Temporal Variations of Heavy Metal Sources in Agricultural Soils in Malta
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of Study Area
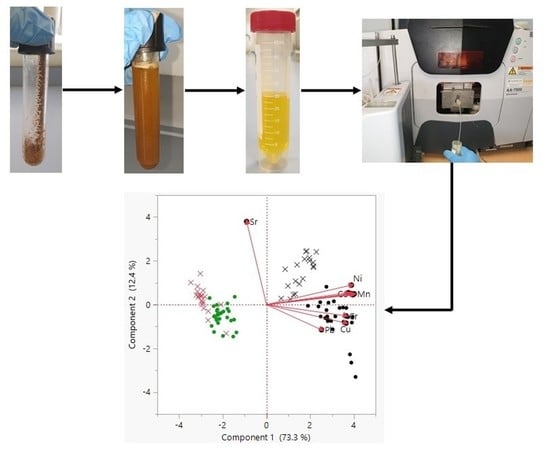
2.2. Sampling and Pre-Treatment
2.3. Physical Analysis
2.4. Chemical Analysis
2.5. Analysis of Heavy Metals Using Spectroscopy
2.6. Statistical Analysis
2.7. Health Risk Assessment
2.8. Potential Ecological Risk Assessment
3. Results and Discussion
3.1. Physical Analysis
3.2. Chemical Analysis
3.2.1. Descriptive Statistics
3.2.2. Correlation Analysis
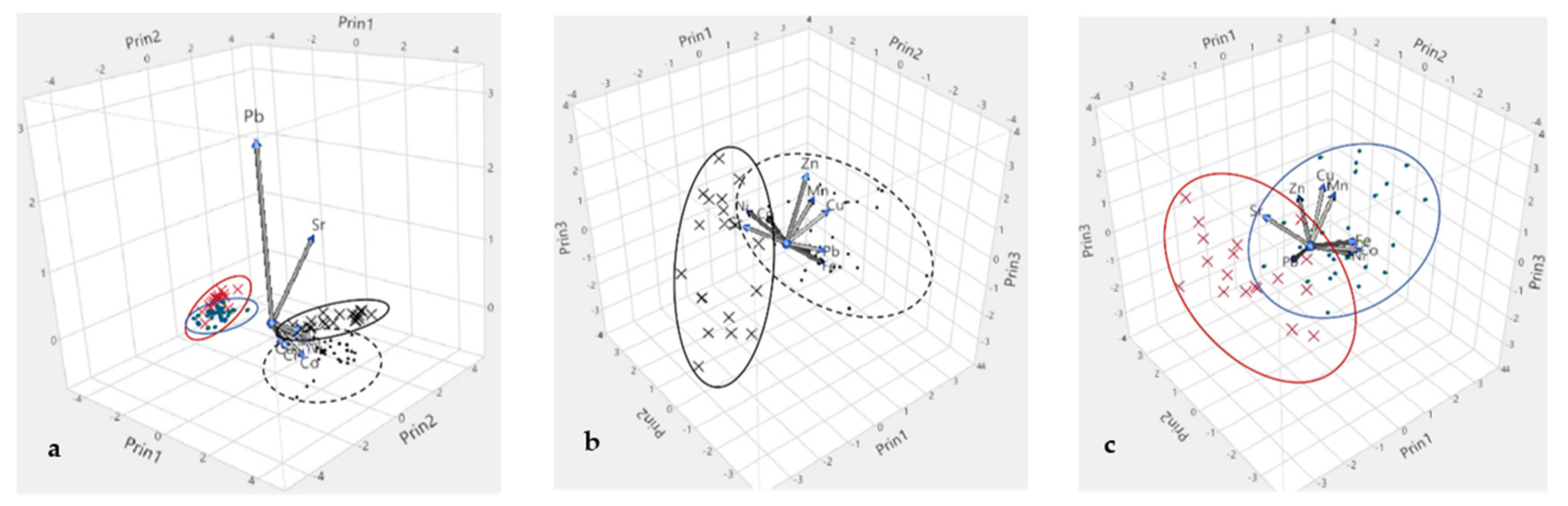
3.2.3. Multivariate Statistical Analysis
| (a) Pre- & Post-Harvesting Stages | (b) Pre-Harvesting Stage | (c) Post-Harvesting Stage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1a | PC2a | F1a | F2a | PC1b | PC2b | F1b | F2b | PC1c | PC2c | F1c | F2c | |
| Co | 0.952 | 0.124 | 0.967 | 0.100 | 0.443 | 0.824 | 0.241 | 0.835 | 0.822 | −0.343 | 0.709 | 0.410 |
| Cr | 0.919 | 0.134 | 0.861 | 0.329 | 0.709 | 0.114 | 0.530 | 0.214 | 0.843 | 0.111 | 0.663 | 0.448 |
| Cu | 0.893 | −0.125 | 0.807 | 0.271 | 0.854 | −0.159 | 0.896 | 0.030 | 0.712 | 0.284 | 0.212 | 0.777 |
| Fe | 0.893 | −0.207 | 0.735 | 0.592 | 0.810 | −0.117 | 0.669 | 0.037 | 0.229 | −0.637 | 0.199 | 0.072 |
| Mn | 0.970 | 0.111 | 0.958 | 0.216 | 0.864 | 0.170 | 0.857 | 0.309 | 0.828 | 0.172 | 0.310 | 0.951 |
| Ni | 0.985 | 0.121 | 0.961 | 0.265 | 0.060 | 0.951 | −0.150 | 0.964 | 0.864 | −0.158 | 0.818 | 0.359 |
| Pb | 0.954 | 0.219 | 0.995 | 0.053 | 0.529 | −0.373 | 0.468 | −0.172 | 0.272 | 0.558 | 0.205 | 0.031 |
| Sr | 0.618 | −0.281 | 0.458 | 0.394 | −0.716 | 0.297 | −0.648 | 0.096 | −0.684 | 0.351 | −0.812 | −0.066 |
| Zn | −0.223 | 0.932 | −0.022 | −0.446 | 0.458 | −0.019 | 0.550 | 0.094 | 0.407 | 0.527 | 0.078 | 0.399 |
3.3. Health Risk Assessment
3.4. Potential Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallett, S.; Sakrabani, R.; Thompson, A.; Deeks, L.; Knox, J. Improving Soil and Water Management for Agriculture: Insights and Innovation from Malta. MCAST J. Appl. Res. Pract. 2017, 1, 40–59. [Google Scholar] [CrossRef]
- Union, E. The EU Environmental Implementation Review 2019 Country Report—MALTA; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Environment and Resources Authority. Maltese Soils. 2020. Available online: https://era.org.mt/topic/maltese-soils/ (accessed on 20 May 2021).
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Paul, E. Soil Microbiology, Ecology and Biochemistry, 4th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Nortcliff, B.; Schulte-Bisping, L. Soil Definition, Function, and Utilization. In Ullmann’s Encyclopaedia; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 400–420. [Google Scholar]
- Mulder, E.G.; VanVeen, W.L. Effect of Microorganisms on the Transformation of Mineral Fractions in Soil; Agricultural University: Wageningen, The Netherlands, 2010. [Google Scholar]
- Ladonin, D.V.; Karpukhin, M.M. Fractional Composition of Nickel, Copper, Zinc, and Lead Compounds in Soils Polluted by Oxides and Soluble Metal Salts. Eurasian Soil Sci. 2011, 44, 874–885. [Google Scholar] [CrossRef]
- Ghosh, M.; Devi, A. Assessment of crop growth, soil properties and crop yield in an upland acidic soil with inorganic fertilizer blended with organic amendments in summer rice cropping seasons. Int. J. Recycl. Org. Waste Agric. 2019, 8, S1–S9. [Google Scholar] [CrossRef]
- Pomazkina, L.V.; Semenova, Y.V. Effects of Climate Changes and Pollution with Heavy Metals on the Transformation of Carbon Compounds in Different Soil Types of Agroecosystems in the Forest-Steppe of Baikal Region. Eurasian Soil Sci. 2018, 51, 576–587. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, K.; Yang, F.; Chen, Y.; Fu, C.; Huang, Y.; Guo, Z.; Li, T. Migration and Transformation of Heavy Metals in the Soil of the Water-Level Fluctuation Zone in the Three Gorges Reservoir under Simulated Nitrogen Deposition. J. Chem. 2021, 2021, 6660661. [Google Scholar] [CrossRef]
- O’Neill, P. Environmental Chemistry, 3rd ed.; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Markiewicz-Patkowska, J.; Hursthouse, A.; Przybyla-Kij, H. The interaction of heavy metals with urban soils: Sorption behaviour of Cd, Cu, Cr, Pb and Zn with a typical mixed brownfield deposit. Environ. Int. 2005, 31, 513–521. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Orroño, D.I.; Lavado, R.S. Distribution of extractable heavy metals in different soil fractions. Chem. Speciat. Bioavailab. 2009, 21, 193–198. [Google Scholar] [CrossRef]
- CustomWeather, Valletta Weather History. 2021. Available online: https://www.timeanddate.com/weather/malta/valletta/historic?month=11&year=2020 (accessed on 28 July 2021).
- Radojevic, M.; Bashkin, V. Practical Environmental Analysis, 2nd ed.; The Royal Society of Chemistry: Dorset, UK, 2006. [Google Scholar]
- Sarkar, D.; Haldar, A. Physical and Chemical Methods in Soil Analysis; New Age International (P) Ltd.: New Delhi, India, 2005. [Google Scholar]
- Shugar, G.; Ballinger, J. Chemical Technicians’ Ready Reference Handbook, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 1996. [Google Scholar]
- Sanz-Medel, A.; Pereiro, R. Atomic Absorption Spectrometry: An Introduction, 2nd ed.; Momentum Press, LLC: New York, NY, USA, 2014. [Google Scholar]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy Metals in Wastewater and Sewage Sludge from Selected Municipal Treatment Plants in Eastern Cape Province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzemińska, E. Human Health Risk Assessment associated with contaminants in the finest fraction of sidewalk dust collected in proximity to trafficked roads. Sci. Rep. 2019, 9, 16364. [Google Scholar] [CrossRef]
- Anyanwu, E.D.; Nwachukwu, E.D. Heavy metal content and health risk assessment of a South-eastern Nigeria River. Appl. Water Sci. 2020, 10, 209–210. [Google Scholar] [CrossRef]
- USEPA IRIS (US Environmental Protection Agency)’s Integrated Risk Information System. Environmental Protection Agency Region I; United States Environmental Protection Agency: Washington, DC, USA, 2011.
- Liang, Y.; Yi, X.; Dang, Z.; Wang, Q.; Luo, H.; Tang, J. Heavy Metal Contamination and Health Risk Assessment in the Vicinity of a Tailing Pond in Guangdong, China. Int. J. Environ. Res. Public Health 2017, 14, 1557. [Google Scholar] [CrossRef]
- Mohammadi AA Zarei, A.; Majidi, S.; Ghaderpoury, A.; Hashempour, Y.; Saghi, M.H.; Ghaderpoori, M. Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodsX 2019, 6, 1642–1651. [Google Scholar] [CrossRef]
- Salhotra, A.M. Chapter Ten—Human Health Risk Assessment for Contaminated Properties. Prog. Mol. Biol. Transl. Sci. 2012, 112, 285–306. [Google Scholar]
- Rouhani, A.; Shahivand, R. Potential ecological risk assessment of heavy metals in archaeology on an example of the Tappe Rivi (Iran). SN Appl. Sci. 2020, 2, 1277. [Google Scholar] [CrossRef]
- Turekian, K.; Wedepohl, K. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Negahban, S.; Mokarram, M. Potential Ecological Risk Assessment of Ni, Cu, Zn, Cd, and Pb in Roadside Soils. Earth Space Sci. 2021, 8, e2020EA001120. [Google Scholar] [CrossRef]
- Al-Edresy, M.; Wasel, S.; Al-Hagibi, H. Ecological risk assessment of heavy metals in coastal sediments between Al-Haymah and Al-Mokha, south red sea, Yemen. Int. J. Hydrol. 2019, 3, 159–173. [Google Scholar] [CrossRef]
- Salah, E.; Turki, A.; Mahal, S.N. Chemometric Evaluation of the Heavy Metals in Urban Soil of Fallujah City, Iraq. J. Environ. Prot. 2015, 6, 1279–1292. [Google Scholar] [CrossRef]
- Serelis, K.; Kafkala, I.; Parpodis, K.; Lazaris, S. Anthropogenic and Geogenic Contamination Due to Heavy Metals in the Vast Area of Vari, Attica. Bull. Geol. Soc. Greece 2017, 43, 2390–2397. [Google Scholar] [CrossRef][Green Version]
- Nunes, J.R.; Ramos-Miras, J.; Lopez-Piñeiro, A.; Loures, L.; Gil, C.; Coelho, J.; Loures, A. Concentrations of Available Heavy Metals in Mediterranean Agricultural Soils and their Relation with Some Soil Selected Properties: A Case Study in Typical Mediterranean Soils. Sustainability 2014, 6, 9124–9138. [Google Scholar] [CrossRef]
- Khatita, A.; Koch, R.; Bamousa, A. Sources identification and contamination assessment of heavy metals in soil of Middle Nile Delta, Egypt. J. Taibah Univ. Sci. 2020, 14, 750–761. [Google Scholar] [CrossRef]
- Briffa, J. Heavy Metals in Maltese Agricultural Soil, Malta. University of Malta Library. 2020. Available online: https://www.um.edu.mt/library/oar/handle/123456789/73391 (accessed on 17 May 2021).
- European Commision, DG Environment. Final Implementation Report for Directive 86/278/EEC on Sewage Sludge: 2013–2015. 2018. Available online: https://ec.europa.eu/environment/archives/waste/reporting/pdf/Final_Implementation_Report_2013_2015_Sewage_Sludge.pdf (accessed on 11 June 2021).
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Dubchak, S. Distribution of Strontium in Soil: Interception, Weathering, Speciation, and Translocation to Plants. In Behaviour of Strontium in Plants and the Environment; Gupta, D., Walther, C., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Štofejová, L.; Fazekaš, J.; Fazekašová, D. Analysis of Heavy Metal Content in Soil and Plants in the Dumping Ground of Magnesite Mining Factory Jelsava-Lubenik (Slovakia). Sustainability 2021, 13, 4508. [Google Scholar] [CrossRef]
- Pedley, H.M.; House, M.R.; Waugh, B. The geology of Malta and Gozo. Geol. Assoc. 1976, 87, 325–341. [Google Scholar] [CrossRef]

| Parameter | Symbol | Unit | Adult Value | Children Value |
|---|---|---|---|---|
| Ingestion Rate | IRingest | mg/day | 100 | 200 |
| Exposure Frequency | EF | days/year | 350 | 350 |
| Exposure Duration | ED | years | 30 | 6 |
| Average Body Weight | BW | kg | 70 | 15 |
| Non-Carcinogens Averaging Time | AT = ED × 365 | days | 10,950 | 2190 |
| Carcinogens Averaging Time | AT = 70 years × 365 | days | 25,550 | 25,550 |
| Unity Conversion Factor | CF | kg/mg | 1 × 10−6 | 1 × 10−6 |
| Exposed Skin Area | SA | cm2 | 5700 | 2800 |
| Adherence Factor | AF | mg/cm3 | 0.07 | 0.07 |
| Dermal Absorption Fraction | ABS | / | 0.001 | 0.001 |
| Metal | RFD 1 by Ingestion (Mg/Kg/Day) | RFD 1 by Dermal Contact (Mg/Kg/Day) | Slope Factor (Mg/Kg/Day) |
|---|---|---|---|
| Fe | 0.007 | Not found | Not found |
| Mn | 0.014 | Not found | Not found |
| Zn | 0.300 | 0.060 | Not found |
| Cr | 3.000 × 10−3 | 6.000 × 10−5 | 0.500 |
| Ni | 0.020 | 5.400 × 10−3 | 0.910 |
| Pb | 3.5 × 10−3 | 5.25 × 10−4 | 0.085 |
| Cu | 0.040 | 0.012 | Not found |
| Geo-Accumulation Index Level | Igeo Class | Igeo Value |
|---|---|---|
| Uncontaminated | 0 | Igeo ≤ 0 |
| Uncontaminated/moderately contaminated | 1 | 0 < Igeo < 1 |
| Moderately contaminated | 2 | 1 < Igeo < 2 |
| Moderately/strongly contaminated | 3 | 2 < Igeo < 3 |
| Strongly contaminated | 4 | 3 < Igeo < 4 |
| Strongly/extremely contaminated | 5 | 4 < Igeo < 5 |
| Extremely contaminated | 6 | 5 < Igeo |
| Risk Factor Level | Ei Value |
|---|---|
| Low potential ecological risk | Ei < 40 |
| Moderate potential ecological risk | 40 ≤ Ei < 80 |
| Considerable potential ecological risk | 80 ≤ Ei < 160 |
| High potential ecological risk | 160 ≤ Ei < 320 |
| Very high potential ecological risk | 320 ≤ Ei |
| Variable | Symbol | Unit | Descriptive Statistics | |||
|---|---|---|---|---|---|---|
| Pre-Harvesting Concentrations | Post-Harvesting Concentrations | Mean | Standard Deviation | |||
| Cobalt | Co | μg/g | 0.150 | 0.047 | 0.100 | 0.055 |
| Chromium | Cr | μg/g | 0.450 | 0.197 | 0.339 | 0.214 |
| Copper | Cu | μg/g | 1.456 | 0.108 | 0.818 | 1.092 |
| Iron | Fe | μg/g | 3814.549 | 729.829 | 2594.401 | 1290.501 |
| Manganese | Mn | μg/g | 66.338 | 15.118 | 41.898 | 27.021 |
| Nickel | Ni | μg/g | 0.824 | 0.0915 | 0.461 | 0.370 |
| Lead | Pb | μg/g | 4.373 | 1.331 | 3.034 | 4.026 |
| Strontium | Sr | μg/g | 10.050 | 10.197 | 9.969 | 1.236 |
| Zinc | Zn | μg/g | 254.888 | 157.437 | 207.262 | 59.237 |
| Element | Mean Concentrations in This Study (mg/g) | Study From Spain by Micó, et al. (2006) (mg/Kg) [4] | Study from Iraq by Salah, et al. (2015) (mg/Kg) [33] | Study from Greece by Serelis, et al. (2010) (mg/Kg) [34] | Study from Portugal-Spain Border by Nunes, et al. (2014) (mg/Kg) [35] | Study from Egypt by Khatita, et al. (2020) (ppm) [36] | Concentration target Values for Soil (ppm) [37] | Target Values for Malta by 86/278/EEC (mg/Kg) [38] |
|---|---|---|---|---|---|---|---|---|
| Co | 0.100 | 7 | 3.430 | 8.300 | - | 31 | 20 | - |
| Cr | 0.339 | 27 | 11.590 | - | 0.850 | 128 | 100 | - |
| Cu | 0.818 | 23 | 2.010 | - | 1.230 | 61.800 | 36 | 100 |
| Fe | 2594.401 | 13,608 | 235.770 | - | - | 62,583 | - | - |
| Mn | 41.898 | 295 | - | - | - | 1099 | 200 | - |
| Ni | 0.461 | 21 | 8.960 | 9.430 | 1.950 | 70.600 | 35 | 70 |
| Pb | 3.034 | 23 | 3.820 | 161.360 | 3.160 | 30.700 | 85 | 100 |
| Sr | 9.969 | - | - | - | - | 308 | - | - |
| Zn | 207.262 | 53 | 5.500 | 3.520 | 0.640 | 143 | 50 | 200 |
| Elements | Hypothesis 1: The Distribution Is the Same Across Field A & Field B. | Hypothesis 2a: The Distribution Is the Same Across Field A. | Hypothesis 2b: The Distribution Is the Same Across Field B. | |
|---|---|---|---|---|
| Pre-Harvesting | Post-Harvesting | Pre- & Post-Harvesting | Pre- & Post-Harvesting | |
| Co | Rejected | Rejected | Rejected | Rejected |
| Cr | Rejected | Rejected | Rejected | Rejected |
| Cu | Rejected | Rejected | Rejected | Rejected |
| Fe | Rejected | Retained (0.828) | Rejected | Rejected |
| Mn | Rejected | Rejected | Rejected | Rejected |
| Ni | Retained (0.276) | Rejected | Rejected | Rejected |
| Pb | Rejected | Rejected | Rejected | Rejected |
| Sr | Rejected | Rejected | Retained (0.165) | Rejected |
| Zn | Retained (0.205) | Retained (0.052) | Rejected | Rejected |
| Test significance level is 0.05. | ||||
| Element | Mean Concentration (mg/g) | HQadults | CRadults | HQchildren | CRchildren | Igeo | CF | Ei |
|---|---|---|---|---|---|---|---|---|
| Co | 0.100 | N/A * | N/A * | N/A * | N/A * | −0.584 | 1.001 | 5.005 |
| Cr | 0.339 | 7.951 × 10−5 | 9.982 × 10−8 | 1.298 × 10−4 | 1.858 × 10−7 | −5.606 | 0.031 | 0.062 |
| Cu | 0.818 | 1.216 × 10−5 | N/A * | 2.248 × 10−5 | N/A * | −2.875 | 0.204 | 1.022 |
| Fe | 2594.401 | N/A * | N/A * | N/A * | N/A * | −1.136 | 0.683 | N/A ** |
| Mn | 41.898 | N/A * | N/A * | N/A * | N/A * | −5.299 | 0.038 | 0.038 |
| Ni | 0.461 | 1.374 × 10−5 | 2.475 × 10−7 | 2.537 × 10−5 | 4.606 × 10−7 | −6.023 | 0.023 | 0.115 |
| Pb | 3.034 | 5.225 × 10−4 | 1.520 × 10−8 | 9.563 × 10−4 | 2.829 × 10−8 | −2.154 | 0.337 | 1.686 |
| Sr | 9.969 | N/A * | N/A * | N/A * | N/A * | −6.520 | 0.016 | N/A ** |
| Zn | 207.262 | 4.137 × 10−4 | N/A * | 7.608 × 10−4 | N/A * | 2.788 | 10.363 | 10.363 |
| HIadults | 1.042 × 10−3 | |||||||
| HIchildren | 1.895 × 10−3 | |||||||
| RIadults | 3.625 × 10−7 | |||||||
| RIchildren | 6.746 × 10−7 | |||||||
| PERI | 18.291 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.; Lia, F. Temporal Variations of Heavy Metal Sources in Agricultural Soils in Malta. Appl. Sci. 2022, 12, 3120. https://doi.org/10.3390/app12063120
Costa C, Lia F. Temporal Variations of Heavy Metal Sources in Agricultural Soils in Malta. Applied Sciences. 2022; 12(6):3120. https://doi.org/10.3390/app12063120
Chicago/Turabian StyleCosta, Christine, and Frederick Lia. 2022. "Temporal Variations of Heavy Metal Sources in Agricultural Soils in Malta" Applied Sciences 12, no. 6: 3120. https://doi.org/10.3390/app12063120
APA StyleCosta, C., & Lia, F. (2022). Temporal Variations of Heavy Metal Sources in Agricultural Soils in Malta. Applied Sciences, 12(6), 3120. https://doi.org/10.3390/app12063120