Abstract
The term “proteome” refers to the total of all proteins expressed in an organism. The term “proteomics” refers to the field of research that includes not only information on the expression levels of individual proteins, but also their higher-order structures, intermolecular interactions, and post-translational modifications. The core technology, matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), is available for protein analysis thanks to the work of Koichi Tanaka and John Fenn, who were awarded the Nobel Prize in Chemistry in 2002. The most successful proteome analysis in clinical practice is rapid microbial identification. This method determines the bacterial species by comparing the proteome profile of the bacteria obtained by matrix-assisted laser desorption ionization-time of flight MS (MALDI-TOF MS) with a database. MS is superior in simplicity, speed, and accuracy to classic speciation by staining and phenotyping. In clinical microbiology, MS has had a large impact on the diagnosis and treatment of infectious disease. Early diagnosis and treatment of infectious disease are important, and rapid identification by MALDI-TOF MS has made a major contribution to this field.
1. Introduction
Mass spectrometry (MS) has progressed remarkably, and its clinical applications are accelerating [1,2,3,4,5,6]. The medical application of MS from a clinical laboratory standpoint can be broadly classified into (1) the search for new diagnostic biomarkers by comprehensive proteome/metabolome analysis and (2) the use of MS in clinical laboratories. Accurate quantification of biomarkers in the former, such as absolute quantification of peptides by selected reaction monitoring, requires MS technology; therefore, the search for new diagnostic biomarkers and MS use in clinical laboratories are linked, but this paper mainly deals with clinical laboratory use. It is only recently that MS has been used in clinical laboratories in hospitals.
Microbial identification using matrix-assisted laser desorption ionization-time of flight MS (MALDI-TOF MS) is based on the analysis by MALDI-TOF-MS of microbial lysates [7,8]. This approach is a new method that is more rapid than conventional methods. For instance, conventional methods call for the isolation and cultivation of microorganisms from specimens using various isolation media; the resulting isolates are then identified by techniques such as analysis of biochemical properties, direct detection of antigens, or amplification/detection of genes carried by the microorganisms. The MALDI microbial identification system mainly consists of a computer equipped with MALDI-TOF MS, a microbial mass spectrum library, and analysis software. The obtained MALDI mass spectra are matched with the microbial mass spectrum library to determine the species [9,10]. In microbiology laboratories, the use of MALDI-TOF MS is becoming more and more widespread for identifying bacteria and yeast-like fungi grown in culture from clinical specimens (blood, urine, feces, sputum, etc.) [11,12,13]. Moreover, direct identification from positive blood cultures is being investigated as an alternative to cultured colonies, to detect and identify organisms that cause bacteremia and sepsis [14,15,16]. For the successful MALDI-TOF MS-based bacterial identification from blood culture bottles, separation of microorganisms from host cells is a critical step, and a number of laboratory-developed and commercially available protocols for this purpose have been reported, as reviewed elsewhere [17]. Currently, to our knowledge, three commercial protocols (including versions for anaerobes and facultative anaerobes) are available: the Sepsityper® kit (Bruker Daltonics) [18], the Vitek MS blood culture kit (bioMerieux, Inc.) [19], and the rapid BACpro® II kit (Nittobo Medical Co., Tokyo, Japan) [20]. The basic principles of the Sepsityper® kit and the rapid BACpro® II kit consist of low-speed centrifugation to remove blood cells, followed by an additional lysis procedure [21].
The clinical applications of MS are diverse, but we will focus on clinical microbiology, which is currently the most widely used application, and outline the rapid identification of microorganisms with this method.
2. History
In a major contribution to MALDI development, Hillenkamp and his colleagues used a mass spectrometer to demonstrate the technique of matrix-assisted laser desorption/ionization (MALDI) in 1985 [22]. John Fenn and Koichi Tanaka were awarded the 2002 Prize in Chemistry for their development of soft desorption ionization methods for mass spectrometric analyses of biological macromolecules; specifically, soft laser desorption by Tanaka and the electrospray by Fenn. The method using MALDI-TOF MS was reported by Cain et al. in 1994 using protein profiling with α-cyano-4-hydroxycinnamic acid as a matrix material and was reported to be useful for rapid identification of bacteria [23]. However, at that time, MALDI-TOF MS was still performed using pretreated bacteria. Later, it was shown that the method could be applied directly to colonies applied to the MS plate without special pretreatment [24,25], paving the way for the method’s practical application. However, it took many years for the results at the laboratory level to reach clinical practice, and it was not until 2009 that the results of the application of this method were first reported by a clinical bacteriology laboratory [26]. The first report from a laboratory was published in 2009, indicating that it takes many years for laboratory-level results to be translated into clinical practice. There are several reasons for this apparent delay, including: (1) concern that the proteome changes dynamically in vivo and is easily affected by culture conditions; (2) doubts about whether the differences in MS spectra are fully consistent with currently established taxonomies; (3) the lack of a complete database; (4) the fact that, from the bacteriologist’s point of view, microbial identification is an arduous task that requires a certain level of training in bacteriology; and (5) early papers were published in journals that may have been too focused on MS techniques (as opposed to applications). Development also required a period of validation for clinical application. Since 2009, the number of clinical report papers has been increasing year by year (Figure 1), and MALDI-TOF MS is gaining attention in hospital bacteriology laboratories.
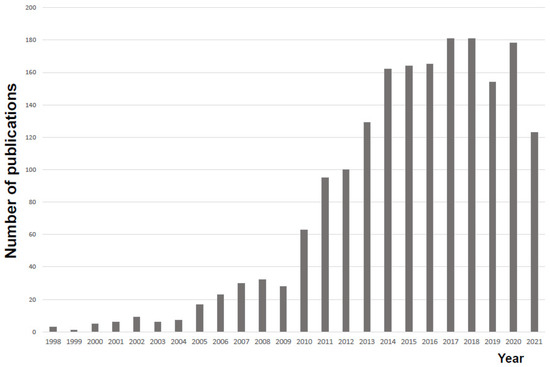
Figure 1.
Trends in research reports on microbial identification using MALDI-TOF mass spectrometry. We used a PubMed literature search as of 17 December 2021, using the terms MALDI-TOF, MS, and Bacterial identification.
3. Measurement Principles and Characteristics
A nitrogen laser (337 nm) is often used to excite the mixed crystal. In such a case, a material with an absorption band at 337 nm is used for the matrix. When the homogeneously mixed crystal of the sample matrix is irradiated with high-density laser light, the matrix first absorbs the laser photons and reaches an excited state. At the same time, the temperature of the crystal rises, and when the mixed crystal reaches the evaporation point, the sample and the matrix evaporate (sublimate). This fact means that, even if the sample does not sublimate well on its own, the sample will sublimate with the help of the matrix. Next, proteins in the evaporated sample undergo chemical ionization by the matrix in a highly excited state. TOF refers to time of flight, one of the ion separation techniques, along with magnetic field and ion trap. The ionized sample is accelerated by an electric field and different masses will gain different velocities. Since ions travel a fixed distance, the molecular weight is determined by measuring the time of flight of each ion. The mixture of sample and MALDI matrix on the MALDI sample plate is desorbed and ionized by a pulsed laser irradiation. The ions formed are accelerated by the electric field applied on the sample plate, and measured by a detector. The arrival time of ions is finally converted to a mass spectrum. In MALDI-TOF MS, the linear mode is used to identify microorganisms, while the reflectron mode is used to detect the ionized components. The linear mode is used for microbial identification in MALDI-TOF MS, because it is more sensitive and can measure proteins with a high molecular weight. Microbial identification is performed in positive ion mode (measures proteins). The optimal and minimum number of laser shots necessary to acquire high-quality spectra in a short time (96 samples/30 min) was automatically determined by the instrument. The main peaks observed in the mass spectrum were derived from the fungal proteins, and their molecular weights ranged from 4000 to 15,000 Da, which are easily detectable using TOF MS. Therefore, the measurement range for microbial identification was approximately 2000–20,000 Da, where the protein-derived peaks are observed. MALDI-TOF MS has become an effective measurement technique in clinical microbiology today [27,28,29]. As mentioned earlier, MALDI Biotyper® from Bruker Daltonics, AXIMA® Microbial Identification System from Shimadzu, and VITEK® MS from Sysmex BioMerieux are the currently available bacterial identification systems. Here, the principles of measurement and operation are introduced using MALDI Biotyper® as an example (Figure 2).
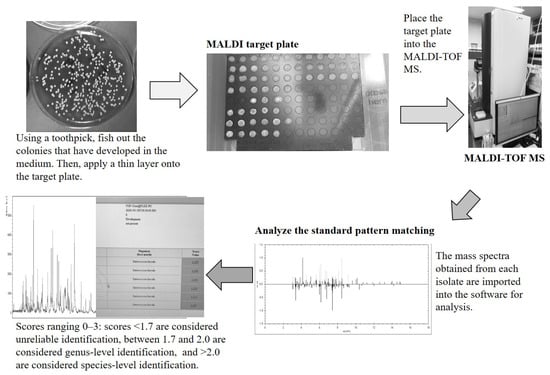
Figure 2.
Flow of bacterial identification by MALDI-TOF MS (example using MALDI Biotyper®). For identification by mass spectrometry (MS), (1) the colony is applied to a MALDI sample plate to be irradiated with a laser. The MALDI matrix is added and dried, and the MALDI sample plate is inserted into the mass spectrometer. (2) The mass spectrometer irradiates the MALDI sample plate with a laser to ionize the proteins (mainly ribosomal proteins) in the bacteria, and the mass spectral pattern is obtained from the mass/charge ratio (m/z) of the constituent proteins and the intensity from the time of flight to the detector. (3) The obtained MALDI mass spectrum pattern is compared to a database for identification. The MALDI matrix used for bacterial identification is typically α-cyano-4-hydroxycinnamic acid (CHCA), which works by (1) absorbing laser energy efficiently, (2) supplying protons to the sample and promoting ionization, and (3) preventing sample decomposition.
MS consists of three basic steps: ionization, mass separation, and detection [9,30,31]. The sample molecules are charged (ionized) to form molecular ions in a gaseous state. The generated ions are sent to the mass separation section, where the ions are separated according to their mass and charge (m/z) ratio using an electric or magnetic field in a vacuum. The separated molecular ions are converted to electrical signals by the mass detector. The MALDI mass spectral data are displayed in a MALDI mass spectrum graph, where m/z is plotted on the horizontal axis and the signal intensity of the ions is plotted on the vertical axis. The signal indicating each ion is called the peak. Since ionization efficiency differs depending on the type of molecule, the ratio of the peak intensities of different ions does not necessarily reflect the concentration ratio of the molecules in the sample. Still, it is possible to compare samples of a given substance.
Since bacterial identification uses a mass spectrometer, an analysis method based on pattern matching is used. The major advantage of the MALDI-TOF MS identification method is that it can be used to identify not only general bacteria, but also anaerobic bacteria, acid-fast bacteria, yeast-like fungi, and filamentous fungi. Rapid identification of bacterial species using MALDI-TOF MS and support for appropriate use of antimicrobial agents based on the results can lead to shorter time to effective antimicrobial therapy, shorter hospital stays, and lower mortality rates. These effects also lead to a reduction in mortality. The sample preparation procedure is as follows: bacteria are scraped from the colonies on the culture medium and applied to the MALDI target plate of the mass spectrometer. A drop of α-cyano-4-hydroxycinnamic acid (CHCA) matrix solution is added to the bacteria on the MALDI target plate; after drying of the solution, the sample is assessed by MALDI-TOF MS. The analytical performance of MALDI-TOF-MS can be improved by using the matrix solution that is most suitable to the compound to be analyzed and the purpose of analysis. DHBA (2,5-dihydroxybenzoic acid) can be applied to compounds such as proteins, peptides, lipids, and carbohydrates. However, there are cases where needle-like mixed crystals are produced, and the sample is severely localized. α-Cyano-4-hydroxycinnamic acid (CHCA) is suitable for sensitive analysis of peptides and is widely used in proteomic studies by MALDI-MS or MALDI-TOF-MS. Sinapinic acid (SA) is also suitable for protein analysis and is useful in microbial analysis. Preparation of MALDI matrix solution was performed. The following is an example of such a process. The matrix solution consisted of pre-dissolved Bruker Matrix HCCA, portioned (Bruker Daltonics). The HCCA matrix was dissolved to a final concentration of 5 mg/mL in a mixture of 50% acetonitrile, 47.5% water, and 2.5% trifluoroacetic acid.
A spot of matrix solution is added to the MALDI sample spot. The samples are desorbed from the MALDI target plate surface by pulsed laser irradiation, ionized, and accelerated in the mass spectrometer, under the influence of the electric field. The ionized protein (bacterial component) flies toward the detector, and the protein mass is determined as a function of its arrival time. As a result, peaks with different masses are detected. The MALDI mass spectrum pattern for each bacterium is different. The amount of bacterial sample required is several micrograms, and identification is possible with 104 to 105 bacteria. Measurement time is only a few minutes, which is a great advantage for rapid bacterial identification. In the sample, the peaks observed in the MALDI mass spectrum are derived primarily from bacterial proteins. Proteins derived from bacterial ribosomes are said to account for 50–70% [32]. This bias reflects that fact that ribosomal proteins are highly expressed, easily ionized, and easily detected by TOF MS in the molecular weight range of approximately 4000–15,000 Da. Therefore, the main component of the mass spectrum is ribosomal protein, which is well conserved in each bacterial species, and different mass spectra can be obtained depending on the bacterial species. According to a study using E. coli, about half of the peaks detected in the range of 4000 to 20,000 Da are ribosomal proteins (including some with post-translational modifications), and the others are DNA-binding proteins, cold-shock proteins, etc. [32]. The MALDI mass spectrum pattern differs depending on the type of bacteria. The MALDI-TOF MS is a pattern-matching method that matches the mass spectra to those of strains in the database [33,34,35]. Candidate strains are displayed in order of increasing score. A score of 2.0 or higher indicates high reliability at the species level (green), and a score of 1.7 or higher but less than 2.0 indicates a match at the genus level (yellow). Scores below that are considered indicative of unidentified isolates (red). The same bacterial species with multiple strains registered is considered identifiable even if there is pattern diversity among the strains. We have cited several references and compiled our own table of the features and advantages of microbial identification by mass spectrometry (Table 1) [8,26,36,37,38,39,40,41,42,43,44]. The mass spectral patterns of molecules, mainly ribosomal proteins, unique to microorganisms obtained by MALD-TOF MS are searched and matched with a library of known standard strains to identify the target bacterial species.

Table 1.
Features and advantages of microbial identification by mass spectrometry.
4. Sample Preparation Strategies for MALDI-Based Bacterial Identification
One of the advantages of MALDI-TOF MS is the use of the same basic measurement technique whether the sample contains aerobic bacteria, anaerobic bacteria, or yeast-like fungi. This makes possible to obtain improvements in test efficiency and reduced wastage of test reagents. There are two main methods of using cultured colonies as a spotting method. One is the cell smear method (smear method), in which colonies are directly applied to a MALDI-TOF MS sample plate. The cell smear method identifies Gram-negative rods such as Enterobacteriaceae with high probability, but Gram-positive bacteria, such as Staphylococcus and Enterococcus, and yeast are insufficiently identified. This may be due to the insufficient extraction of proteins from the bacterial body due to differences in cell wall structure, resulting in a lack of the spectral information necessary for identification. The identification probability is increased using the formic acid extraction method (on-plate method), in which the colony is applied to a sample plate, and formic acid is subsequently applied to the colony to promote cell wall destruction.
The cell smear method (direct smear method) is used in routine testing [45,46,47,48,49]. When evaluating bacteria grown in culture medium, the causative organism is estimated by considering the patient’s clinical information, the epidemiological information of the infection, the gram staining results, active inflammatory findings, whether the causative organism was observed, and the number of bacteria that developed.
The identification probability typically is more accurate when performed using freshly grown colonies. For fast-growing bacteria, colonies that form after one day of culturing are the best substrates; empirically, identification is possible within 2–3 days. For slow-growing bacteria, the timing for identification depends on when a colony is observed. The recommended medium is listed on each instrument for colonies used for identification, but these recommendations differ little from the medium used in daily activities of the typical clinical laboratory. The colony identification method (processing operation on the MALDI sample plate) is shown below.
4.1. Cell Smear Method (Direct Smear Method)
The colonies grown in the medium are recovered with a toothpick and applied thinly to the MALDI sample plate. A drop of the MALDI matrix is applied to the colonies smeared on the MALDI sample plate; the liquid then is dried in preparation for measurement.
4.2. On-Plate Method
Formic acid is added before applying a drop of the MALDI matrix in the cell smear method [50,51]. The colonies grown on the medium are recovered with a toothpick and applied to the MALDI sample plate in a thin layer. A drop of formic acid is applied and allowed to dry. The cell smear method identifies gram-negative rods, such as Enterobacteriaceae, with a high probability, but is less accurate for the identification of gram-positive bacteria, such as Staphylococcus and Enterococcus, and of yeasts. This distinction may reflect insufficient extraction of proteins from the bacterial body due to differences in the cell wall structure, resulting in a lack of spectral information for identification. The formic acid extraction (on-plate) method, in which the colony is applied to a MALDI sample plate and then formic acid is applied to the colony to promote cell wall destruction, increases the identification probability.
4.3. Ethanol and Formic Acid Extraction Method
In some instance, the on-plate method still does not provide a sufficient MALDI mass spectrum, presumably as a result of low amounts of extracted protein. Under these circumstances, the ethanol-formic acid extraction method is recommended [52,53]. This method is the basic approach for processing Nocardia, acid-fast bacteria, and fungi, which have cell wall structures that differ from general bacteria [54]. For example, beads may be used to physically break the cell wall [54].
The MALDI Biotyper™ identifies bacteria using MALDI-TOF MS by pattern matching between mass spectra and bacteria registered in a database (Figure 2). A score of 2.0 or higher indicates high reliability at the species level, and a score of 1.7 to 2.0 indicates a match at the genus level. Since multiple strains of the same species are registered, accurate identification is possible even with pattern diversity among strains [30,55,56,57].
5. Direct Identification of Bacterial Species from the Blood Culture Medium
In general, after the liquid culture becomes positive, the sample is subcultured on solid medium for a day and a night to permit the growth of colonies. While most identification is performed using plate culture, the most clinically useful application of MALDI-TOF MS is in the identification of bacterial species directly from blood culture medium. The rapidity of this method is particularly useful in blood culture, where identification results can be obtained within one hour after a positive reaction in the blood culture bottle. This method is expected to improve the prognosis of patients and shorten their hospital stay because this approach can identify the organism 1.5 to 2 days earlier than the conventional method. Pretreatment to separate and concentrate the bacteria from the blood culture medium is necessary, but this procedure can be done in a short time.
Pretreatment methods for bacterial identification using MS techniques are required for direct identification of microorganisms from blood culture bottles [58,59,60,61,62,63]. Kits have been developed for this purpose. The MALDI Sepsityper® kit (Bruker Daltonics), which collects bacteria by lysing blood cell components in lysis buffer followed by high-speed centrifugation, is widely used in Japan and overseas [64,65,66,67,68]. An alternative method is to capture and collect bacteria using positively charged polyacrylamide (cationic particles) [69]. The cationic particle technique has been improved and put to practical use; following clinical evaluation, a kit incorporating this technology is sold in Japan and overseas as the pretreatment rapid kit BACpro® II (Nitto-Bo Medical Inc.) [20,70,71,72]. However, these methods cannot be used for drug susceptibility testing, given that the bacteria are killed during the pretreatment process, which is a major drawback. In contrast, if viable bacteria can be recovered efficiently, these organisms can be used for species identification and rapid drug susceptibility testing using automated equipment. Therefore, further improvement of new pretreatment kits is expected.
Gene-level methods for rapid identification from blood culture-positive bottles have also progressed [73]. An automated multi-item simultaneous gene-related testing system capable of simultaneous detection of causative organisms and drug resistance genes has been reported, and comparative studies with MS-based methods have begun [68]. However, both methods are still in their early stages, and further investigations of the use of genetic and protein level methods in clinical practice will be necessary.
6. Direct Identification from Other Matrices (Cerebrospinal Fluid, Urine)
6.1. Rapid Identification from Spinal Fluid Specimens
The spinal fluid is examined for color, clarity, and the presence of suspended particles. Under normal conditions, the spinal fluid is clear and colorless, but in meningitis, it may be cloudy white (increased white blood cells), yellow (increased protein), or red, indicating hemorrhage. In some cases, centrifugation is performed to observe the color of the supernatant. Then, we use a microscope to look at the cells (red blood cells and white blood cells) that are present. Glucose, proteins, albumin, and globulins such as IgG are measured. We also culture the bacteria and use special stains to look for infectious agents. Direct and rapid identification is desirable for bacterial meningitis, which requires rapid diagnosis and appropriate administration of antimicrobial agents. Direct identification of bacteria in spinal fluid can provide a bacteriological diagnosis and enable early and appropriate treatment. Segawa et al. performed direct identification of bacteria from the spinal fluid of patients with bacterial meningococcal disease using MALDI-TOF MS [69]. Those authors reported that MALDI-TOF MS is an important technique for clinical microbiology laboratories because the method enables appropriate treatment [69]. The technique involves placing 1 mL of spinal fluid in a microcentrifuge tube, centrifuging at 13,500× g for 5 min, and discarding the supernatant and leukocyte layer. Then, ethanol-formic acid extraction is used to process/treat the sediment (pellet) [69]. Bishop et al. evaluated the potential of MALDI-TOF-MS for the rapid identification of bacteria from smear-positive cerebrospinal fluid (CSF) in a cohort of meningitis patients [74]. It was suggested that MALDI-TOF-MS is useful for the rapid identification of Gram-negative rods, but not Gram-positive bacteria [74].
6.2. Direct Identification from Urine Samples
In the diagnosis of urinary tract infections, direct identification is possible if the quantity of bacteria exceeds a certain level (104–105 or more) [75]. However, in the future, handling multiple bacteria will be an issue.
7. Identification of Acid-Fast Bacteria and Fungi
7.1. Acid-Fast Bacilli
In this paper, we discuss the identification of acid-fast bacteria and fungi using the zirconia/silica bead method. Heat treatment for inactivation and physical disruption of mycobacteria using the zirconia/silica bead method to increase the amount of protein extracted resulted in superior antimicrobial identification compared to the conventional method.
7.2. Nocardia
The ethanol-formic acid extraction method is the main method for processing of Nocardia. Although commercial databases are regularly updated, the commercial database for the genus Nocardia contains insufficient data. Therefore, Segawa et al. [54] developed the Nocardia Extraction Method at the Department of Clinical Laboratory at Chiba University Hospital and added it to the database of mass spectra on Nocardia strains.
7.3. Filamentous Fungi
Filamentous fungi, like the tubercle bacillus group, require special handling because there are many highly infectious Biosafety Level 3 (BSL3) fungi that are causative agents of important mycoses. The on-plate method for filamentous fungi should be performed in a safety cabinet because static electricity can disperse spores. Fungal infections typically are respiratory, resulting from inhalation of spores; therefore, culturing in a liquid medium that does not produce spores is (generally) considered safer and preferable.
Since new species are added to the library as needed, it is important to interpret the identification results by noting which version of the database is used at the institution or the laboratory performing the identification.
7.4. Yeast-Like Fungi
Yeast-like fungi exist in species with hard-surface cell walls, and peaks may not be obtained when colonies are applied directly. For stable operation for routine purposes, pretreatment to break the cell wall is necessary. For pretreatment, the colonies are smeared on a sample plate, and a drop of formic acid solution is added. The sample and acid solution are mixed on the sample plate and then dried. After drying, the matrix is added to the sample plate, and measurements are made. The ethanol/formic acid extraction method may be used for some yeast-like fungi when mass spectral peaks cannot be obtained. The procedure is as follows: (1) suspend the colonies in sterile distilled water in an Eppendorf tube and mix (vortex); (2) add pure ethanol (vortex) and centrifuge; (3) discard the supernatant, add formic acid to the sediment, and mix (vortex); (4) add acetonitrile, mix (vortex), and centrifuge; and finally, (5) place the supernatant on a sample plate dedicated to MS, dry, and then perform MS measurement. Note that the supernatant is placed on the MS sample plate and dried, and then the matrix is placed on the plate and dried before MS measurement.
8. Pitfalls in Bacterial Identification by MS
Bacteria with high homology at the genetic level cannot be differentiated and are distinguished by their biochemical properties [76]. Similarly, Salmonella typhi, Salmonella paratyphi A, and Salmonella spp. cannot be differentiated and are all identified as Salmonella spp. by MS. Other examples of pairs of species that cannot be distinguished by MS include Bacillus cereus and Bacillus anthracis, as well as Yersinia pestis and Yersinia pseudotuberculosis. Bacterial identification by MS is based on matching with the MALDI mass spectrum of the species (strains) listed in the database. If the species is not listed in the database or is a new species, such an organism will not be identified and instead must be classified by another method. One of the pitfalls of bacterial identification is that identification by mass spectrometry involves a matching of the mass spectra of the bacteria (strains) listed in the database; therefore, bacteria that are not listed in the database cannot be identified. Thus, if a species cannot be identified, it must be identified using another method. However, if the species to be identified is not listed in the database, the score value of a related species is high, and if the species is presented at species level, it is an erroneous identification.
9. Maintenance, Inspection, and Accuracy Control
In the same way that deterioration of the light source lamp of clinical testing equipment must be monitored, establishing an accurate control method for mass spectrometers is needed to detect attenuation of laser intensity. Current recommendations suggest that users follow maintenance, inspection, and accuracy control methods (e.g., calibration using E. coli) defined by each company; the measurement of standard strains is essential for accurate control of clinical microbiology tests [77,78]. The inspection work must be performed by skilled personnel who have acquired knowledge and skills related to MALDI-TOF MS. If, as a result of the inspection, any item is found to be incompatible with the state recommended by the manufacturer where the spectra necessary for microbial identification can be obtained, repair or adjustment to the state recommended by the manufacturer is required in principle. It is also important to check the resolution of the linear mode, the laser power, and the connection of the vacuum system joint, which are essential for measurement.
10. Future Prospects
In order to provide appropriate treatment for infectious diseases, quick identification of the causative microorganism is important, for which MALDI-TOF-MS plays an important role. In the future, MALDI-TOF-MS will become a key identification method, not only in clinical microbiology, but also in the broader field of microbiology, and will contribute greatly to the development of this field. However, some issues with the method remain. These include difficulty in differentiating related species and the inability to perform drug susceptibility tests, which may be solved with further improvements that are still expected to be a long way off. Therefore, it is essential to identify the organisms based on their basic characteristics, such as the initial appearance under Gram stain, and observation of colonies and biochemical characteristics, while considering the clinical symptoms, pathogenesis, and course of treatment.
11. Conclusions
The MS technique is increasingly used as a screening method because pretreatment is simpler than conventional methods, and there is no need for select reagents for individual genera and species of bacteria and fungi. In addition, although basic knowledge of MS is required for bacterial identification, the method does not require the high level of expertise needed for conventional bacteriological tests and, thus, can be used widely. In clinical microbiology, MS technology is having a large impact on the diagnosis and treatment of infectious diseases. Early diagnosis and treatment of infectious diseases are important, and rapid identification by MALDI-TOF MS is making a large contribution in this context. This technology has already been shown to shorten hospital stays and reduce medical costs. The method is also expected to prevent the increase of resistant bacteria and to improve patient prognosis. It is hoped that further research will lead to the day when MS technology can be used routinely for bacterial typing and the detection of resistant bacteria.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors express their appreciation for the contributions of members of the Divisions of Laboratory Medicine, Department of Pathology and Microbiology, Nihon University School of Medicine, Tokyo, Japan. This work was supported by a Grant-in-Aid for Scientific Research (Grant No. 18K09593).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nomura, F. Proteome-based bacterial identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS): A revolutionary shift in clinical diagnostic microbiology. Biochim. Biophys. Acta 2015, 1854, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Nomura, F.; Tsuchida, S.; Murata, S.; Satoh, M.; Matsushita, K. Mass spectrometry-based microbiological testing for blood stream infection. Clin. Proteom. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Jannetto, P.J.; Fitzgerald, R.L. Effective Use of Mass Spectrometry in the Clinical Laboratory. Clin. Chem. 2016, 62, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry in Clinical Microbiology. Clin. Infect. Dis. 2013, 57, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Dingle, T.C.; Butler-Wu, S.M. Maldi-tof mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Rolain, J.M.; Fournier, P.E.; La Scola, B.; Drancourt, M.; Raoult, D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010, 5, 1733–1754. [Google Scholar] [CrossRef]
- Bizzini, A.; Greu, G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin. Microbiol. Infect. 2010, 16, 1614–1619. [Google Scholar] [CrossRef]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: A fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef]
- Han, S.S.; Jeong, Y.S.; Choi, S.K. Current Scenario and Challenges in the Direct Identification of Microorganisms Using MALDI TOF MS. Microorganisms 2021, 9, 1917. [Google Scholar] [CrossRef]
- Patel, R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef]
- Opota, O.; Croxatto, A.; Prod’hom, G.; Greub, G. Blood culture-based diagnosis of bacteraemia: State of the art. Clin. Microbiol. Infect. 2015, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Chui, H.; Domish, L.; Hernandez, D.; Wang, G. Recent development of mass spectrometry and proteomics applications in identification and typing of bacteria. Proteom. Clin. Appl. 2016, 10, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Robin, P. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4. [Google Scholar]
- Bettina, S.; Guido, V.B.; Reinhard, Z.; Erik, C.B.; Michael, H. Evaluation of the Bruker MALDI Biotyper for identification of gram positive rods: Development of a diagnostic algorithm for the clinical laboratory. J. Clin. Microbiol. 2013, 52, 1089–1097. [Google Scholar]
- Biswas, S.; Rolain, J.M. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. J. Microbiol. Meth. 2013, 92, 14–24. [Google Scholar] [CrossRef]
- Buchan, B.W.; Riebe, K.M.; Ledeboer, N.A. Comparison of the MALDI Biotyper system using Sepsityper specimen processing to routine microbiological methods for identification of bacteria from positive blood culture bottles. J. Clin. Microbiol. 2012, 50, 346–352. [Google Scholar] [CrossRef]
- Faron, M.L.; Buchan, B.W.; Ledeboer, N.A. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for use with positive blood cultures: Methodology, performance, and optimization. J. Clin. Microbiol. 2017, 55, 3328–3338. [Google Scholar] [CrossRef]
- Schubert, S.; Weinert, K.; Wagner, C.; Gunzl, B.; Wieser, A.; Maier, T.; Kostrzewa, M. Novel, improved sample preparation for rapid, direct identification from positive blood cultures using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. J. Mol. Diagn 2011, 13, 701–706. [Google Scholar] [CrossRef]
- Fothergill, A.; Kasinathan, V.; Hyman, J.; Walsh, J.; Drake, T.; Wang, Y.F. Rapid identification of bacteria and yeasts from positive-blood-culture bottles by using a lysis-filtration method and matrix-assisted laser desorption ionization-time of flight mass spectrum analysis with the SARAMIS database. J. Clin. Microbiol. 2013, 51, 805–809. [Google Scholar] [CrossRef]
- Ashizawa, K.; Murata, S.; Terada, T.; Ito, D.; Bunya, M.; Watanabe, K.; Teruuchi, Y.; Tsuchida, S.; Satoh, M.; Nishimura, M.; et al. Applications of copolymer for rapid identification of bacteria in blood culture broths using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Methods 2017, 139, 54–60. [Google Scholar] [CrossRef]
- Prod’hom, G.; Bizzini, A.; Durussel, C.; Bille, J.; Greub, G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J. Clin. Microbiol. 2010, 48, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Bachmann, D.; Hillenkamp, F. Influence of the Wavelength in High-Irradiance Ultraviolet Laser Desorption Mass Spectrometry of Organic Molecules. Anal. Chem. 1985, 57, 2935–2939. [Google Scholar] [CrossRef]
- Cain, T.C.; Lubman, D.M.; Weber, W.J., Jr. Differentiation of bacteria using protein profiles from matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid. Commun. Mass Spectrom. 1994, 8, 1026–1030. [Google Scholar] [CrossRef]
- Holland, R.D.; Wilkes, J.G.; Rafii, F.; Sutherland, J.B.; Persons, C.C.; Voorhees, K.J.; Lay, J.O., Jr. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-offlight mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 1227–1232. [Google Scholar] [CrossRef]
- Claydon, M.A.; Davey, S.N.; Edwards-Jones, V.; Gordon, D.B. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 1996, 14, 1584–1586. [Google Scholar] [CrossRef]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 543–555. [Google Scholar] [CrossRef]
- Vrioni, G.; Tsiamis, C.; Oikonomidis, G.; Theodoridou, K.; Kapsimali, V.; Tsakris, A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018, 6, 240. [Google Scholar] [CrossRef]
- Angeletti, S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J. Microbiol. Methods 2017, 138, 20–29. [Google Scholar] [CrossRef]
- Posteraro, B.; De Carolis, E.; Vella, A.; Sanguinetti, M. MALDI-TOF mass spectrometry in the clinical mycology laboratory: Identification of fungi and beyond. Expert. Rev. Proteom. 2013, 10, 151–164. [Google Scholar] [CrossRef]
- Jang, K.S.; Kim, Y.H. Rapid and robust MALDI-TOF MS techniques for microbial identification: A brief overview of their diverse applications. J. Microbiol. 2018, 56, 209–216. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for identification of environmental bacteria: A review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, V.; Fenselau, C. Characterization of the protein subset desorbed by MALDI from whole bacterial cells. Anal. Chem. 2001, 73, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Topić Popović, N.; Kazazić, S.P.; Bojanić, K.; Strunjak-Perović, I.; Čož-Rakovac, R. Sample preparation and culture condition effects on MALDI-TOF MS identification of bacteria: A review. Mass. Spectrom. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.G.; Cornet, M.; Hennebique, A.; Rasamoelina, T.; Caspar, Y.; Pondérand, L.; Bidart, M.; Durand, H.; Jacquet, M.; Garnaud, C.; et al. MALDI-TOF MS in a Medical Mycology Laboratory: On Stage and Backstage. Microorganisms 2021, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.G.; Romero, C.; Dard, C.; Garnaud, C.; Cognet, O.; Girard, T.; Rasamoelina, T.; Cornet, M.; Maubon, D. Evaluation of ID Fungi Plates Medium for Identification of Molds by MALDI Biotyper. J. Clin. Microbiol. 2020, 58, e01687-19. [Google Scholar] [CrossRef]
- Strejcek, M.; Smrhova, T.; Junkova, P.; Uhlik, O. Whole-Cell MALDI-TOF MS Versus 16S rRNA Gene Analysis for Identification and Dereplication of Recurrent Bacterial Isolates. Front. Microbiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Li, Y.; Shan, M.; Zhu, Z.; Mao, X.; Yan, M.; Chen, Y.; Zhu, Q.; Li, H.; Gu, B. Application of MALDI-TOF MS to rapid identification of anaerobic bacteria. BMC Infect. Dis. 2019, 19, 941. [Google Scholar] [CrossRef]
- Ge, M.C.; Kuo, A.J.; Liu, K.L.; Wen, Y.H.; Chia, J.H.; Chang, P.Y.; Lee, M.H.; Wu, T.L.; Chang, S.C.; Lu, J.J. Routine identification of microorganisms by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: Success rate, economic analysis, and clinical outcome. J. Microbiol. Immunol. Infect. 2017, 50, 662–668. [Google Scholar] [CrossRef]
- Tran, A.; Alby, K.; Kerr, A.; Jones, M.; Gilligan, P.H. Cost Savings Realized by Implementation of Routine Microbiological Identification by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2015, 53, 2473–2479. [Google Scholar] [CrossRef]
- Hou, T.Y.; Chiang-Ni, C.; Teng, S.H. Current status of MALDI-TOF mass spectrometry in clinical microbiology. J. Food Drug Anal. 2019, 27, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Cuénod, A.; Foucault, F.; Pflüger, V.; Egli, A. Factors Associated With MALDI-TOF Mass Spectral Quality of Species Identification in Clinical Routine Diagnostics. Front. Cell. Infect. Microbiol. 2021, 11, 646648. [Google Scholar] [CrossRef] [PubMed]
- Oberle, M.; Wohlwend, N.; Jonas, D.; Maurer, F.P.; Jost, G.; Tschudin-Sutter, S.; Vranckx, K.; Egli, A. The Technical and Biological Reproducibility of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) Based Typing: Employment of Bioinformatics in a Multicenter Study. PLoS ONE 2016, 11, e0164260. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.M.; Cheung, W.L.; Wong, K.S.; Xie, M.; Luk, C.Y.; Wong, F.L.; Li, M.W.; Tsai, S.N.; To, W.T.; Chan, L.Y.; et al. High-Throughput Mass Spectrometric Analysis of the Whole Proteome and Secretome from Sinorhizobium fredii Strains CCBAU25509 and CCBAU45436. Front. Microbiol. 2019, 10, 2569. [Google Scholar] [CrossRef]
- Shafer, D.; Liu, H.; Dong, J.; Liu, W.; Loft, J.; Phelps, T.; Zhang, Y. Comparison of direct smear and chemical extraction methods for MALDI-TOF mass spectrometry identification of clinical relevant anaerobic bacteria. Front. Lab. Med. 2017, 1, 27–30. [Google Scholar] [CrossRef]
- Khot, P.D.; Couturier, M.R.; Wilson, A.; Croft, A.; Fisher, M.A. Optimization of matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis for bacterial identification. J. Clin. Microbiol. 2012, 50, 3845–3852. [Google Scholar] [CrossRef]
- Fournier, R.; Wallet, F.; Grandbastien, B.; Dubreuil, L.; Courcol, R.; Neut, C.; Dessein, R. Chemical extraction versus direct smear for MALDI-TOF mass spectrometry identification of anaerobic bacteria. Anaerobe 2012, 18, 294–297. [Google Scholar] [CrossRef]
- Doellinger, J.; Schneider, A.; Stark, T.D.; Ehling-Schulz, M.; Lasch, P. Evaluation of MALDI-ToF Mass Spectrometry for Rapid Detection of Cereulide from Bacillus cereus Cultures. Front. Microbiol. 2020, 11, 511674. [Google Scholar] [CrossRef]
- Mestas, J.; Quias, T.; Bard, J.D. Direct Identification of Aerobic Bacteria by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry Is Accurate and Robust. J. Clin. Lab. Anal. 2016, 30, 543–551. [Google Scholar] [CrossRef]
- Guo, J.; Lai, W.; Li, B.; Tang, L.; Wu, Y.; Luo, Y.; Liu, C.; Lu, W.; Mu, X. Rapid identification of Brucella sepsis/osteomyelitis in a 6-year old febrile patient with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry directly from positive blood culture: A case report. BMC. Infect. Dis. 2019, 19, 240. [Google Scholar] [CrossRef]
- Chen, X.F.; Hou, X.; Xiao, M.; Zhang, L.; Cheng, J.W.; Zhou, M.L.; Huang, J.J.; Zhang, J.J.; Xu, Y.C.; Hsueh, P.R. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) Analysis for the Identification of Pathogenic Microorganisms: A Review. Microorganisms 2021, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.A.; Stokes, M.G.; Lukaszewski, R.A. Observations on the Inactivation Efficacy of a MALDI-TOF MS Chemical Extraction Method on Bacillus anthracis Vegetative Cells and Spores. PLoS ONE 2015, 10, e0143870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulthess, B.; Brodner, K.; Bloemberg, G.V.; Zbinden, R.; Böttger, E.C.; Hombach, M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: Comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J. Clin. Microbiol. 2013, 51, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.; Nishimura, M.; Sogawa, K.; Tsuchida, S.; Murata, S.; Watanabe, M.; Matsushita, K.; Kamei, K.; Nomura, F. Identification of Nocardia species using matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Clin. Proteom. 2015, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Watanabe, M.; Sato, K.; Segawa, S.; Ishii, C.; Miyabe, A.; Murata, S.; Saito, T.; Nomura, F. Use of the MALDI BioTyper system with MALDI-TOF mass spectrometry for rapid identification of microorganisms. Anal. Bioanal. Chem. 2011, 400, 1905–1911. [Google Scholar] [CrossRef]
- Sogawa, K.; Watanabe, M.; Sato, K.; Segawa, S.; Miyabe, A.; Murata, S.; Saito, T.; Nomura, F. Rapid identification of microorganisms by mass spectrometry: Improved performances by incorporation of in-house spectral data into a commercial database. Anal. Bioanal. Chem. 2012, 403, 1811–1822. [Google Scholar] [CrossRef]
- Nagy, E.; Becker, S.; Kostrzewa, M.; Barta, N.; Urbán, E. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. J. Med. Microbiol. 2012, 61, 1393–1400. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Bai, Y.; Song, Z.; Chu, W.; Zhao, M.; Hao, Y.; Lu, Z. Rapid method for direct identification of positive blood cultures by MALDI-TOF MS. Exp. Ther. Med. 2020, 20, 235. [Google Scholar] [CrossRef]
- Yonetani, S.; Ohnishi, H.; Ohkusu, K.; Matsumoto, T.; Watanabe, T. Direct identification of microorganisms from positive blood cultures by MALDI-TOF MS using an in-house saponin method. Int. J. Infect. Dis. 2016, 52, 37–42. [Google Scholar] [CrossRef]
- Haigh, J.D.; Green, I.M.; Ball, D.; Eydmann, M.; Millar, M.; Wilks, M. Rapid identification of bacteria from bioMerieux BacT/ALERT blood culture bottles by MALDI-TOF MS. Br. J. Biomed. Sci. 2013, 70, 149–155. [Google Scholar] [CrossRef]
- McIver, C.J.; Er, N.; Mukerjee, C.; Tokis, S.; Taylor, P. A simplified and rapid method for the direct identification of microorganisms in positive BacT/ALERT blood culture bottles using MALDI-TOF MS. Pathology 2018, 50, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Keness, Y.; Nitzan, O.; Pastukh, N.; Tkhawkho, L.; Freidus, V.; Peretz, A. Cheap and rapid in-house method for direct identification of positive blood cultures by MALDI-TOF MS technology. BMC. Infect. Dis. 2019, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, Q.; Kudinha, T.; Sun, L.; Zhang, R.; Liu, C.; Yu, S.; Xiao, M.; Kong, F.; Zhao, Y.; et al. An Improved In-house MALDI-TOF MS Protocol for Direct Cost-Effective Identification of Pathogens from Blood Cultures. Front. Microbiol. 2017, 8, 1824. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhou, Z.; Li, J.; Chen, X.; Zhou, M. Rapid identification of microorganisms from positive blood cultures in pediatric patients by MALDI-TOF MS: Sepsityper kit versus short-term subculture. J. Microbiol. Methods. 2020, 172, 105894. [Google Scholar] [CrossRef] [PubMed]
- Cordovana, M.; Zignoli, A.; Ambretti, S. Rapid Sepsityper in clinical routine: 2 years’ successful experience. J. Med. Microbiol. 2020, 69, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Di Gaudio, F.; Indelicato, S.; Indelicato, S.; Tricoli, M.R.; Stampone, G.; Bongiorno, D. Improvement of a rapid direct blood culture microbial identification protocol using MALDI-TOF MS and performance comparison with SepsiTyper kit. J. Microbiol. Methods 2018, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ponderand, L.; Pavese, P.; Maubon, D.; Giraudon, E.; Girard, T.; Landelle, C.; Maurin, M.; Caspar, Y. Evaluation of Rapid Sepsityper protocol and specific MBT-Sepsityper module (Bruker Daltonics) for the rapid diagnosis of bacteremia and fungemia by MALDI-TOF-MS. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.A.; Denys, G.A. Parallel Evaluation of the MALDI Sepsityper and Verigene BC-GN Assays for Rapid Identification of Gram-Negative Bacilli from Positive Blood Cultures. J. Clin. Microbiol. 2017, 55, 2708–2718. [Google Scholar] [CrossRef]
- Segawa, S.; Sawai, S.; Murata, S.; Nishimura, M.; Beppu, M.; Sogawa, K.; Watanabe, M.; Satoh, M.; Matsutani, T.; Kobayashi, M.; et al. Direct application of MALDI-TOF mass spectrometry to cerebrospinal fluid for rapid pathogen identification in a patient with bacterial meningitis. Clin. Chim Acta. 2014, 435, 59–61. [Google Scholar] [CrossRef]
- Yonezawa, T.; Watari, T.; Ashizawa, K.; Hanada, D.; Yanagiya, T.; Watanabe, N.; Terada, T.; Tomoda, Y.; Fujii, S. Dvelopment of an improved rapid BACpro® protocol and a method for direct identification from blood-culture-positive bottles using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Methods 2018, 148, 138–144. [Google Scholar] [CrossRef]
- Tsuchida, S.; Murata, S.; Miyabe, A.; Satoh, M.; Takiwaki, M.; Ashizawa, K.; Terada, T.; Ito, D.; Matsushita, K.; Nomura, F. Application of the biocopolymer preparation system, rapid BACpro II kit, for mass-spectrometry-based bacterial identification from positive blood culture bottles by the MALDI Biotyper system. J. Microbiol. Methods 2018, 152, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kayin, M.; Mert, B.; Aydemir, S.; Özenci, V. Comparison of rapid BACpro II, Sepsityper kit and in-house preparation methods for direct identification of bacteria from blood cultures by MALDI-TOF MS with and without Sepsityper module analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Niimi, H.; Ueno, T.; Hayashi, S.; Abe, A.; Tsurue, T.; Mori, M.; Tabata, H.; Minami, H.; Goto, M.; Akiyama, M.; et al. Melting temperature mapping method: A novel method for rapid identification of unknown pathogenic microorganisms within three hours of sample collection. Sci. Rep. 2015, 28, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Bishop, B.; Geffen, Y.; Plaut, A.; Kassis, O.; Bitterman, R.; Paul, M.; Neuberger, A. Clin The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for rapid bacterial identification in patients with smear-positive bacterial meningitis. Microbiol. Infect. 2018, 24, 171–174. [Google Scholar] [CrossRef]
- Ferreira, L.; Sánchez-Juanes, F.; González-Avila, M.; Cembrero-Fuciños, D.; Herrero-Hernández, A.; González-Buitrago, J.M.; Muñoz-Bellido, J.L. Direct identification of urinary tract pathogens from urine samples by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2010, 48, 2110–2115. [Google Scholar] [CrossRef]
- Khot, P.D.; Fisher, M.A. Novel approach for differentiating Shigella species and Escherichia coli by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 3711–3716. [Google Scholar] [CrossRef]
- Ilina, E.N.; Borovskaya, A.D.; Malakhova, M.M.; Vereshchagin, V.A.; Kubanova, A.A.; Kruglov, A.N.; Svistunova, T.S.; Gazarian, A.O.; Maier, T.; Kostrzewa, M.; et al. Direct bacterial profiling by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for identification of pathogenic Neisseria. J. Mol. Diagn. 2009, 11, 75–86. [Google Scholar] [CrossRef]
- Lo, C.I.; Fall, B.; Sambe-Ba, B.; Diawara, S.; Gueye, M.W.; Mediannikov, O.; Sokhna, C.; Faye, N.; Diemé, Y.; Wade, B.; et al. MALDI-TOF Mass Spectrometry: A Powerful Tool for Clinical Microbiology at Hopital Principal de Dakar, Senegal (West Africa). PLoS ONE 2015, 10, e0145889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).