Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
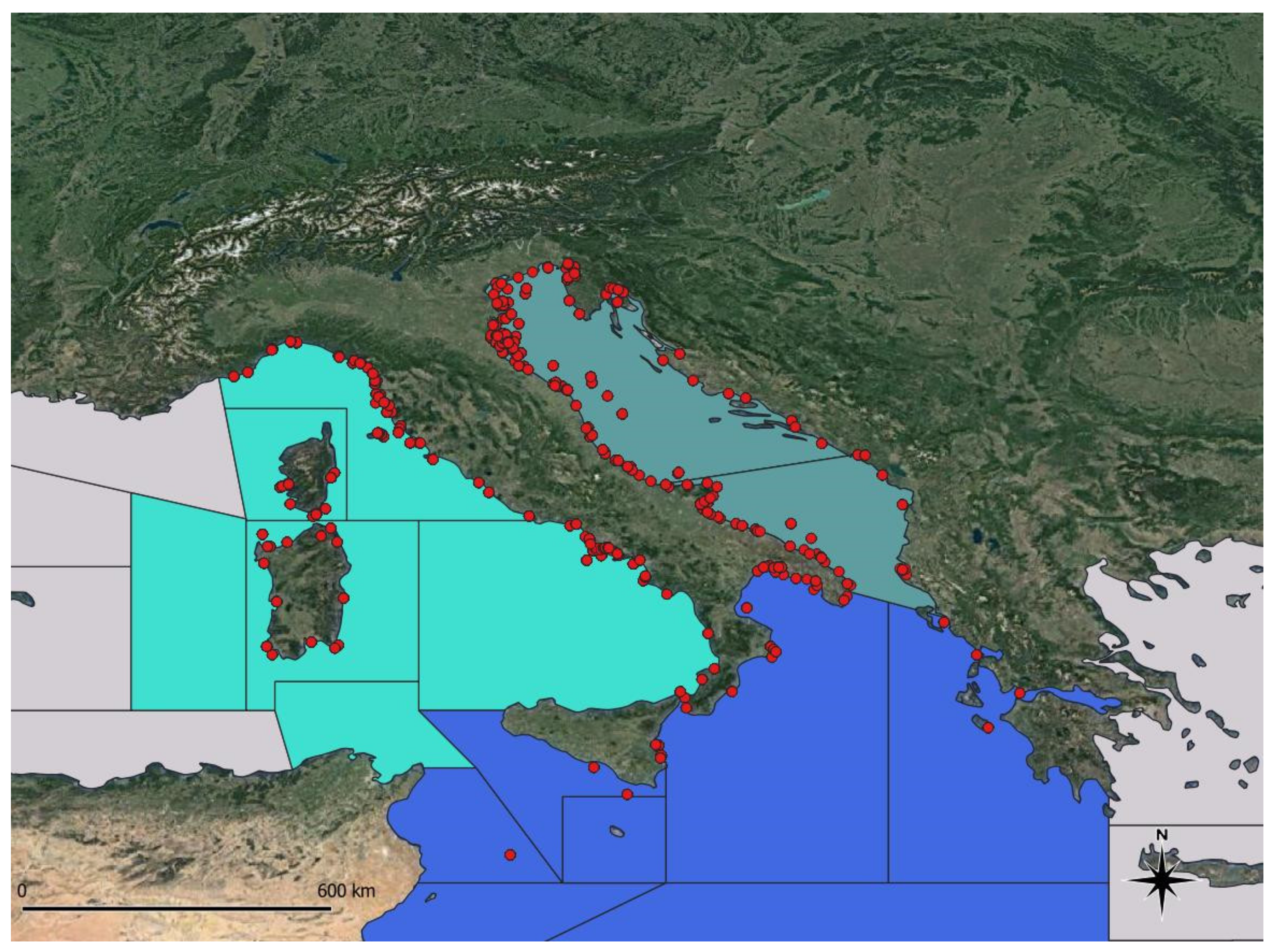
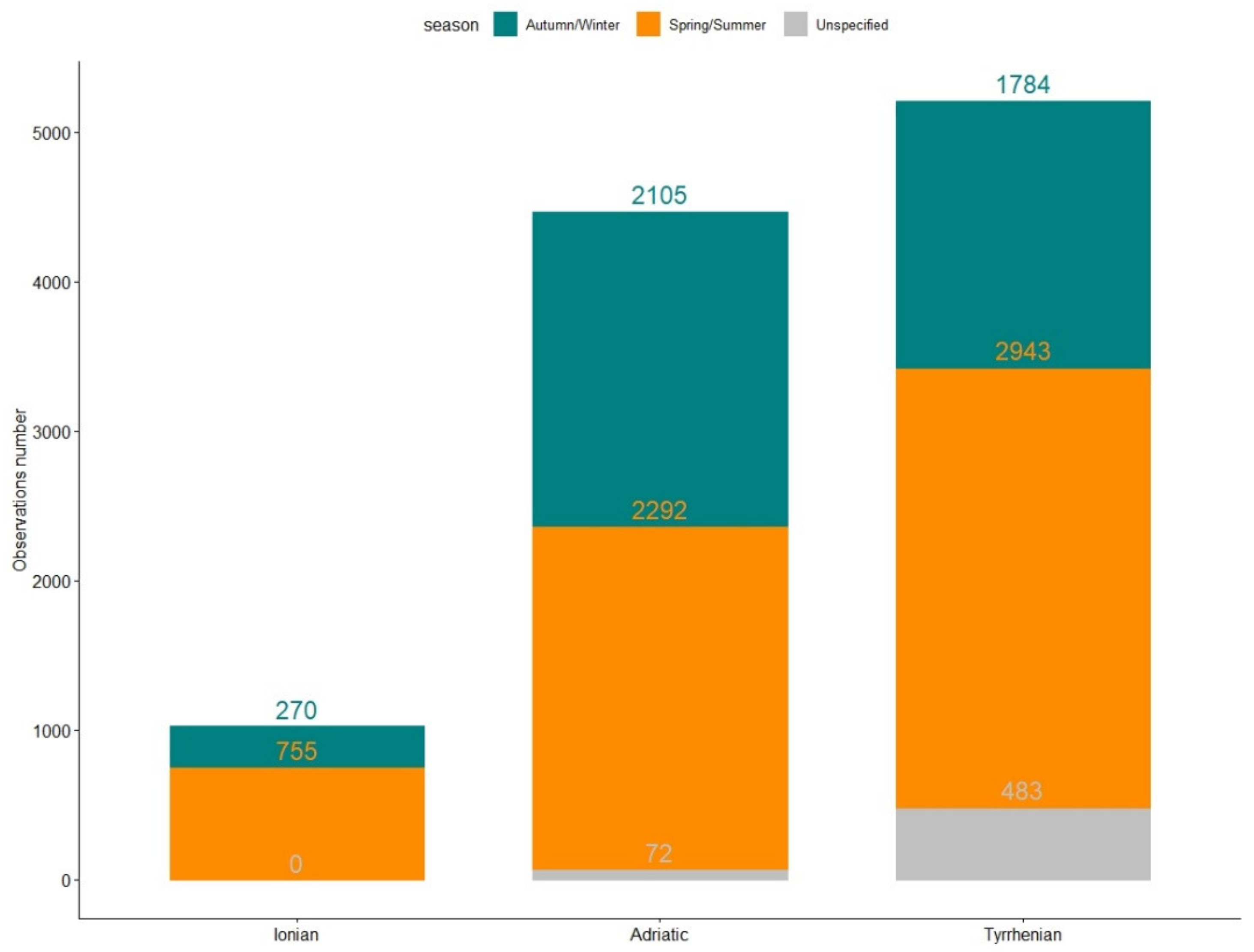
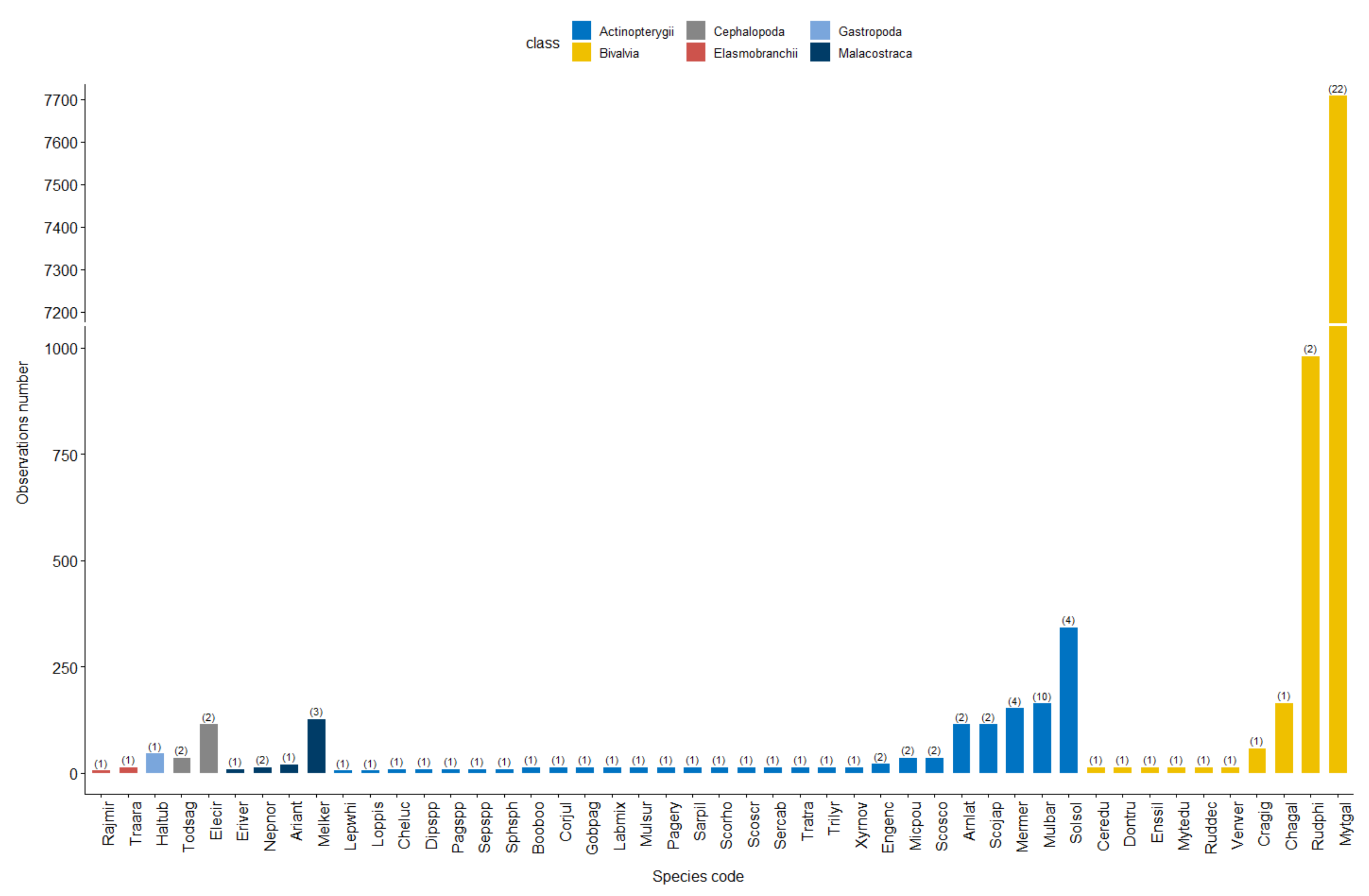
3.1. Database Description
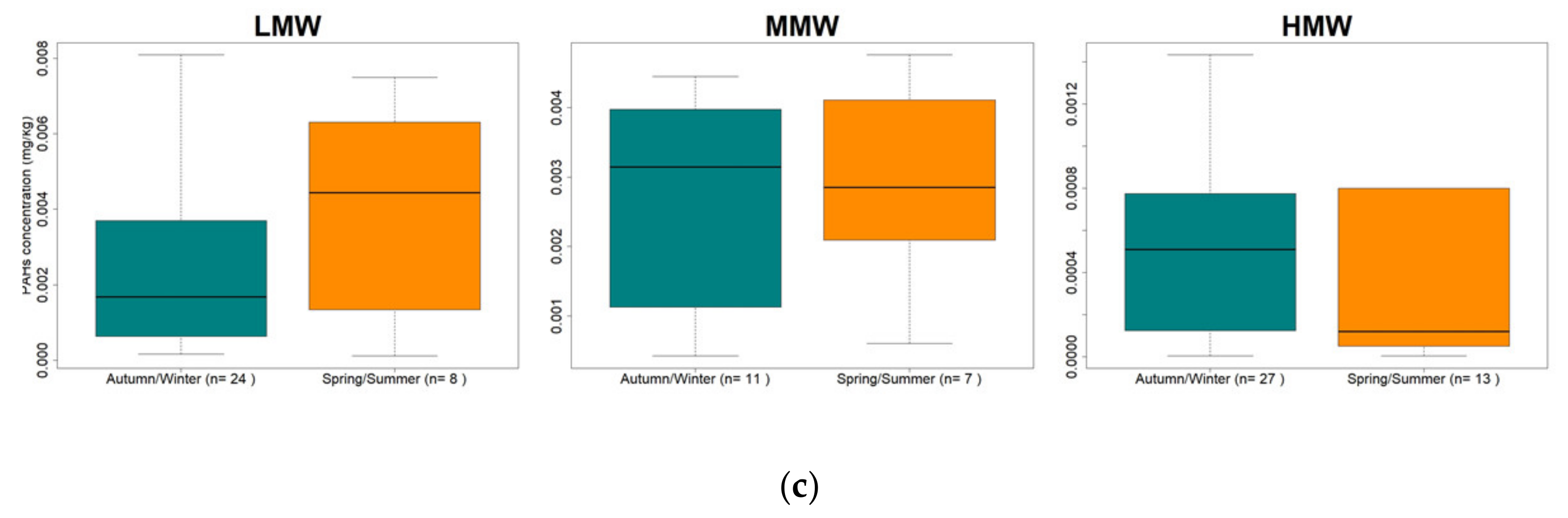
3.2. PAHs Concentration by Molecular Weight and by Season
3.3. Latitude, Depth and Sampling Year Effect in Mediterranean Mussel
3.3.1. Adriatic
3.3.2. Tyrrhenian
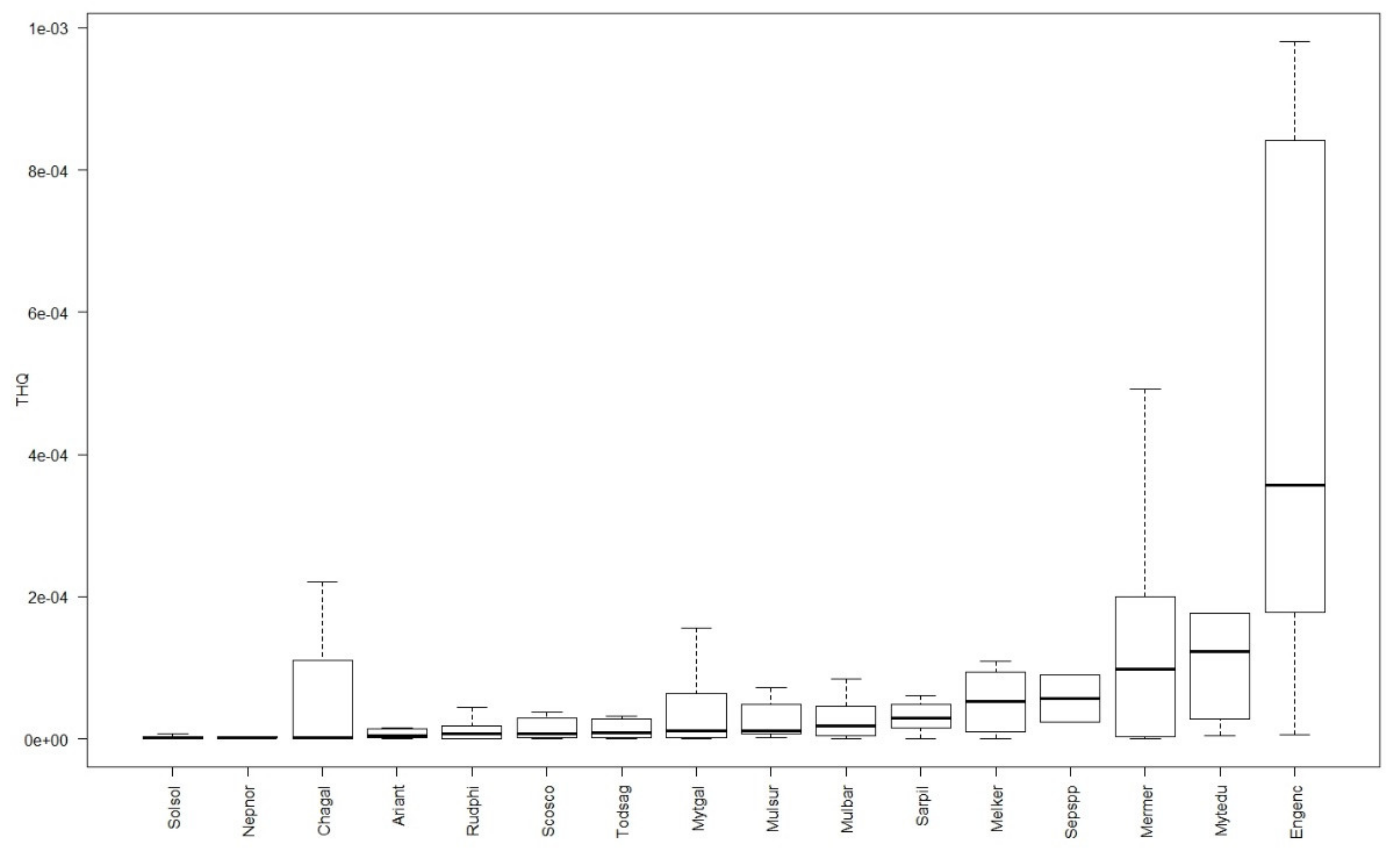
3.4. Human Health Risks Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavanello, S.; Campisi, M.; Mastrangelo, G.; Hoxha, M.; Bollati, V. The effects of everyday-life exposure to polycyclic aromatic hydrocarbons on biological age indicators. Environ. Health 2020, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; van Ruth, S.M. Infrared Spectroscopy: Applications. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 424–431. ISBN 978-0-12-384953-3. [Google Scholar]
- Sakshi; Haritash, A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef] [PubMed]
- Ifegwu, O.C.; Anyakora, C. Polycyclic Aromatic Hydrocarbons. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 72, pp. 277–304. [Google Scholar] [CrossRef]
- Lucas, J.; Percelay, I.; Larcher, T.; Lefrançois, C. Effects of pyrolytic and petrogenic polycyclic aromatic hydrocarbons on swimming and metabolic performance of zebrafish contaminated by ingestion. Ecotoxicol. Environ. Saf. 2016, 132, 145–152. [Google Scholar] [CrossRef]
- Mercogliano, R.; Santonicola, S.; De Felice, A.; Anastasio, A.; Murru, N.; Ferrante, M.C.; Cortesi, M.L. Occurrence and distribution of polycyclic aromatic hydrocarbons in mussels from the gulf of Naples, Tyrrhenian Sea, Italy. Mar. Pollut. Bull. 2016, 104, 386–390. [Google Scholar] [CrossRef]
- Grigoriou, C.; Costopoulou, D.; Vassiliadou, I.; Chrysafidis, D.; Tzamtzis, V.; Bakeas, E.; Leondiadis, L. Monitoring of Polycyclic Aromatic Hydrocarbon Levels in Mussels (Mytilus galloprovincialis) from Aquaculture Farms in Central Macedonia Region, Greece, Using Gas Chromatography—Tandem Mass Spectrometry Method. Molecules 2021, 26, 5953. [Google Scholar] [CrossRef]
- Bua, R.O.; Contino, A.; Giuffrida, A. Polycyclic aromatic hydrocarbons in Mullus surmuletus from the Catania Gulf (Sicily, Italy): Distribution and potential health risks. Environ. Sci. Pollut. Res. 2020, 28, 7756–7765. [Google Scholar] [CrossRef]
- SCF. Opinion of the Scientific Committee on Food on the risks to human health of Polycyclic Aromatic Hydrocarbons in food. EFSA J. 2008, 724, 1–114. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maxi-mum Levels for Polycyclic Aromatic Hydrocarbons in FoodstuffsText with EEA Relevance. 5. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0004:0008:En:PDF (accessed on 15 January 2022).
- Frapiccini, E.; Cocci, P.; Annibaldi, A.; Panfili, M.; Santojanni, A.; Grilli, F.; Marini, M.; Palermo, F.A. Assessment of seasonal relationship between polycyclic aromatic hydrocarbon accumulation and expression patterns of oxidative stress-related genes in muscle tissues of red mullet (M. barbatus) from the Northern Adriatic Sea. Environ. Toxicol. Pharmacol. 2021, 88, 103752. [Google Scholar] [CrossRef]
- González-Fernández, C.; Albentosa, M.; Campillo, J.A.; Viñas, L.; Romero, D.; Franco, A.; Bellas, J. Effect of nutritive status on Mytilus galloprovincialis pollution biomarkers: Implications for large-scale monitoring programs. Aquat. Toxicol. 2015, 167, 90–105. [Google Scholar] [CrossRef]
- Conte, F.; Copat, C.; Longo, S.; Conti, G.O.; Grasso, A.; Arena, G.; Dimartino, A.; Brundo, M.V.; Ferrante, M. Polycyclic aromatic hydrocarbons in Haliotis tuberculata (Linnaeus, 1758) (Mollusca, Gastropoda): Considerations on food safety and source investigation. Food Chem. Toxicol. 2016, 94, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Recabarren-Villalón, T.; Ronda, A.C.; Oliva, A.L.; Cazorla, A.L.; Marcovecchio, J.E.; Arias, A.H. Seasonal distribution pattern and bioaccumulation of Polycyclic aromatic hydrocarbons (PAHs) in four bioindicator coastal fishes of Argentina. Environ. Pollut. 2021, 291, 118125. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Canzanella, S.; Lambiase, S.; Scaramuzzo, A.; La Nucara, R.; Bruno, T.; Picazio, G.; Colarusso, G.; Brunetti, R.; Gallo, P. Organic pollutants (PCBs, PCDD/Fs, PAHs) and toxic metals in farmed mussels from the Gulf of Naples (Italy): Monitoring and human exposure. Reg. Stud. Mar. Sci. 2020, 40, 101497. [Google Scholar] [CrossRef]
- Fiorito, F.; Amoroso, M.G.; Lambiase, S.; Serpe, F.P.; Bruno, T.; Scaramuzzo, A.; Maglio, P.; Fusco, G.; Esposito, M. A relationship between environmental pollutants and enteric viruses in mussels (Mytilus galloprovincialis). Environ. Res. 2018, 169, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Baali, A.; Yahyaoui, A. Polycyclic Aromatic Hydrocarbons (PAHs) and Their Influence to Some Aquatic Species. In Biochemical Toxicology-Heavy Metals and Nanomaterials; Ince, M., Kaplan Ince, O., Ondrasek, G., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-696-6. [Google Scholar]
- Perugini, M.; Visciano, P.; Manera, M.; Turno, G.; Lucisano, A.A.; Amorena, M. Polycyclic Aromatic Hydrocarbons in Marine Organisms from the Gulf of Naples, Tyrrhenian Sea. J. Agric. Food Chem. 2007, 55, 2049–2054. [Google Scholar] [CrossRef]
- Corsi, I.; Mariottini, M.; Menchi, V.; Sensini, C.; Balocchi, C.; Focardi, S. Monitoring a Marine Coastal Area: Use of Mytilus galloprovincialis and Mullus barbatus as Bioindicators. Mar. Ecol. 2002, 23, 138–153. [Google Scholar] [CrossRef]
- Vassura, I.; Foschini, F.; Baravelli, V.; Fabbri, D. Distribution of alternant and non-alternant polycyclic aromatic hydrocarbons in sediments and clams of the Pialassa Baiona Lagoon (Ravenna, Italy). Chem. Ecol. 2005, 21, 415–424. [Google Scholar] [CrossRef]
- Fabbri, D.; Baravelli, V.; Giannotti, K.; Donnini, F.; Fabbri, E. Bioaccumulation of cyclopenta[cd]pyrene and benzo[ghi]fluoranthene by mussels transplanted in a coastal lagoon. Chemosphere 2006, 64, 1083–1092. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Giammarino, A.; Manera, M.; Di Nardo, W.; Amorena, M. Polycyclic aromatic hydrocarbons in marine organisms from the Adriatic Sea, Italy. Chemosphere 2007, 66, 1904–1910. [Google Scholar] [CrossRef]
- Bihari, N.; Fafanđel, M.; Piškur, V. Polycyclic Aromatic Hydrocarbons and Ecotoxicological Characterization of Seawater, Sediment, and Mussel Mytilus galloprovincialis from the Gulf of Rijeka, the Adriatic Sea, Croatia. Arch. Environ. Contam. Toxicol. 2007, 52, 379–387. [Google Scholar] [CrossRef]
- Della Torre, C.; Corsi, I.; Nardi, F.; Perra, G.; Tomasino, M.P.; Focardi, S. Transcriptional and post-transcriptional response of drug-metabolizing enzymes to PAHs contamination in red mullet (Mullus barbatus, Linnaeus, 1758): A field study. Mar. Environ. Res. 2010, 70, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsi, I.; Tabaku, A.; Nuro, A.; Beqiraj, S.; Marku, E.; Perra, G.; Tafaj, L.; Baroni, D.; Bocari, D.; Guerranti, C.; et al. Ecotoxicological Assessment of Vlora Bay (Albania) by a Biomonitoring Study Using an Integrated Approach of Sublethal Toxicological Effects and Contaminant Levels in Bioindicator Species. J. Coast. Res. 2011, 270, 116–120. [Google Scholar] [CrossRef]
- Trisciani, A.; Corsi, I.; Della Torre, C.; Perra, G.; Focardi, S. Hepatic biotransformation genes and enzymes and PAH metabolites in bile of common sole (Solea solea, Linnaeus, 1758) from an oil-contaminated site in the Mediterranean Sea: A field study. Mar. Pollut. Bull. 2011, 62, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Barone, G.; Perrone, V.G.; Storelli, A. Risk characterization for polycyclic aromatic hydrocarbons and toxic metals associated with fish consumption. J. Food Compos. Anal. 2013, 31, 115–119. [Google Scholar] [CrossRef]
- Gomiero, A.; Volpato, E.; Nasci, C.; Perra, G.; Viarengo, A.; Dagnino, A.; Spagnolo, A.; Fabi, G. Use of multiple cell and tissue-level biomarkers in mussels collected along two gas fields in the northern Adriatic Sea as a tool for long term environmental monitoring. Mar. Pollut. Bull. 2015, 93, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Grazioli, E.; Focardi, S.; Renzi, M.; Perra, G. Levels of chemicals in two fish species from four Italian fishing areas. Mar. Pollut. Bull. 2016, 111, 449–452. [Google Scholar] [CrossRef]
- Glad, M.; Bihari, N.; Jaksic, Z.; Fafanđel, M. Comparison between resident and caged mussels: Polycyclic aromatic hydrocarbon accumulation and biological response. Mar. Environ. Res. 2017, 129, 195–206. [Google Scholar] [CrossRef]
- Cacciatore, F.; Bernarello, V.; Brusà, R.B.; Sesta, G.; Franceschini, G.; Maggi, C.; Gabellini, M.; Lamberti, C.V. PAH (Polycyclic Aromatic Hydrocarbon) bioaccumulation and PAHs/shell weight index in Ruditapes philippinarum (Adams & Reeve, 1850) from the Vallona lagoon (northern Adriatic Sea, NE Italy). Ecotoxicol. Environ. Saf. 2018, 148, 787–798. [Google Scholar] [CrossRef]
- Frapiccini, E.; Annibaldi, A.; Betti, M.; Polidori, P.; Truzzi, C.; Marini, M. Polycyclic aromatic hydrocarbon (PAH) accumulation in different common sole (Solea solea) tissues from the North Adriatic Sea peculiar impacted area. Mar. Pollut. Bull. 2018, 137, 61–68. [Google Scholar] [CrossRef]
- Bajt, O.; Ramšak, A.; Milun, V.; Andral, B.; Romanelli, G.; Scarpato, A.; Mitrić, M.; Kupusović, T.; Kljajić, Z.; Angelidis, M.; et al. Assessing chemical contamination in the coastal waters of the Adriatic Sea using active mussel biomonitoring with Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 141, 283–298. [Google Scholar] [CrossRef]
- Frapiccini, E.; Panfili, M.; Guicciardi, S.; Santojanni, A.; Marini, M.; Truzzi, C.; Annibaldi, A. Effects of biological factors and seasonality on the level of polycyclic aromatic hydrocarbons in red mullet (Mullus barbatus). Environ. Pollut. 2019, 258, 113742. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Marcotrigiano, G.O. Polycyclic Aromatic Hydrocarbons in Mussels (Mytilus galloprovincialis) from the Ionian Sea, Italy. J. Food Prot. 2001, 64, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.O.; Copat, C.; Ledda, C.; Fiore, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Evaluation of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Mullus barbatus from Sicily Channel and Risk-Based Consumption Limits. Bull. Environ. Contam. Toxicol. 2012, 88, 946–950. [Google Scholar] [CrossRef]
- Marrone, R.; Smaldone, G.; Pepe, T.; Mercogliano, R.; De Felice, A.; Anastasio, A. Polycyclic Aromatic Hydrocarbons (Pahs) in Seafoods Caught in Corigliano Calabro Gulf (Cs, Italy). Ital. J. Food Saf. 2012, 1, 41–46. [Google Scholar] [CrossRef]
- Traina, A.; Ausili, A.; Bonsignore, M.; Fattorini, D.; Gherardi, S.; Gorbi, S.; Quinci, E.; Romano, E.; Manta, D.S.; Tranchida, G.; et al. Organochlorines and Polycyclic Aromatic Hydrocarbons as fingerprint of exposure pathways from marine sediments to biota. Mar. Pollut. Bull. 2021, 170, 112676. [Google Scholar] [CrossRef] [PubMed]
- Cocchieri, R.A.; Arnese, A.; Minicucci, A.M. Polycyclic aromatic hydrocarbons in marine organisms from Italian central Mediterranean coasts. Mar. Pollut. Bull. 1990, 21, 15–18. [Google Scholar] [CrossRef]
- Piccardo, M.; Coradeghini, R.; Valerio, F. Polycyclic Aromatic Hydrocarbon Pollution in Native and Caged Mussels. Mar. Pollut. Bull. 2001, 42, 951–956. [Google Scholar] [CrossRef]
- Amoroso, S.; Arnese, A.; Cirillo, T.; Montuori, P.; Triassi, M.; Amodio-Cocchieri, R. Pollution by Mercury, Arsenic, Lead, Chromium, Cadmium, and Polycyclic Aromatic Hydrocarbons of Fish and Mussels from the Gulf of Naples, Italy. Bull. Environ. Contam. Toxicol. 2003, 71, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Serpe, F.P.; Esposito, M.; Gallo, P.; Salini, M.; Maglio, P.; Hauber, T.; Serpe, L. Determination of heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in Mytilus galloprovincialis from Campania coasts, Italy. Fresenius Environ. Bull. 2010, 19, 2292–2296. [Google Scholar]
- Serpe, F.P.; Esposito, M.; Gallo, P.; Serpe, L. Optimisation and validation of an HPLC method for determination of polycyclic aromatic hydrocarbons (PAHs) in mussels. Food Chem. 2010, 122, 920–925. [Google Scholar] [CrossRef]
- Marrone, R.; Mercogliano, R.; Palma, G.; Chirollo, C.; Smaldone, G.; Anastasio, A. Polycyclic aromatic hydrocarbons (PAHS) in seafood caught off in napoli gulf (Italy). Ital. J. Food Saf. 2011, 1, 61–65. [Google Scholar] [CrossRef]
- Ferrante, M.; Zanghì, G.; Cristaldi, A.; Copat, C.; Grasso, A.; Fiore, M.; Signorelli, S.S.; Zuccarello, P.; Conti, G.O. PAHs in seafood from the Mediterranean Sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Arnese, A.; Esposito, F.; Albano, L.; Masucci, A.; Capelli, C.; Cirillo, T.; Nardone, A. Evaluation of the impact of anthropogenic activities on arsenic, cadmium, chromium, mercury, lead, and polycyclic aromatic hydrocarbon levels in seafood from the Gulf of Naples, Italy. J. Environ. Sci. Health Part A 2018, 53, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Arienzo, M.; Toscanesi, M.; Trifuoggi, M.; Ferrara, L.; Stanislao, C.; Donadio, C.; Grazia, V.; Gionata, D.V.; Carella, F. Contaminants bioaccumulation and pathological assessment in Mytilus galloprovincialis in coastal waters facing the brownfield site of Bagnoli, Italy. Mar. Pollut. Bull. 2019, 140, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, S.; Ariano, A.; Serpe, F.P.; Scivicco, M.; Velotto, S.; Esposito, M.; Severino, L. Polycyclic aromatic hydrocarbons (PAHs), arsenic, chromium and lead in warty crab (Eriphia verrucosa): Occurrence and risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 35305–35315. [Google Scholar] [CrossRef]
- Baumard, P.; Budzinski, H.; Garrigues, P. Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean sea. Environ. Toxicol. Chem. 1998, 17, 765–776. [Google Scholar] [CrossRef]
- Cinnirella, S.; Bruno, D.E.; Pirrone, N.; Horvat, M.; Živković, I.; Evers, D.C.; Johnson, S.; Sunderland, E.M. Mercury concentrations in biota in the Mediterranean Sea, a compilation of 40 years of surveys. Sci. Data 2019, 6, 205–211. [Google Scholar] [CrossRef] [Green Version]
- US-EPA. Guidance for Assessing Chemical Contamination Data for Use in Fish Advisories, Volume 2. Risk Assessment and Fish Consumption Limits; EPA/823-B94-004; Office of Science and Technology Office of Water U.S. Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Grilli, F.; Accoroni, S.; Acri, F.; Aubry, F.B.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
- Navarra, S.; Carbonari, F.; Bambi, C. Ismea il Pesce a Tavola: Percezioni e Stili di Consumo degli Italiani, 2011. Ismea Mercati web site. Available online: https://www.ismea.it/flex/cm/pages/ServeAttachment.php/L/IT/D/f%252Fb%252F5%252FD.914a4f340b52602fcc38/P/BLOB%3AID%3D6191/E/pdf (accessed on 15 January 2022).
- Galgani, F.; Martínez-Gómez, C.; Giovanardi, F.; Romanelli, G.; Caixach, J.; Cento, A.; Scarpato, A.; Benbrahim, S.; Messaoudi, S.; Deudero, S.; et al. Assessment of polycyclic aromatic hydrocarbon concentrations in mussels (Mytilus galloprovincialis) from the Western basin of the Mediterranean Sea. Environ. Monit. Assess. 2010, 172, 301–317. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, C.; Villa, S.; Chimera, C.; Finizio, A.; Monti, G. Spatial and temporal trends in the ecological risk posed by polycyclic aromatic hydrocarbons in Mediterranean Sea sediments using large-scale monitoring data. Ecol. Indic. 2021, 129, 107923. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Karayücel, S.; Karayücel, I.; Öztürk, R.; Eyüboğlu, B. Meat Yield, Condition Index, and Biochemical Composition of Mussels (Mytilus galloprovincialis Lamarck, 1819) in Sinop, South of the Black Sea. J. Aquat. Food Prod. Technol. 2012, 21, 198–205. [Google Scholar] [CrossRef]
- Prato, E.; Danieli, A.; Maffia, M.; Biandolino, F. Lipid and Fatty Acid Compositions of Mytilus galloprovincialis Cultured in the Mar Grande of Taranto (Southern Italy): Feeding Strategies and Trophic Relationships. Zool. Stud. 2010, 49, 211–219. [Google Scholar]
- Bongiorno, T.; Iacumin, L.; Tubaro, F.; Marcuzzo, E.; Sensidoni, A.; Tulli, F. Seasonal changes in technological and nutritional quality of Mytilus galloprovincialis from suspended culture in the Gulf of Trieste (North Adriatic Sea). Food Chem. 2015, 173, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Shimada, K.; Sugiyama, T.; Sato, K.; Takami, A.; Chan, C.K.; Kim, I.S.; Kim, Y.P.; Lin, N.-H.; Hatakeyama, S. Seasonal and annual changes in PAH concentrations in a remote site in the Pacific Ocean. Sci. Rep. 2019, 9, 12591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Ding, X.; He, Q.; Yang, W.; Zhu, M.; Li, S.; Zhang, R.; Shen, R.; Zhang, Y.; Bi, X.; et al. Nationwide increase of polycyclic aromatic hydrocarbons in ultrafine particles during winter over China revealed by size-segregated measurements. Atmos. Chem. Phys. 2020, 20, 14581–14595. [Google Scholar] [CrossRef]
- Guigue, C.; Tedetti, M.; Ferretto, N.; Garcia, N.; Méjanelle, L.; Goutx, M. Spatial and seasonal variabilities of dissolved hydrocarbons in surface waters from the Northwestern Mediterranean Sea: Results from one year intensive sampling. Sci. Total Environ. 2014, 466-467, 650–662. [Google Scholar] [CrossRef]
- Marini, M.; Frapiccini, E. Persistence of polycyclic aromatic hydrocarbons in sediments in the deeper area of the Northern Adriatic Sea (Mediterranean Sea). Chemosphere 2012, 90, 1839–1846. [Google Scholar] [CrossRef]
- Kodnik, D.; Carniel, F.C.; Licen, S.; Tolloi, A.; Barbieri, P.; Tretiach, M. Seasonal variations of PAHs content and distribution patterns in a mixed land use area: A case study in NE Italy with the transplanted lichen Pseudevernia furfuracea. Atmos. Environ. 2015, 113, 255–263. [Google Scholar] [CrossRef]
- Witt, G. Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Mar. Pollut. Bull. 1995, 31, 237–248. [Google Scholar] [CrossRef]
- Frapiccini, E.; Marini, M. Polycyclic Aromatic Hydrocarbon Degradation and Sorption Parameters in Coastal and Open-Sea Sediment. Water Air Soil Pollut. 2015, 226, 246. [Google Scholar] [CrossRef]
- Guigue, C.; Tedetti, M.; Dang, D.H.; Mullot, J.-U.; Garnier, C.; Goutx, M. Remobilization of polycyclic aromatic hydrocarbons and organic matter in seawater during sediment resuspension experiments from a polluted coastal environment: Insights from Toulon Bay (France). Environ. Pollut. 2017, 229, 627–638. [Google Scholar] [CrossRef]
- Mathieu, A.; Lemaire, P.; Carriere, S.; Drai, P.; Giudicelli, J.; Lafaurie, M. Seasonal and Sex-Linked Variations in Hepatic and Extrahepatic Biotransformation Activities in Striped Mullet (Mullus barbatus). Ecotoxicol. Environ. Saf. 1991, 22, 45–57. [Google Scholar] [CrossRef]
- Marini, M.; Jones, B.H.; Campanelli, A.; Grilli, F.; Lee, C.M. Seasonal variability and Po River plume influence on biochemical properties along western Adriatic coast. J. Geophys. Res. Earth Surf. 2008, 113, C05S90. [Google Scholar] [CrossRef]
- Marini, M.; Campanelli, A.; Sanxhaku, M.; Kljajić, Z.; Betti, M.; Grilli, F. Late Spring Characterization of Different Coastal Areas of the Adriatic Sea. Acta Adriat. 2015, 56, 27–46. [Google Scholar]
- Dachs, J.; Bayona, J.M.; Raoux, C.; Albaigés, J. Spatial, Vertical Distribution and Budget of Polycyclic Aromatic Hydrocarbons in the Western Mediterranean Seawater. Environ. Sci. Technol. 1997, 31, 682–688. [Google Scholar] [CrossRef]
- Iacono, R.; Napolitano, E.; Palma, M.; Sannino, G. The Tyrrhenian Sea Circulation: A Review of Recent Work. Sustainability 2021, 13, 6371. [Google Scholar] [CrossRef]
- Marine Traffic Density Map. Available online: https://ec.europa.eu/maritimeaffairs/atlas/maritime_atlas/#lang=EN;p=w;bkgd=1;theme=2:0.75,5007:0.75;c=1417747.2062308616,4941995.124365145;z=6;e=t (accessed on 10 February 2022).
- Barale, V.; Dusart, J.; Assouline, M.; Niceta, F. European Atlas of the Seas: “A picture is worth a thousand words”. J. Coast. Conserv. 2017, 22, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Pensa, A.; Pinton, A.; Vita, L.; Bonamico, A.; De Benedetti, A.A.; Giordano, G. Atlas of Italian Submarine Volcanic Structures. In Memorie Descrittive della Carta Geologica d’Italia; ISPRA—Servizio Geologico d’Italia: Rome, Italy, 2019; Volume 104. [Google Scholar]
- Saroni, A.; Sciarra, A.; Grassa, F.; Eich, A.; Weber, M.; Lott, C.; Ferretti, G.; Ivaldi, R.; Coltorti, M. Shallow submarine mud volcano in the northern Tyrrhenian sea, Italy. Appl. Geochem. 2020, 122, 104722. [Google Scholar] [CrossRef]
- Kozak, K.; Ruman, M.; Kosek, K.; Karasiński, G.; Stachnik, Ł.; Polkowska, Ż. Impact of Volcanic Eruptions on the Occurrence of PAHs Compounds in the Aquatic Ecosystem of the Southern Part of West Spitsbergen (Hornsund Fjord, Svalbard). Water 2017, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Remizovschi, A.; Carpa, R.; Forray, F.L.; Chiriac, C.; Roba, C.-A.; Beldean-Galea, S.; Andrei, A.-S.; Szekeres, E.; Baricz, A.; Lupan, I.; et al. Mud volcanoes and the presence of PAHs. Sci. Rep. 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo, J.L. Nutrients and Chemical Pollutants in Fish and Shellfish. Balancing Health Benefits and Risks of Regular Fish Consumption. Crit. Rev. Food Sci. Nutr. 2014, 56, 979–988. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, A.; Giuliani, C.; Marini, M.; Luiselli, D. Methylmercury and Polycyclic Aromatic Hydrocarbons in Mediterranean Seafood: A Molecular Anthropological Perspective. Appl. Sci. 2021, 11, 11179. [Google Scholar] [CrossRef]


| Foodstuffs | Maximum Levels for BaP (mg/kg) | Maximum Levels for PAH4 1 (mg/kg) |
|---|---|---|
| Muscle meat of smoked fish and smoked fishery products | 0.002 | 0.012 |
| Bivalve mollusks (fresh, chilled or frozen) | 0.005 | 0.030 |
| Bivalve mollusks (smoked) | 0.006 | 0.035 |
| Species | PAHs Class 1 | Cmean 2 | Cmin 3 | Cmax 4 |
|---|---|---|---|---|
| Mytilus galloprovincialis | LMW | 0.00942 | 0.00000 | 3.96000 |
| MMW | 0.01068 | 0.00000 | 1.05960 | |
| HMW | 0.00570 | 0.00000 | 0.34500 | |
| Ruditapes philippinarum | LMW | 0.00137 | 0.00000 | 0.03770 |
| MMW | 0.00197 | 0.00041 | 0.00810 | |
| HMW | 0.00058 | 0.00000 | 0.00690 | |
| Solea solea | LMW | 0.00079 | 0.00006 | 0.01000 |
| MMW | 0.00189 | 0.00009 | 0.01037 | |
| HMW | 0.00914 | 0.00000 | 0.73500 | |
| Mullus barbatus | LMW | 0.01030 | 0.00011 | 0.09385 |
| MMW | 0.00299 | 0.00042 | 0.01050 | |
| HMW | 0.00090 | 0.00000 | 0.00539 |
| Species | Period | PAHs Class 1 | Cmean 2 | Cmin 3 | Cmax 4 | Depthmin 5 | Deptmax 6 | Yearmin 7 | Yearmax 8 |
|---|---|---|---|---|---|---|---|---|---|
| Mytilus galloprovincialis | Autumn/Winter | LMW | 0.00434 | 0.00000 | 0.18090 | 0.00 | 77.60 | 1995 | 2018 |
| MMW | 0.01292 | 0.00000 | 0.96000 | 0.00 | 118.80 | 1995 | 2018 | ||
| HMW | 0.00512 | 0.00000 | 0.34500 | 0.00 | 118.80 | 1995 | 2018 | ||
| Spring/Summer | LMW | 0.01155 | 0.00000 | 3.96000 | 0.00 | 108.00 | 1981 | 2017 | |
| MMW | 0.00908 | 0.00000 | 1.05960 | 0.00 | 108.00 | 1981 | 2017 | ||
| HMW | 0.00601 | 0.00000 | 0.18040 | 0.00 | 108.00 | 1981 | 2017 | ||
| Ruditapes philippinarum | Autumn/Winter | LMW | 0.00177 | 0.00000 | 0.03770 | - | - | 2005 | 2013 |
| MMW | 0.00228 | 0.00080 | 0.00770 | - | - | 2005 | 2013 | ||
| HMW | 0.00066 | 0.00000 | 0.00690 | - | - | 2005 | 2013 | ||
| Spring/Summer | LMW | 0.00087 | 0.00000 | 0.00890 | - | - | 2001 | 2014 | |
| MMW | 0.00160 | 0.00041 | 0.00810 | - | - | 2001 | 2014 | ||
| HMW | 0.00047 | 0.00000 | 0.00600 | - | - | 2001 | 2014 | ||
| Mullus barbatus | Autumn/Winter | LMW | 0.00649 | 0.00016 | 0.09265 | 70 | 70 | 2004 | 2019 |
| MMW | 0.00315 | 0.00042 | 0.01050 | 70 | 70 | 2004 | 2019 | ||
| HMW | 0.00088 | 0.00000 | 0.00539 | 70 | 70 | 2004 | 2019 | ||
| Spring/Summer | LMW | 0.01477 | 0.00011 | 0.09385 | 70 | 70 | 2004 | 2019 | |
| MMW | 0.00334 | 0.00060 | 0.00749 | 70 | 70 | 2004 | 2019 | ||
| HMW | 0.00118 | 0.00000 | 0.00536 | 70 | 70 | 2004 | 2019 |
| Period | PAHs Class 1 | Cmean 2 | Cmin 3 | Cmax 4 | Depthmin 5 | Depthmax 6 | Yearmin 7 | Yearmax 8 | Latmin 9 | Latmax 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Autumn/Winter | LMW | 0.00463 | 0.00002 | 0.18090 | 1.20 | 24.80 | 2006 | 2009 | 41.60 | 45.76 |
| MMW | 0.00591 | 0.00015 | 0.11010 | 1.20 | 24.80 | 2005 | 2009 | 41.60 | 45.76 | |
| HMW | 0.00382 | 0.00002 | 0.34500 | 1.20 | 24.80 | 2005 | 2009 | 41.60 | 45.76 | |
| Spring/Summer | LMW | 0.01093 | 0.00000 | 1.15500 | 0.01 | 35.00 | 2006 | 2011 | 40.20 | 45.77 |
| MMW | 0.00396 | 0.00003 | 0.03780 | 0.01 | 30.60 | 2005 | 2017 | 40.20 | 45.77 | |
| HMW | 0.00209 | 0.00000 | 0.03150 | 0.01 | 30.60 | 2005 | 2017 | 40.20 | 45.77 |
| Period | PAHs Class 1 | Cmean 2 | Cmin 3 | Cmax 4 | Depthmin 5 | Depthmax 6 | Yearmin 7 | Yearmax 8 | Latmin 9 | Latmax 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Autumn/Winter | LMW | 0.00434 | 0.00000 | 0.12000 | 0.10 | 49.02 | 1999 | 2017 | 38.54 | 43.50 |
| MMW | 0.02649 | 0.00000 | 0.96000 | 0.10 | 49.02 | 1999 | 2017 | 38.54 | 43.50 | |
| HMW | 0.00953 | 0.00000 | 0.20237 | 0.10 | 49.02 | 1999 | 2017 | 38.54 | 43.50 | |
| Spring/Summer | LMW | 0.00685 | 0.00000 | 0.20000 | 0.19 | 108.00 | 1981 | 2017 | 38.54 | 44.42 |
| MMW | 0.01234 | 0.00000 | 1.05960 | 0.19 | 108.00 | 1981 | 2017 | 38.97 | 44.42 | |
| HMW | 0.00766 | 0.00000 | 0.18040 | 0.19 | 108.00 | 1981 | 2017 | 38.54 | 44.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giovanni, A.; Abondio, P.; Frapiccini, E.; Luiselli, D.; Marini, M. Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood. Appl. Sci. 2022, 12, 2776. https://doi.org/10.3390/app12062776
De Giovanni A, Abondio P, Frapiccini E, Luiselli D, Marini M. Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood. Applied Sciences. 2022; 12(6):2776. https://doi.org/10.3390/app12062776
Chicago/Turabian StyleDe Giovanni, Andrea, Paolo Abondio, Emanuela Frapiccini, Donata Luiselli, and Mauro Marini. 2022. "Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood" Applied Sciences 12, no. 6: 2776. https://doi.org/10.3390/app12062776
APA StyleDe Giovanni, A., Abondio, P., Frapiccini, E., Luiselli, D., & Marini, M. (2022). Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood. Applied Sciences, 12(6), 2776. https://doi.org/10.3390/app12062776









