from stroke. Brain Res. Rev. 2001, 36, 169–174. [Google Scholar] [CrossRef]
- Scotto di Luzio, F.; Cordella, F.; Lauretti, C.; Draicchio, F.; Zollo, L. Assessment of muscular activation patterns in 3D upper limb robot-aided rehabilitation. In International Conference on NeuroRehabilitation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 349–353. [Google Scholar]
- Lauretti, C.; Cordella, F.; Ciancio, A.L.; Trigili, E.; Catalan, J.M.; Badesa, F.J.; Crea, S.; Pagliara, S.M.; Sterzi, S.; Vitiello, N.; et al. Learning by demonstration for motion planning of upper-limb exoskeletons. Front. Neurorobot. 2018, 12, 5. [Google Scholar] [CrossRef]
- Simonetti, D.; Zollo, L.; Milighetti, S.; Miccinilli, S.; Bravi, M.; Ranieri, F.; Magrone, G.; Guglielmelli, E.; Di Lazzaro, V.; Sterzi, S. Literature review on the effects of tDCS coupled with robotic therapy in post stroke upper limb rehabilitation. Front. Hum. Neurosci. 2017, 11, 268. [Google Scholar] [CrossRef]
- Tan, C.K.; Kadone, H.; Watanabe, H.; Marushima, A.; Yamazaki, M.; Sankai, Y.; Suzuki, K. Lateral symmetry of synergies in lower limb muscles of acute post-stroke patients after robotic intervention. Front. Neurosci. 2018, 12, 276. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; van der Smagt, P. Evidence of muscle synergies during human grasping. Biol. Cybern. 2013, 107, 233–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, E.J.; Flanders, M. Muscular and postural synergies of the human hand. J. Neurophysiol. 2004, 92, 523–535. [Google Scholar] [CrossRef]
- Israely, S.; Leisman, G.; Machluf, C.C.; Carmeli, E. Muscle synergies control during hand-reaching tasks in multiple directions post-stroke. Front. Comput. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef]
- Gloreha IDROGENET srl, Italy. Available online: https://www.gloreha.com/ (accessed on 19 November 2021).
- Dean, C.; Mackey, F. Motor assessment scale scores as a measure of rehabilitation outcome following stroke. Aust. J. Physiother. 1992, 38, 31–35. [Google Scholar] [CrossRef]
- Singer, B.; Garcia-Vega, J. The Fugl-Meyer upper extremity scale. J. Physiother. 2017, 63, 53. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, Y.J. Nonnegative matrix factorization: A comprehensive review. IEEE Trans. Knowl. Data Eng. 2012, 25, 1336–1353. [Google Scholar] [CrossRef]



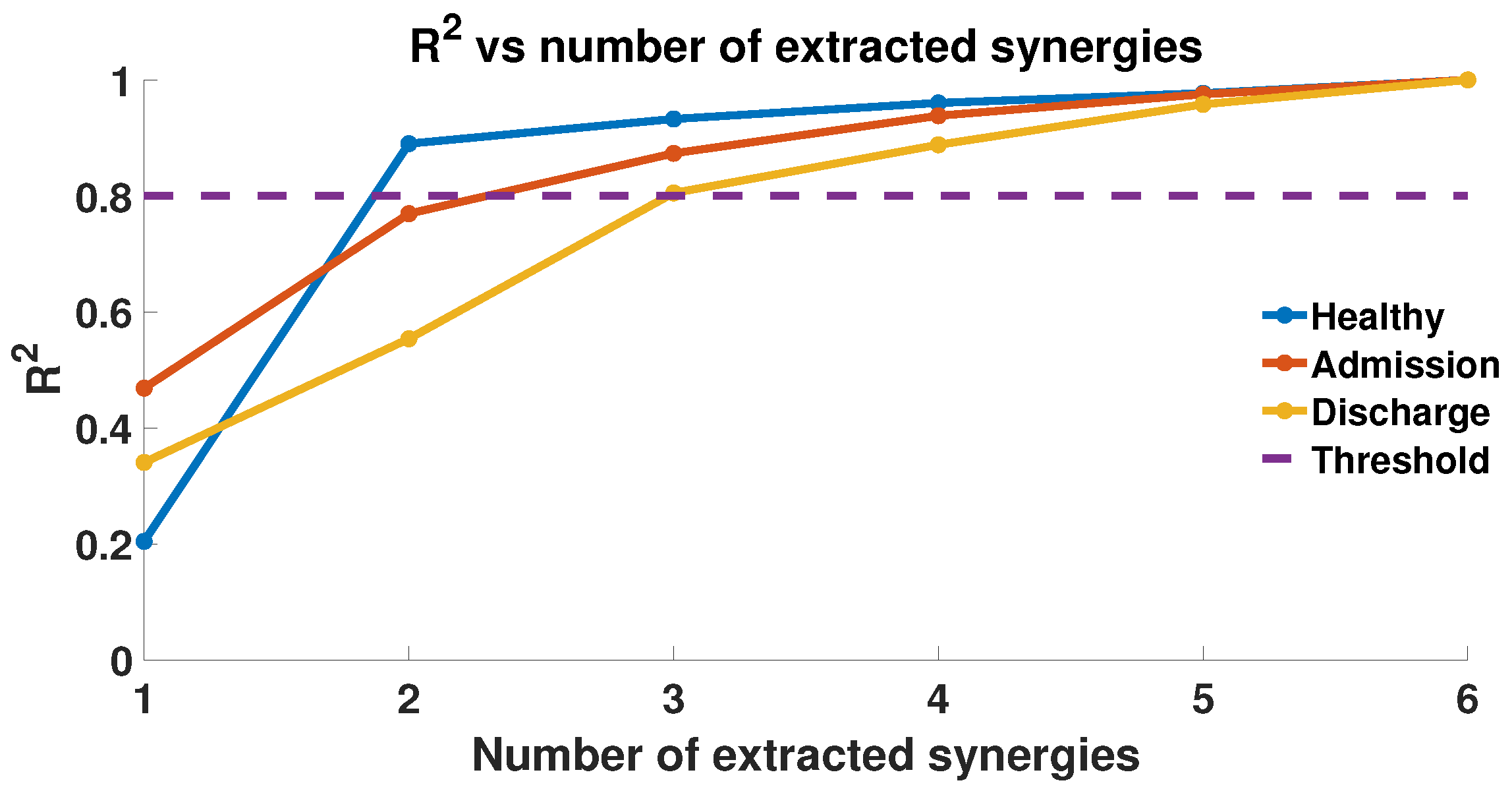

| Age | Sex | Stroke | Time from Stroke [Months] | |
|---|---|---|---|---|
| 1 | 56 | M | ischemic | 68 |
| 2 | 53 | F | hemorrhagic | 96 |
| 3 | 40 | M | ischemic | 75 |
| 4 | 79 | F | ischemic | 18 |
| 5 | 61 | M | hemorrhagic | 49 |
| 6 | 56 | M | hemorrhagic | 30 |
| 7 | 72 | M | ischemic | 33 |
| FM * | MP * | |||
|---|---|---|---|---|
| BR | AR | BR | AR | |
| 1 | 39 | 45 | 5 | 10 |
| 2 | 37 | 48 | 9 | 12 |
| 3 | 36 | 48 | 6 | 12 |
| 4 | 45 | 51 | 15 | 18 |
| 5 | 34 | 37 | 4 | 8 |
| 6 | 31 | 34 | 16 | 16 |
| 7 | 26 | 36 | 14 | 15 |
| CS | SI | |||||
|---|---|---|---|---|---|---|
| H-BR * | H-AR * | BR-AR * | H-BR * | H-AR * | BR-AR * | |
| P | ||||||
| S | ||||||
| C | ||||||
| B | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotto di Luzio, F.; Cordella, F.; Bravi, M.; Santacaterina, F.; Bressi, F.; Sterzi, S.; Zollo, L. Modification of Hand Muscular Synergies in Stroke Patients after Robot-Aided Rehabilitation. Appl. Sci. 2022, 12, 3146. https://doi.org/10.3390/app12063146
Scotto di Luzio F, Cordella F, Bravi M, Santacaterina F, Bressi F, Sterzi S, Zollo L. Modification of Hand Muscular Synergies in Stroke Patients after Robot-Aided Rehabilitation. Applied Sciences. 2022; 12(6):3146. https://doi.org/10.3390/app12063146
Chicago/Turabian StyleScotto di Luzio, Francesco, Francesca Cordella, Marco Bravi, Fabio Santacaterina, Federica Bressi, Silvia Sterzi, and Loredana Zollo. 2022. "Modification of Hand Muscular Synergies in Stroke Patients after Robot-Aided Rehabilitation" Applied Sciences 12, no. 6: 3146. https://doi.org/10.3390/app12063146
APA StyleScotto di Luzio, F., Cordella, F., Bravi, M., Santacaterina, F., Bressi, F., Sterzi, S., & Zollo, L. (2022). Modification of Hand Muscular Synergies in Stroke Patients after Robot-Aided Rehabilitation. Applied Sciences, 12(6), 3146. https://doi.org/10.3390/app12063146








