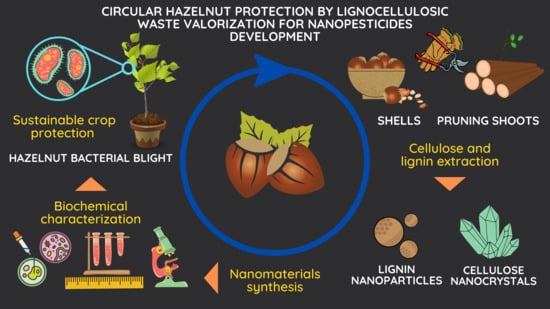
Circular Hazelnut Protection by Lignocellulosic Waste Valorization for Nanopesticides Development
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanomaterialssynthesis
2.2.1. Cellulose Extraction and CNC Synthesis
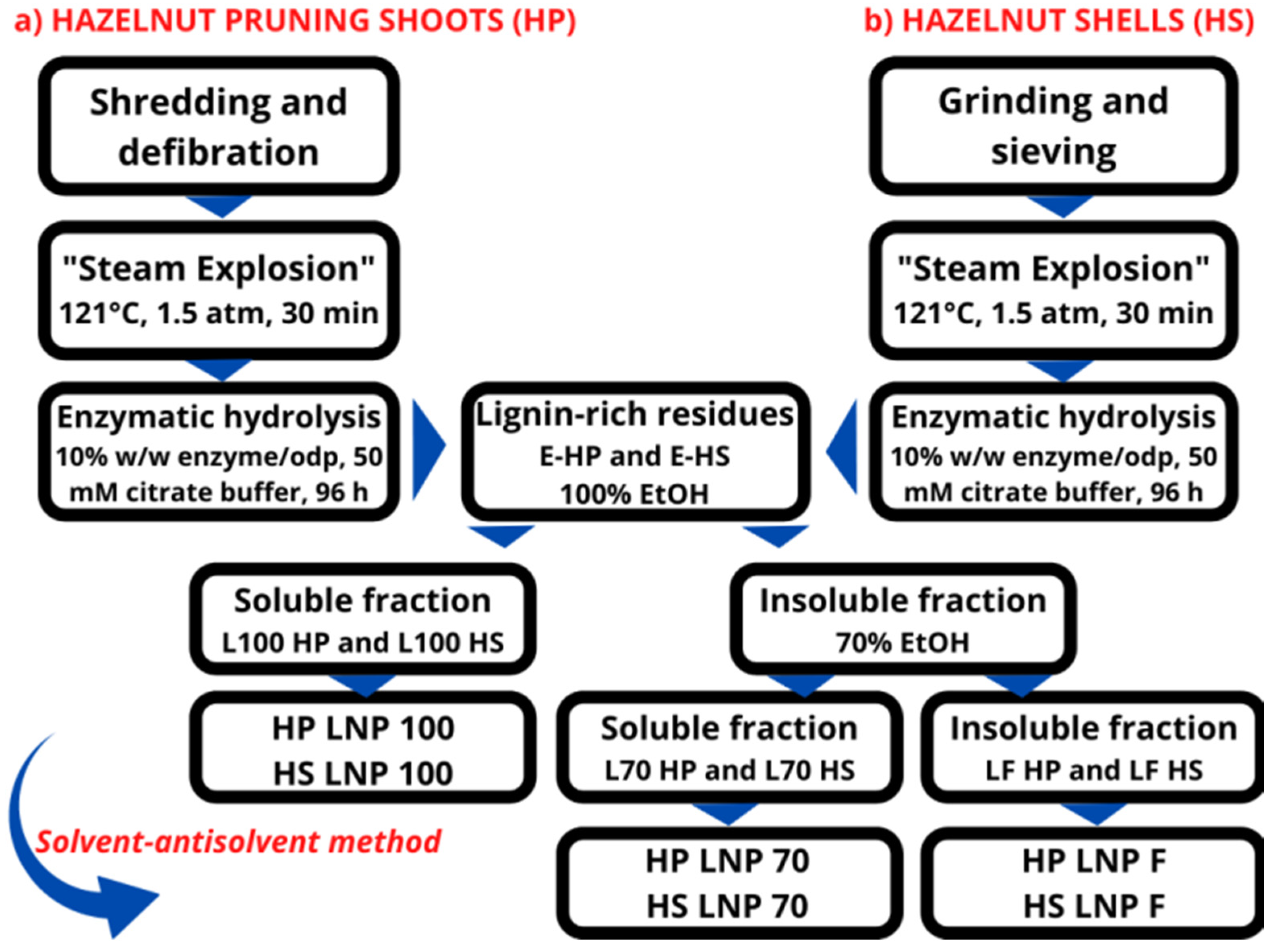
2.2.2. Lignin Extraction and LNP Synthesis
2.3. Nanomaterials Characterization
2.3.1. CNC Size
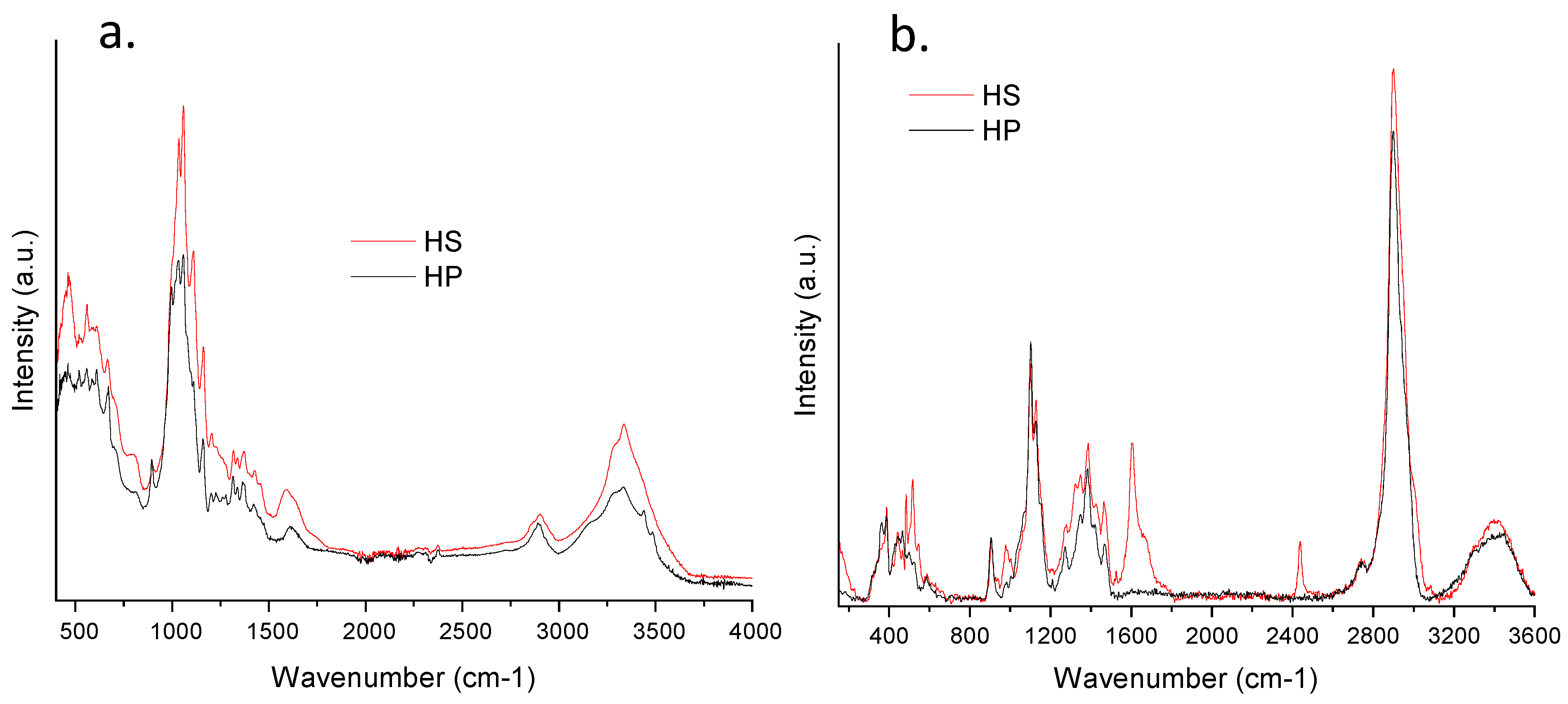
2.3.2. IR and Raman Spectroscopy
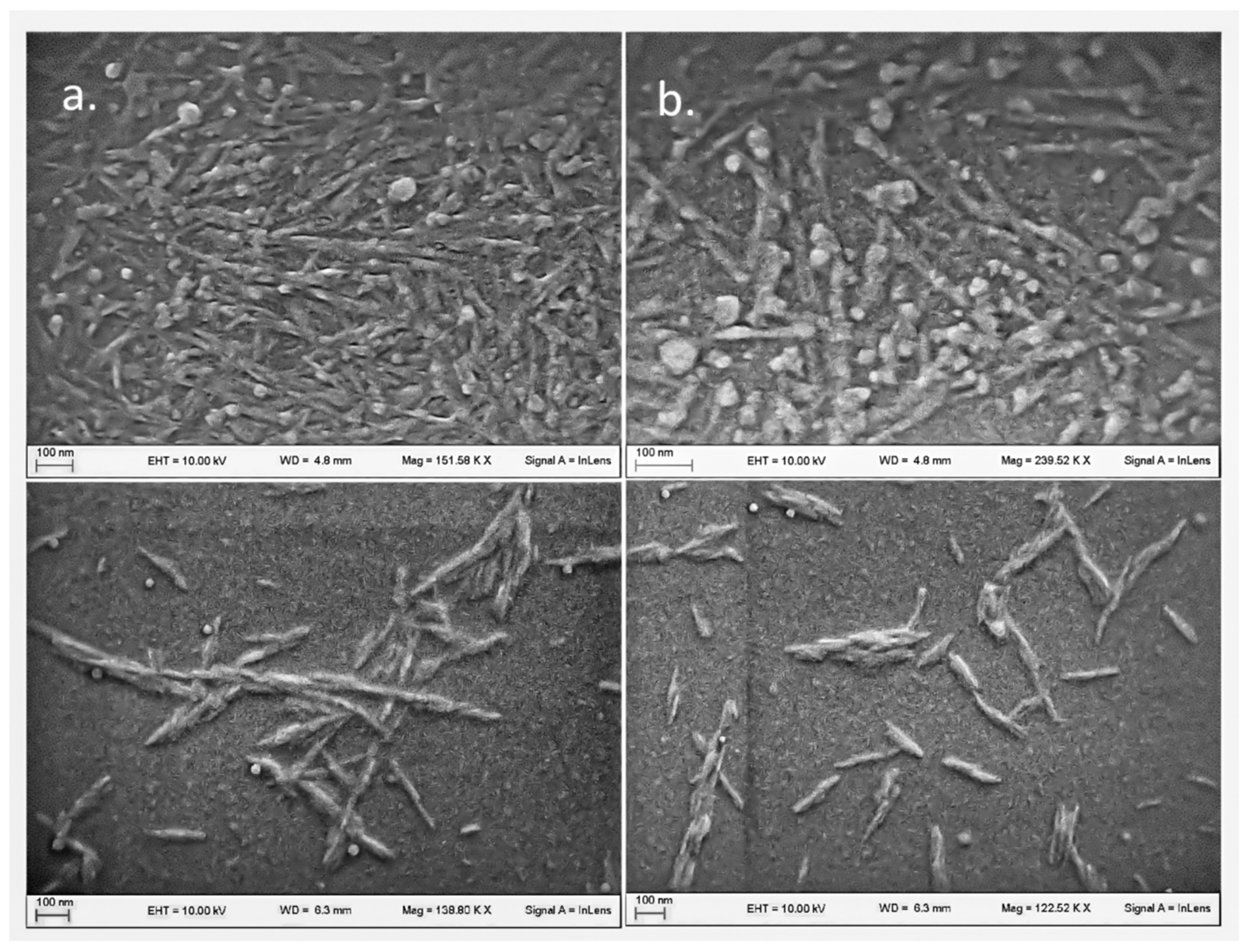
2.3.3. Morphology
2.3.4. Diffractometric Analysis
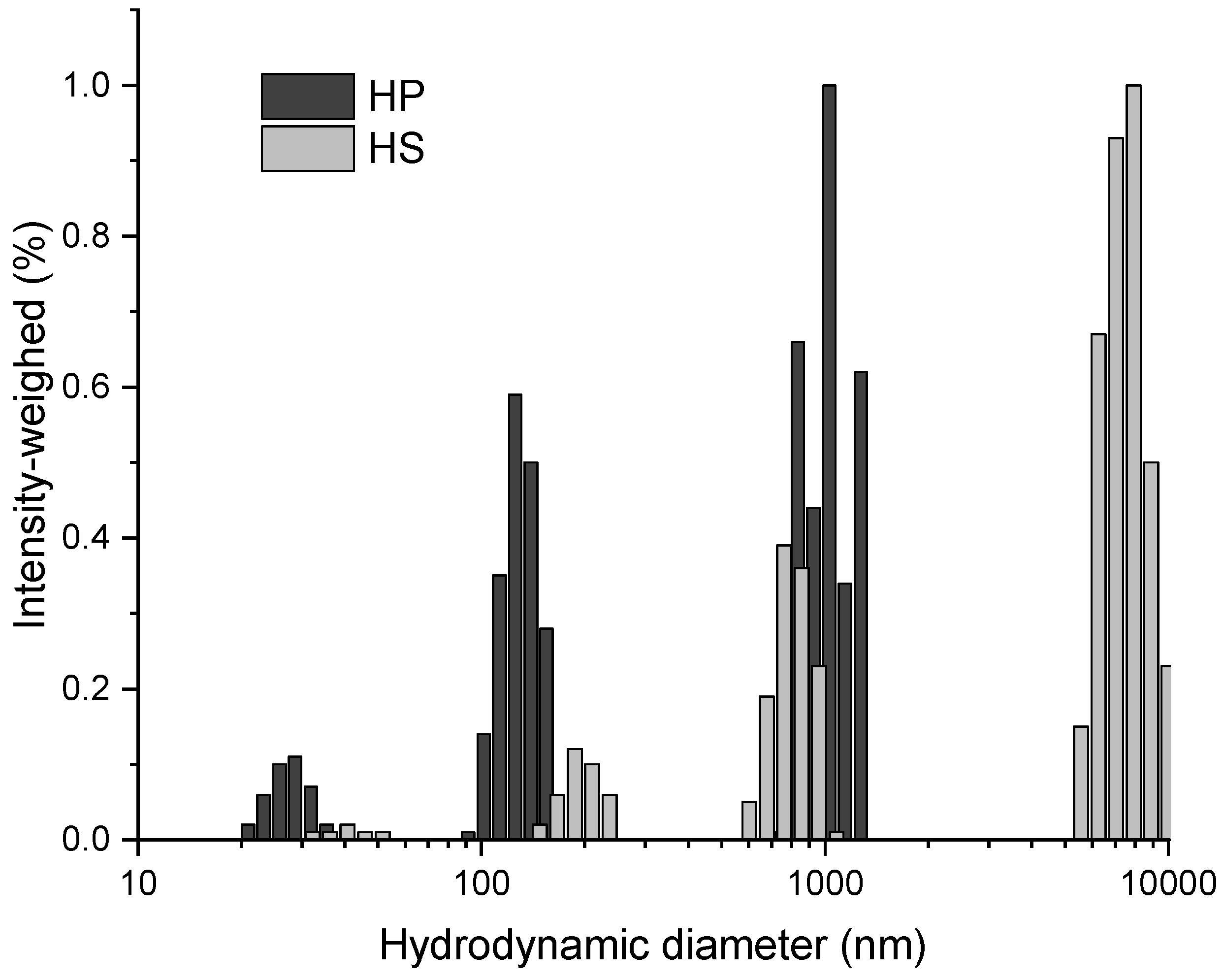
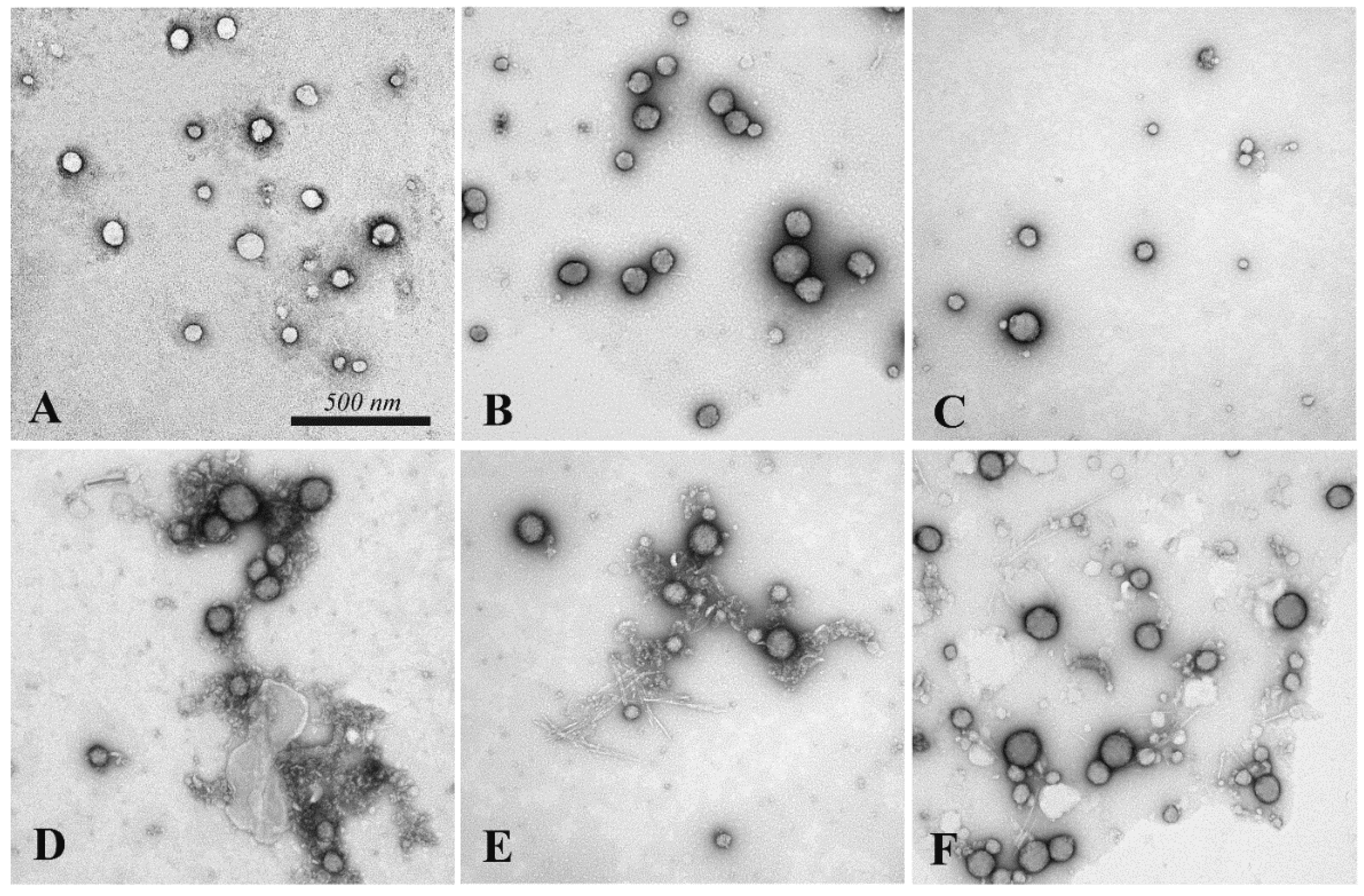
2.3.5. LNP Morphology and Dimension
2.3.6. LNP Total Phenolic Content
2.4. In Vitro Antibacterial Activity
2.5. In Vivo Antibacterial Activity
2.6. In Vivo Phytobiological Compatibility
2.7. Statistical Ananlysis
3. Results and Discussion
3.1. CNC and LNP Synthesis
3.2. CNC and LNP Characterization
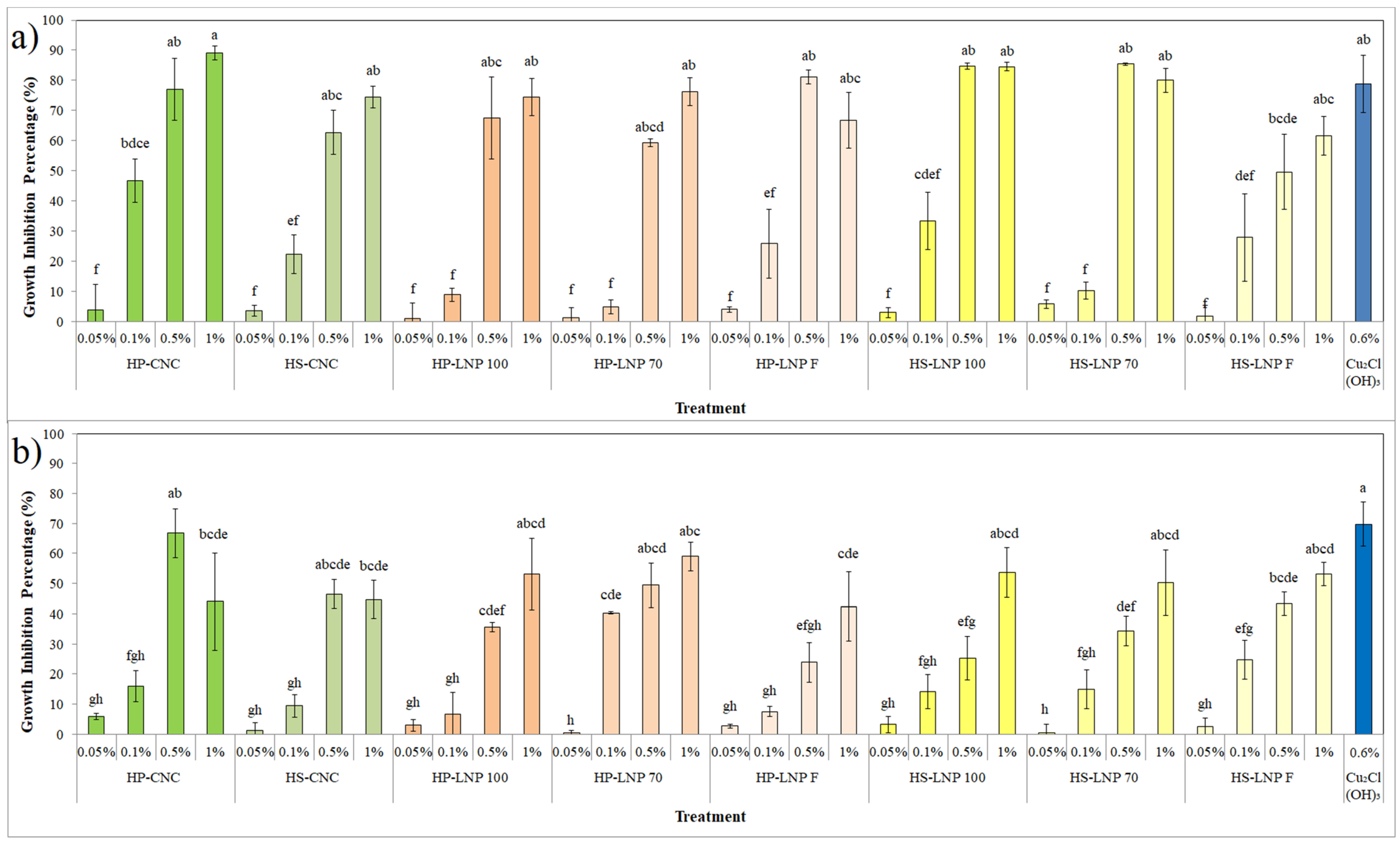
3.3. In Vitro Antibacterial Activity
3.4. In Vivo Antibacterial Activity
3.5. Phytobiological Compatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef]
- Vauterin, L.; Hoste, B.; Kersters, K.; Swings, J. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 1995, 45, 472–489. [Google Scholar] [CrossRef]
- Scortichini, M.; Rossi, M.P.; Marchesi, U. Genetic, phenotypic and pathogenic diversity of Xanthomonas arboricola pv. corylina strains question the representative nature of the type strain. Plant Pathol. 2002, 51, 374–381. [Google Scholar] [CrossRef]
- EPPO Data Sheets on Quarantine Pests: Xanthomonas arboricola pv. corylina. EPPO Bull. 2004, 34, 179–181. [CrossRef]
- Puławska, J.; Kałuzna, M.; Kołodziejska, A.; Sobiczewski, P. Identification and characterization of Xanthomonas arboricola pv. corylina causing bacterial blight of hazelnut: A new disease in Poland. J. Plant. Pathol. 2010, 92, 803–806. [Google Scholar]
- Webber, J.B.; Putnam, M.; Serdani, M.; Pscheidt, J.W.; Wiman, N.G.; Stockwell, V.O. Characterization of isolates of Xanthomonas arboricola pv. corylina, the causal agent of bacterial blight, from Oregon hazelnut orchards. J. Plant Pathol. 2020, 102, 799–812. [Google Scholar] [CrossRef]
- Kałużna, M.; Fischer-Le Saux, M.; Pothier, J.F.; Jacques, M.A.; Obradović, A.; Tavares, F.; Stefani, E. Xanthomonas arboricola pv. juglandis and pv. corylina: Brothers or distant relatives? Genetic clues, epidemiology, and insights for disease management. Mol. Plant Pathol. 2021, 22, 1481–1499. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Fabi, A.; Varvaro, L. Summer heat and low soil organic matter influence severity of hazelnut Cytospora canker. Phytopathology 2014, 104, 387–395. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Fabi, A.; Ridolfi, R.; Varvaro, L. Epidemiological Study of Hazelnut Bacterial Blight in Central Italy by Using Laboratory Analysis and Geostatistics. PLoS ONE 2013, 8, e56298. [Google Scholar] [CrossRef]
- Pisetta, M.; Albertin, I.; Petriccione, M.; Scortichini, M. Effects of hot water treatment to control Xanthomonas arboricola pv. corylina on hazelnut (Corylus avellana L.) propagative material. Sci. Hortic. 2016, 211, 187–193. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Varvaro, L. Xanthomonas arboricola disease of hazelnut: Current status and future perspectives for its management. Plant Pathol. 2014, 63, 243–254. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Varympopi, A.; Dimopoulou, A.; Theologidis, I.; Karamanidou, T.; Kaldeli Kerou, A.; Vlachou, A.; Karfaridis, D.; Papafotis, D.; Hatzinikolaou, D.G.; Tsouknidas, A.; et al. Bactericides Based on Copper Nanoparticles Restrain Growth of Important Plant Pathogens. Pathogens 2020, 9, 1024. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Goto, M.; Hikota, T.; Nakajima, M.; Takikawa, Y.; Tsuyumu, S. Occurrence and Properties of Copper-resistance in Plant Pathogenic Bacteria. Jpn. J. Phytopathol. 1994, 60, 147–153. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Chen, H.; Yada, R. Nanotechnologies in agriculture: New tools for sustainable development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Giovanale, G.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Cellulose nanocrystals from Actinidia deliciosa pruning residues combined with carvacrol in PVA_CH films with antioxidant/antimicrobial properties for packaging applications. Int. J. Biol. Macromol. 2017, 104, 43–55. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crops Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Hamed, O.A.; Fouad, Y.; Hamed, E.M.; Al-Hajj, N. Cellulose powder from olive industry solid waste. BioResources 2012, 7, 4190–4201. [Google Scholar] [CrossRef]
- Schiavi, D.; Balbi, R.; Giovagnoli, S.; Camaioni, E.; Botticella, E.; Sestili, F.; Balestra, G.M. A green nanostructured pesticide to control tomato bacterial speck disease. Nanomaterials 2021, 11, 1852. [Google Scholar] [CrossRef]
- Balestra, G.M.; Fortunati, E. Nanotechnology-Based Sustainable Alternatives for the Managements of Plant Diseases; Balestra, G.M., Fortunati, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128233948. [Google Scholar]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Fortunati, E.; Verma, D.; Luzi, F.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Novel nanoscaled materials from lignocellulosic sources: Potential applications in the agricultural sector. Handb. Ecomater. 2019, 4, 2657–2679. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Erdocia, X.; Charrier-El Bouhtoury, F.; Prado, R.; Labidi, J. Multistage treatment of almonds waste biomass: Characterization and assessment of the potential applications of raw material and products. Waste Manag. 2018, 80, 40–50. [Google Scholar] [CrossRef]
- Crus Lopes, L.P.; Martins, J.; Esteves, B.; Texeira De Lemos, L. New Products from Hazelnut Shell. In Proceedings of the Ecowood 2012—5th International Conferences on Environmentally-Compatible Forest Products, Porto, Portugal, 5–7 September 2012; pp. 83–90. [Google Scholar]
- Demirbaş, A. Estimating of structural composition of wood and non-wood biomass samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Di Michele, A.; Pagano, C.; Allegrini, A.; Blasi, F.; Cossignani, L.; Di Raimo, E.; Faieta, M.; Oliva, E.; Pittia, P.; Primavilla, S.; et al. Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization. Molecules 2021, 26, 6607. [Google Scholar] [CrossRef] [PubMed]
- Lelli, V.; Molinari, R.; Merendino, N.; Timperio, A.M. Detection and comparison of bioactive compounds in different extracts of two hazelnut skin varieties, tonda gentile romana and tonda di giffoni, using a metabolomics approach. Metabolites 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Fortunati, E.; Balestra, G.M. Lignocellulosic Materials as Novel Carriers, Also at Nanoscale, of Organic Active Principles for Agri-Food Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081024263. [Google Scholar]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- La Ferla, B.; Zoia, L.; Bigini, P.; Di Gennaro, P. Cellulose nanocrystals as promising nano-devices in the biomedical field. AIP Conf. Proc. 2018, 1990, 020019. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Fortunati, E.; Yang, W.; Luzi, F.; Kenny, J.; Torre, L.; Puglia, D. Lignocellulosic nanostructures as reinforcement in extruded and solvent casted polymeric nanocomposites: An overview. Eur. Polym. J. 2016, 80, 295–316. [Google Scholar] [CrossRef]
- Ngo, T.D.; Ho, T. Lignocellulosic biomasses, sustainable platform for bio-based materials with polypropylene. In Polypropylene: Properties, Uses and Benefits; Garcia, P., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 175–210. [Google Scholar]
- Gu, H.; Zhang, Y.; Li, X.; Li, W. Lignin improves release behavior of slow-release fertilizers with high content of urea. J. Appl. Polym. Sci. 2019, 136, 48238. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-based nanoparticles: A review on their preparations and applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Cailotto, S.; Gigli, M.; Bonini, M.; Rigoni, F.; Crestini, C. Sustainable Strategies in the Synthesis of Lignin Nanoparticles for the Release of Active Compounds: A Comparison. ChemSusChem 2020, 13, 4759–4767. [Google Scholar] [CrossRef] [PubMed]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro- to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Zikeli, F.; Vinciguerra, V.; Sennato, S.; Scarascia Mugnozza, G.; Romagnoli, M. Preparation of Lignin Nanoparticles with Entrapped Essential Oil as a Bio-Based Biocide Delivery System. ACS Omega 2020, 5, 358–368. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Mugnozza, G.S. Preparation of lignin nanoparticles from wood waste for wood surface treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef]
- Yang, W.; Kenny, J.M.; Puglia, D. Structure and properties of biodegradable wheat gluten bionanocomposites containing lignin nanoparticles. Ind. Crops Prod. 2015, 74, 348–356. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Nicolini, A.; Gelosia, M.; Coccia, V.; Petrozzi, A.; Brinchi, L. Lignin as co-product of second generation bioethanol production from ligno-cellulosic biomass. Energy Procedia 2014, 45, 52–60. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Processing of PLA nanocomposites with cellulose nanocrystals extracted from Posidonia oceanica waste: Innovative reuse of coastal plant. Ind. Crops Prod. 2015, 67, 439–447. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Jafari, S.M.; Kashaninejad, M.; Barani Motlagh, M. Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int. J. Biol. Macromol. 2018, 120, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.J. Biodegradable UV-Blocking Films through Core-Shell Lignin-Melanin Nanoparticles in Poly(butylene adipate- co-terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12, 2038. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Haven, M.Ø.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and Cellic® CTec2: Saccharification/fermentation of wheat straw, solid-liquid partition and potential of enzyme recycling by alkaline washing. Enzyme Microb. Technol. 2015, 79–80, 70–77. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A simple and effective approach to fabricate lignin nanoparticles with tunable sizes based on lignin fractionation. Green Chem. 2020, 22, 2011–2017. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Lu, S.; Qiu, X.; Qian, Y.; Li, P. Encapsulating TiO2 in Lignin-Based Colloidal Spheres for High Sunscreen Performance and Weak Photocatalytic Activity. ACS Sustain. Chem. Eng. 2019, 7, 6234–6242. [Google Scholar] [CrossRef]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the Interpretation of X-Ray Diffraction Powder Patterns in Terms of the Nanostructure of Cellulose I Fibres. Macromol. Chem. Phys. 2005, 1568–1575. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Zgoda, J.R.; Porter, J.R. A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Del Prete, M.S.; Fortuna, M.; Caselli, F.; Scalise, G. In vitro susceptibility tests for cationic peptides: Comparison of broth microdilution methods for bacteria that grow aerobically. Antimicrob. Agents Chemother. 2000, 44, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Fabi, A.; Varvaro, L. Severe Outbreak of Bacterial Blight Caused by Xanthomonas arboricola pv. corylina on Hazelnut cv. Tonda di Giffoni in Central Italy. Plant Dis. 2012, 96, 15–17. [Google Scholar] [CrossRef]
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G.M. Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot. 2013, 46, 18–22. [Google Scholar] [CrossRef]
- Canzoniere, P.; Francesconi, S.; Giovando, S.; Balestra, G.M. Antibacterial activity of tannins towards Pseudomonas syringae pv. tomato, and their potential as biostimulants on tomato plants. Phytopathol. Mediterr. 2021, 60, 23–36. [Google Scholar] [CrossRef]
- Gao, J.-C.; Guo, G.-J.; Guo, Y.-M. Measuring Plant Leaf Area by Scanner and ImageJ Software. China Veg. 2011, 1, 73–77. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Fuso, A.; Risso, D.; Rosso, G.; Rosso, F.; Manini, F.; Manera, I.; Caligiani, A. Potential valorization of hazelnut shells through extraction, purification and structural characterization of prebiotic compounds: A critical review. Foods 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative–Qualitative Analysis and Chromatographic Purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, S.; Sardar, H.H.; Neogi, S. Greener extraction of highly crystalline and thermally stable cellulose micro-fibers from sugarcane bagasse for cellulose nano-fibrils preparation. Carbohydr. Polym. 2021, 251, 117030. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Atanasio, P.; Schiavi, D.; Lorenzo, V.D.; Scaramuzzo, F.A.; Passeri, D.; Balestra, G.M.; Rossi, M. Preliminary results towards the mechanical characterization of cellulose nanofibers using HarmoniX mode atomic force microscopy Preliminary Results Towards the Mechanical Characterization of Cellulose Nanofibers Using HarmoniX Mode Atomic Force Microscopy. AIP Conf. Proc. 2021, 2416, 020011. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem.-Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Gracia, N.; McDonagh, B.H.; Domingues, F.C.; Nerín, C.; Chinga-Carrasco, G. Antimicrobial activity of biocomposite films containing cellulose nanofibrils and ethyl lauroyl arginate. J. Mater. Sci. 2019, 54, 12159–12170. [Google Scholar] [CrossRef]
- D’Orazio, G.; Munizza, L.; Zampolli, J.; Forcella, M.; Zoia, L.; Fusi, P.; Di Gennaro, P.; La Ferla, B. Cellulose nanocrystals are effective in inhibiting host cell bacterial adhesion. J. Mater. Chem. B 2017, 5, 7018–7020. [Google Scholar] [CrossRef]
- Sun, X.; Danumah, C.; Liu, Y.; Boluk, Y. Flocculation of bacteria by depletion interactions due to rod-shaped cellulose nanocrystals. Chem. Eng. J. 2012, 198–199, 476–481. [Google Scholar] [CrossRef]
- Noronha, V.T.; Camargos, C.H.M.; Jackson, J.C.; Souza Filho, A.G.; Paula, A.J.; Rezende, C.A.; Faria, A.F. Physical membrane-stress-mediated antimicrobial properties of cellulose nanocrystals. ACS Sustain. Chem. Eng. 2021, 9, 3203–3212. [Google Scholar] [CrossRef]
- Sun, X.; Lu, Q.; Boluk, Y.; Liu, Y. The impact of cellulose nanocrystals on the aggregation and initial adhesion of Pseudomonas fluorescens bacteria. Soft Matter 2014, 10, 8923–8931. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin nanoparticles and their nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathogenic and spoilage microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Sedem Dzah, C.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Carvalho, R.; Duman, K.; Jones, J.B.; Paret, M.L. Bactericidal Activity of Copper-Zinc Hybrid Nanoparticles on Copper-Tolerant Xanthomonas perforans. Sci. Rep. 2019, 9, 20124. [Google Scholar] [CrossRef]
- de Lima, P.H.C.; Antunes, D.R.; de L. Forini, M.M.; da S. Pontes, M.; Mattos, B.D.; Grillo, R. Recent Advances on Lignocellulosic-Based Nanopesticides for Agricultural Applications. Front. Nanotechnol. 2021, 3, 809329. [Google Scholar] [CrossRef]
- Di Lorenzo, V.; Schiavi, D.; Petrucci, C.; Balestra, G.M. Organic Nanoparticles from Lignocellulosic Agricultural Residues for Plant Protection Applications. AIP Conf. Proc. 2021, 2416, 020013. [Google Scholar] [CrossRef]
- Doan, H.K.; Ngassam, V.N.; Gilmore, S.F.; Tecon, R.; Parikh, A.N.; Leveau, J.H.J. Topography-Driven Shape, Spread, and Retention of Leaf Surface Water Impacts Microbial Dispersion and Activity in the Phyllosphere. Phytobiomes J. 2020, 4, 268–280. [Google Scholar] [CrossRef]
- Arya, G.C.; Harel, A. Phyllosphere and Its Potential Role in Sustainable Agriculture. In Microbial genomics in sustainable Agroecosystems; Springer: Singapore, 2019; pp. 39–65. [Google Scholar] [CrossRef]

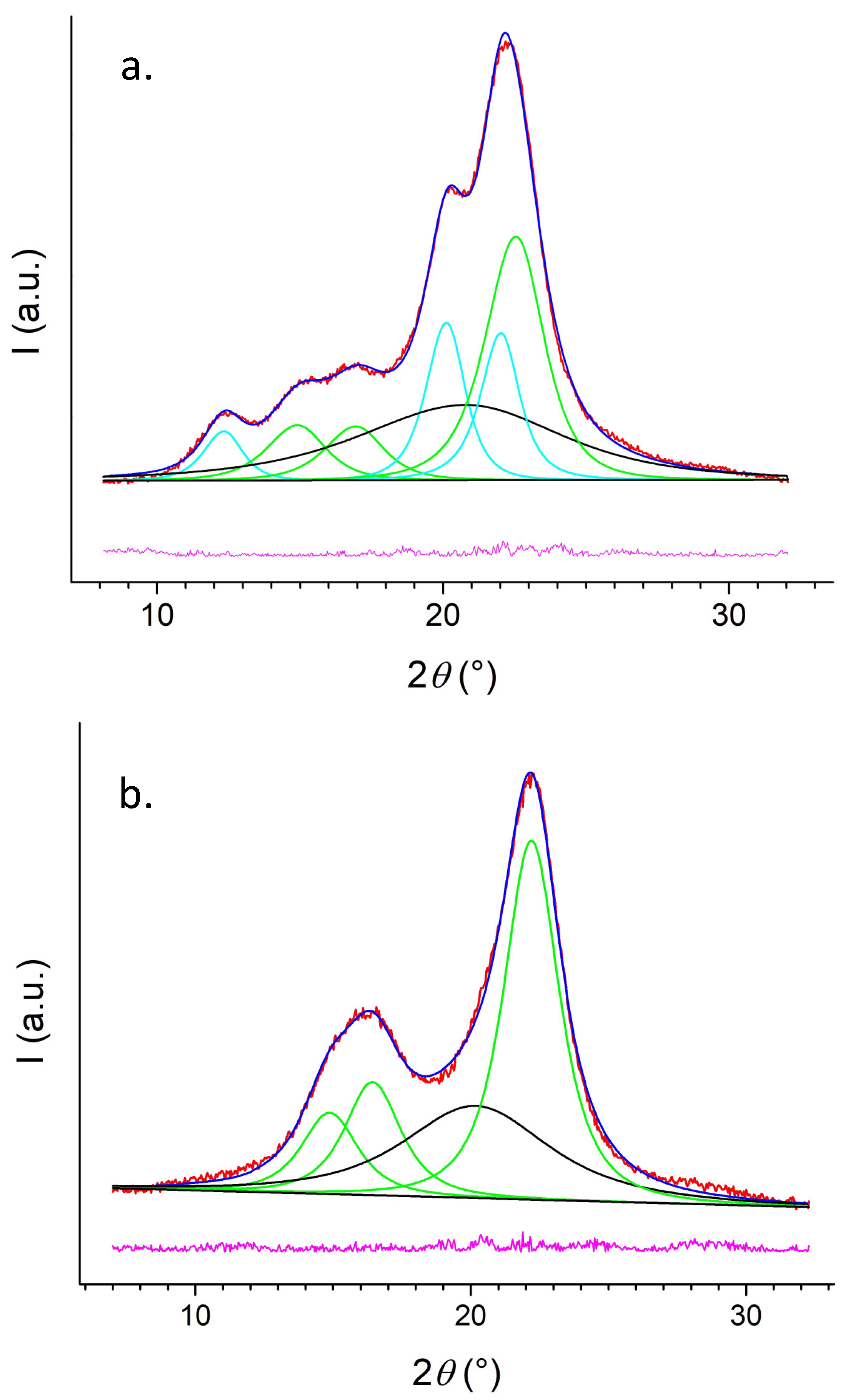


| CNC Extract | Crystalline Phase | Main Peaks (°2θ) | Crystalline Content (%) | Amorphous Content (%) |
|---|---|---|---|---|
| HP | Iβ | 14.8 16.5 20.2 | 42 | 30 |
| II | 12.3 20.1 22.0 | 28 | ||
| HS | Iβ | 14.9 16.4 22.2 | 71 | 29 |
| Treatment | 21 dpi | |
|---|---|---|
| Disease Incidence (%) (p < 0.01) | Disease Severity (Necrosis cm−2) (p < 0.05) | |
| Water | 48.8 ± 6.0 a | 1.02 ± 0.08 a |
| Copper oxychloride | 24.3 ± 4.1 b | 0.67 ± 0.06 b |
| HP CNC | 29.9 ± 4.2 b | 0.90 ± 0.04 a |
| HS CNC | 25.7 ± 1.6 b | 0.90 ± 0.03 a |
| HP LNP | 30.0 ± 2.5 b | 0.92 ± 0.10 a |
| HS LNP | 24.8 ± 2.1 b | 1.08 ± 0.09 a |
| Treatment | Biological Parameters | Days post Treatment | |||
|---|---|---|---|---|---|
| 1 dpt | 7 dpt | 14 dpt | 21 dpt | ||
| Water | Leaf Area (cm2) | 42.9 ± 4.4 | 60.7 ± 3.2 | 71.2 ± 1.7 | 71.6 ± 3.1 |
| Chlorophyll Content (DU) | 26.9 ± 1.4 | 22.0 ± 0.8 | 23.9 ± 1.2 | 23.0 ± 0.7 | |
| Flavonols Content (DU) | 0.46 ± 0.03 | 0.49 ± 0.02 | 0.46 ± 0.01 | 0.40 ± 0.02 | |
| Nitrogen Balance Index | 59.3 ± 2.3 | 46.2 ± 2.8 | 52.3 ± 1.9 | 60.3 ± 4.0 | |
| Copper oxychloride | Leaf Area (cm2) | 43.6 ± 5.2 | 60.5 ± 4.5 | 63.8 ± 1.2 | 63.0 ± 4.1 |
| Chlorophyll Content (DU) | 26.8 ± 1.0 | 21.6 ± 0.8 | 24.3 ± 1.6 | 22.7 ± 0.7 | |
| Flavonols Content (DU) | 0.47 ± 0.02 | 0.48 ± 0.02 | 0.45 ± 0.02 | 0.41 ± 0.03 | |
| Nitrogen Balance Index | 58.7 ± 2.5 | 46.2 ± 2.4 | 54.5 ± 3.2 | 61.4 ± 5.1 | |
| HP CNC | Leaf Area (cm2) | 54.1 ± 4.7 | 69.4 ± 5.3 | 74.6 ± 4.6 | 67.2 ± 5.5 |
| Chlorophyll Content (DU) | 21.4 ± 1.1 | 24.5 ± 1.0 | 26.5 ± 1.0 | 24.5 ± 1.2 | |
| Flavonols Content (DU) | 0.43 ± 0.03 | 0.43 ± 0.01 | 0.37 ± 0.01 | 0.42 ± 0.03 | |
| Nitrogen Balance Index | 53.3 ± 3.6 | 58.1 ± 2.6 | 74.4 ± 4.5 | 63.5 ± 4.4 | |
| HS CNC | Leaf Area (cm2) | 43.1 ± 0.1 | 58.1 ± 3.9 | 64.8 ± 4.1 | 65.5 ± 5.7 |
| Chlorophyll Content (DU) | 23.8± 0.6 | 24.6 ± 0.8 | 23.1 ± 0.8 | 22.6 ± 1.0 | |
| Flavonols Content (DU) | 0.40 ± 0.01 | 0.38 ± 0.03 | 0.39 ± 0.02 | 0.40 ± 0.02 | |
| Nitrogen Balance Index | 61.0 ± 2.7 | 70.0 ± 4.2 | 62.5 ± 4.0 | 58.1 ± 3.4 | |
| HP LNP | Leaf Area (cm2) | 36.8 ± 3.6 | 56.8 ± 4.0 | 78.6 ± 6.0 | 65.0 ± 5. |
| Chlorophyll Content (DU) | 27.1 ± 2.0 | 20.0 ± 0.9 | 21.3 ± 0.9 | 23.2 ± 1.1 | |
| Flavonols Content (DU) | 0.58 ± 0.05 | 0.53 ± 0.03 | 0.47 ± 0.01 | 0.46 ± 0.01 | |
| Nitrogen Balance Index | 57.5 ± 8.6 | 39.9 ±3.0 | 46.0 ± 2.1 | 50.9 ± 2.9 | |
| HS LNP | Leaf Area (cm2) | 55.0 ± 4.9 | 71.3 ± 4.9 | 69.1 ± 4.1 | 61.4 ± 3.8 |
| Chlorophyll Content (DU) | 22.8 ± 0.7 | 19.5 ± 0.9 | 20.9 ± 0.7 | 21.8 ± 1.0 | |
| Flavonols Content (DU) | 0.57 ± 0.05 | 0.50 ± 0.02 | 0.46 ± 0.02 | 0.42 ± 0.02 | |
| Nitrogen Balance Index | 46.8 ± 4.0 | 39.9 ±2.8 | 46.9 ± 2.9 | 54.1 ± 3.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavi, D.; Ronchetti, R.; Di Lorenzo, V.; Salustri, M.; Petrucci, C.; Vivani, R.; Giovagnoli, S.; Camaioni, E.; Balestra, G.M. Circular Hazelnut Protection by Lignocellulosic Waste Valorization for Nanopesticides Development. Appl. Sci. 2022, 12, 2604. https://doi.org/10.3390/app12052604
Schiavi D, Ronchetti R, Di Lorenzo V, Salustri M, Petrucci C, Vivani R, Giovagnoli S, Camaioni E, Balestra GM. Circular Hazelnut Protection by Lignocellulosic Waste Valorization for Nanopesticides Development. Applied Sciences. 2022; 12(5):2604. https://doi.org/10.3390/app12052604
Chicago/Turabian StyleSchiavi, Daniele, Riccardo Ronchetti, Veronica Di Lorenzo, Mirko Salustri, Camilla Petrucci, Riccardo Vivani, Stefano Giovagnoli, Emidio Camaioni, and Giorgio M. Balestra. 2022. "Circular Hazelnut Protection by Lignocellulosic Waste Valorization for Nanopesticides Development" Applied Sciences 12, no. 5: 2604. https://doi.org/10.3390/app12052604
APA StyleSchiavi, D., Ronchetti, R., Di Lorenzo, V., Salustri, M., Petrucci, C., Vivani, R., Giovagnoli, S., Camaioni, E., & Balestra, G. M. (2022). Circular Hazelnut Protection by Lignocellulosic Waste Valorization for Nanopesticides Development. Applied Sciences, 12(5), 2604. https://doi.org/10.3390/app12052604