Preparation and Properties of CO2 Micro-Nanobubble Water Based on Response Surface Methodology
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
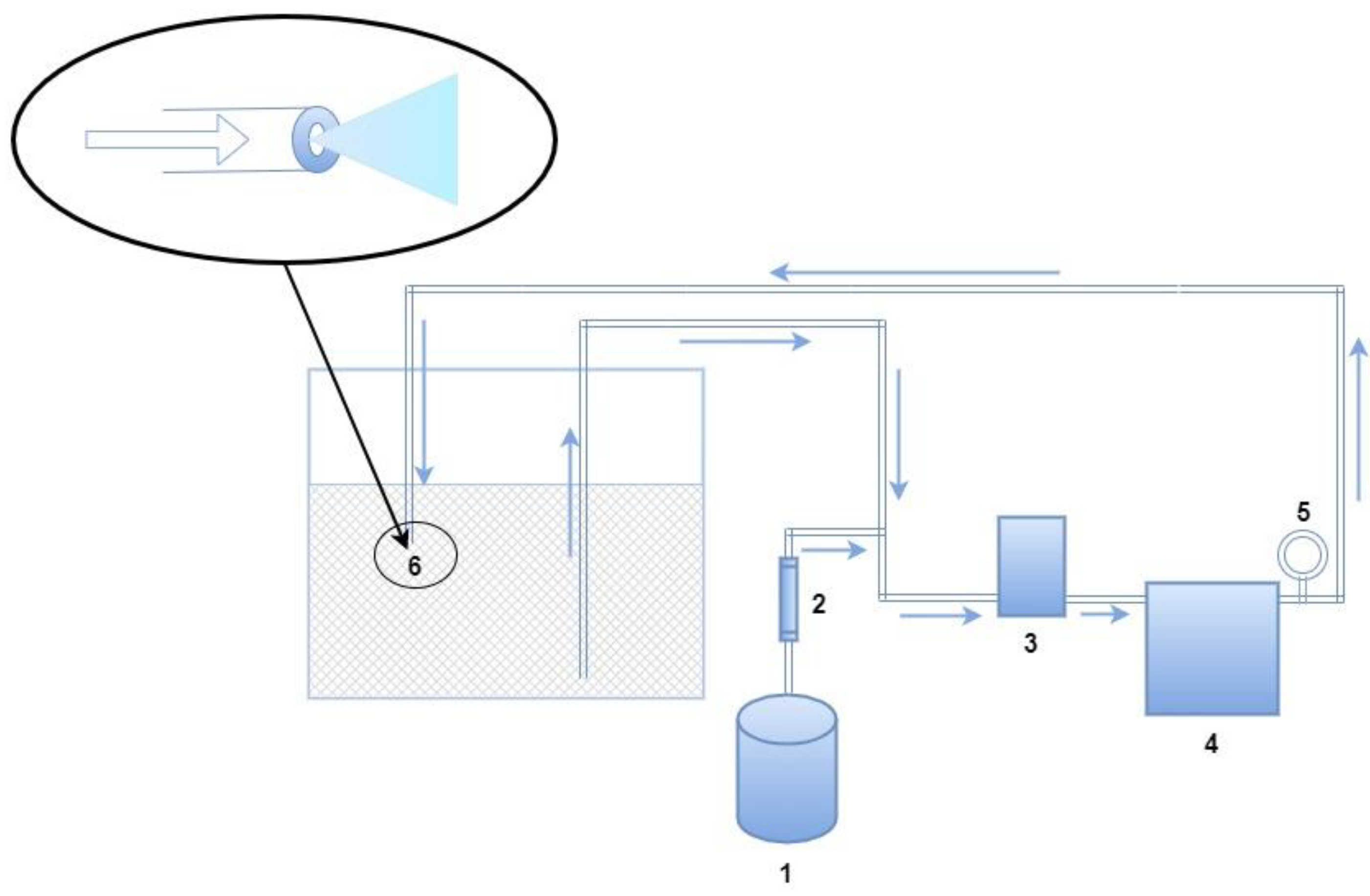
2.1. Experiment Material and Equipment
2.2. Experiment Design and Methods
2.2.1. Single-Factor Experiment on CO2 MNB Water Preparation
- (1)
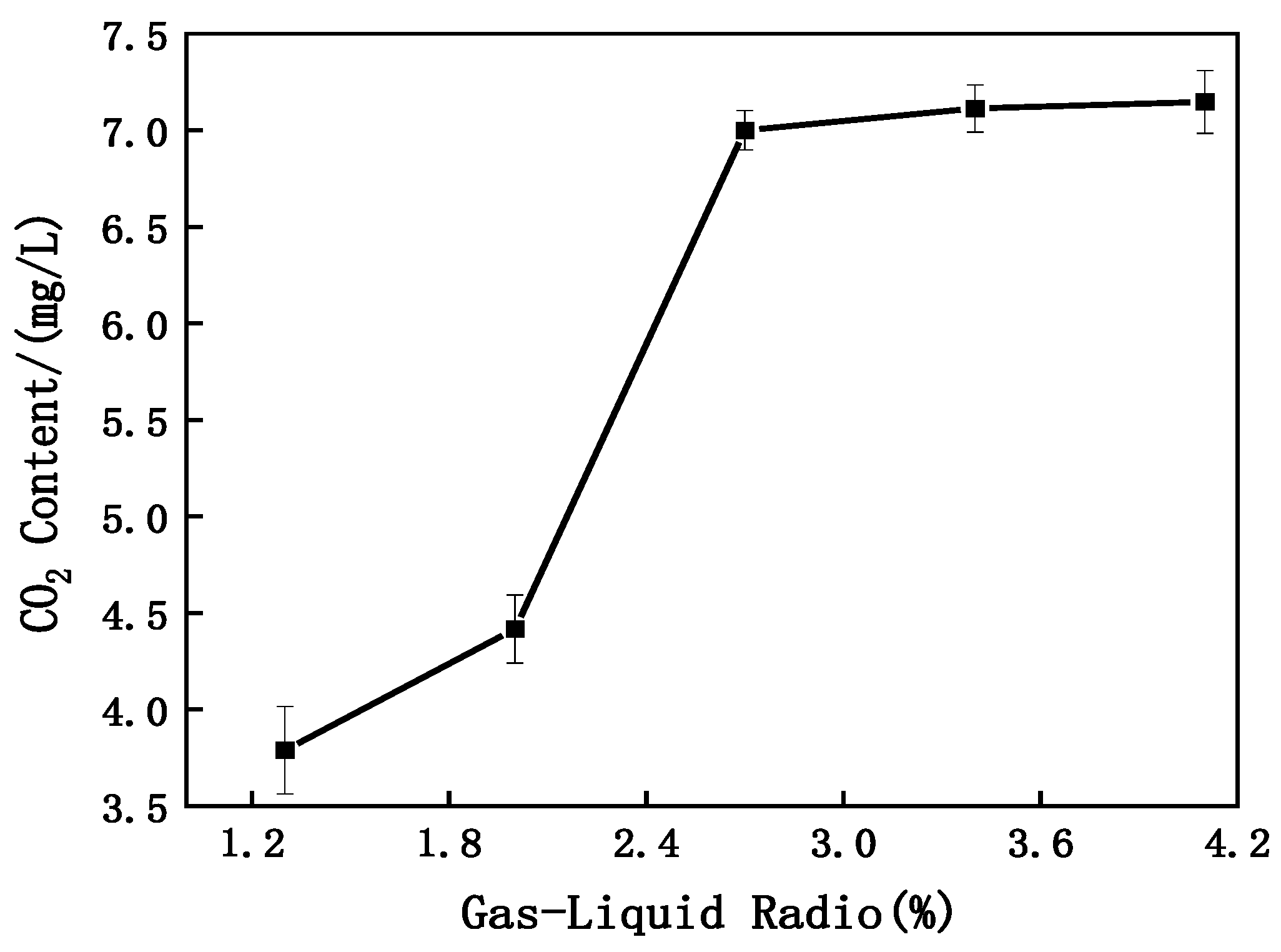
- Impact of Gas–liquid Ratio on CO2 Content
- (2)
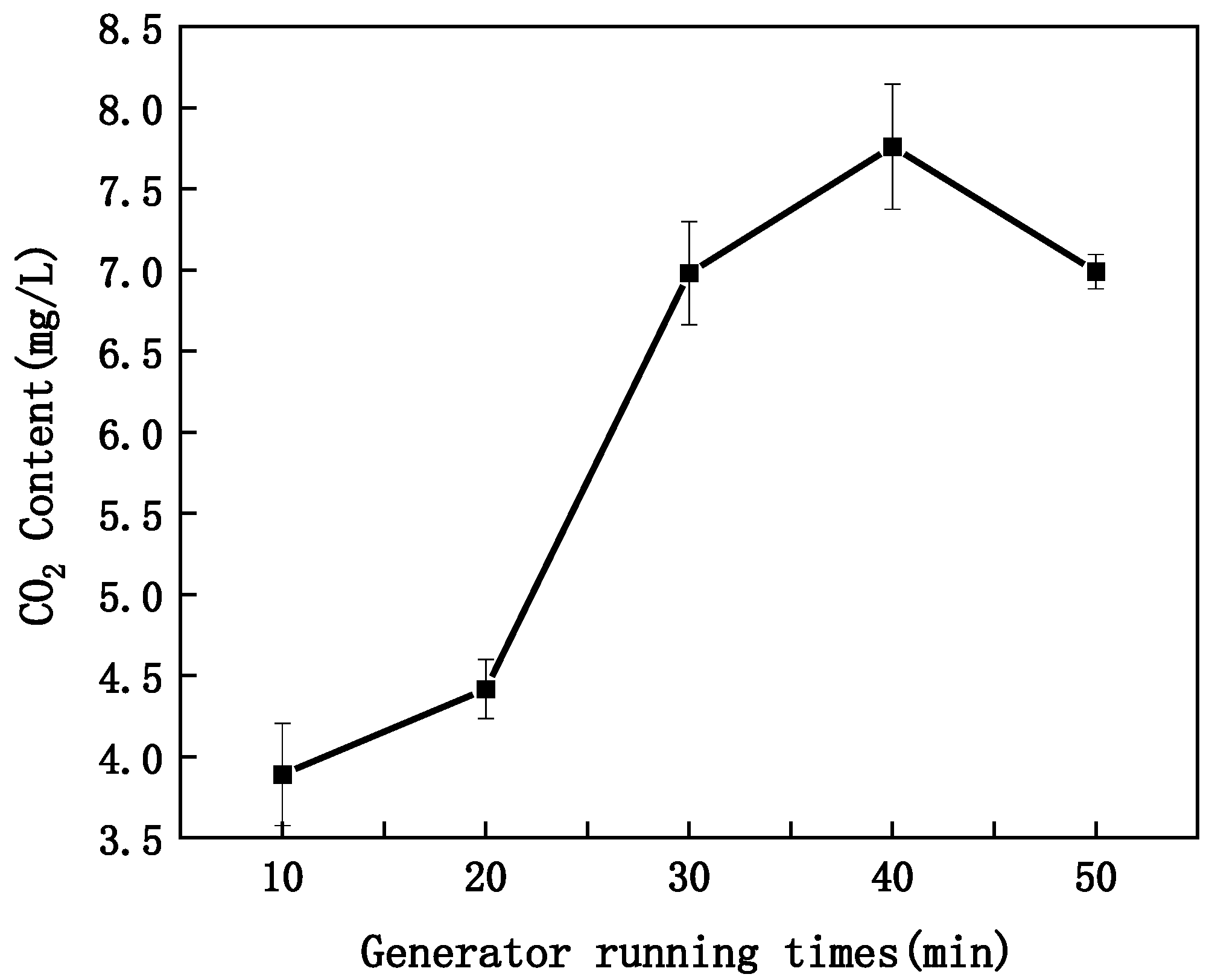
- Impact of Equipment Running Time on CO2 Content
- (3)
- Impact of Inlet Temperature of Water on CO2 Content
2.2.2. Optimal CO2 MNB Water Preparation Based on Response Surface Methodology
2.2.3. Measurement of CO2 Content in MNB Water
- (1)
- Standard Solution Preparation
- (2)
- Standard Sample Calibration
- (3)
- Sample Measurement
2.2.4. Characterization of CO2 MNB Water
- (1)
- Particle Size and Distribution of CO2 MNBs Based on Dissolved Gas Release Method
- (2)
- Stability of CO2 MNBs
- (3)
- pH of CO2 MNB Water
2.3. Statistical Analysis
3. Results and Discussion
3.1. Single-Factor Experimental Result Analysis
- (1)
- Analysis of Gas–Liquid Ratio Effect on CO2 Concentration in MNB Water
- (2)
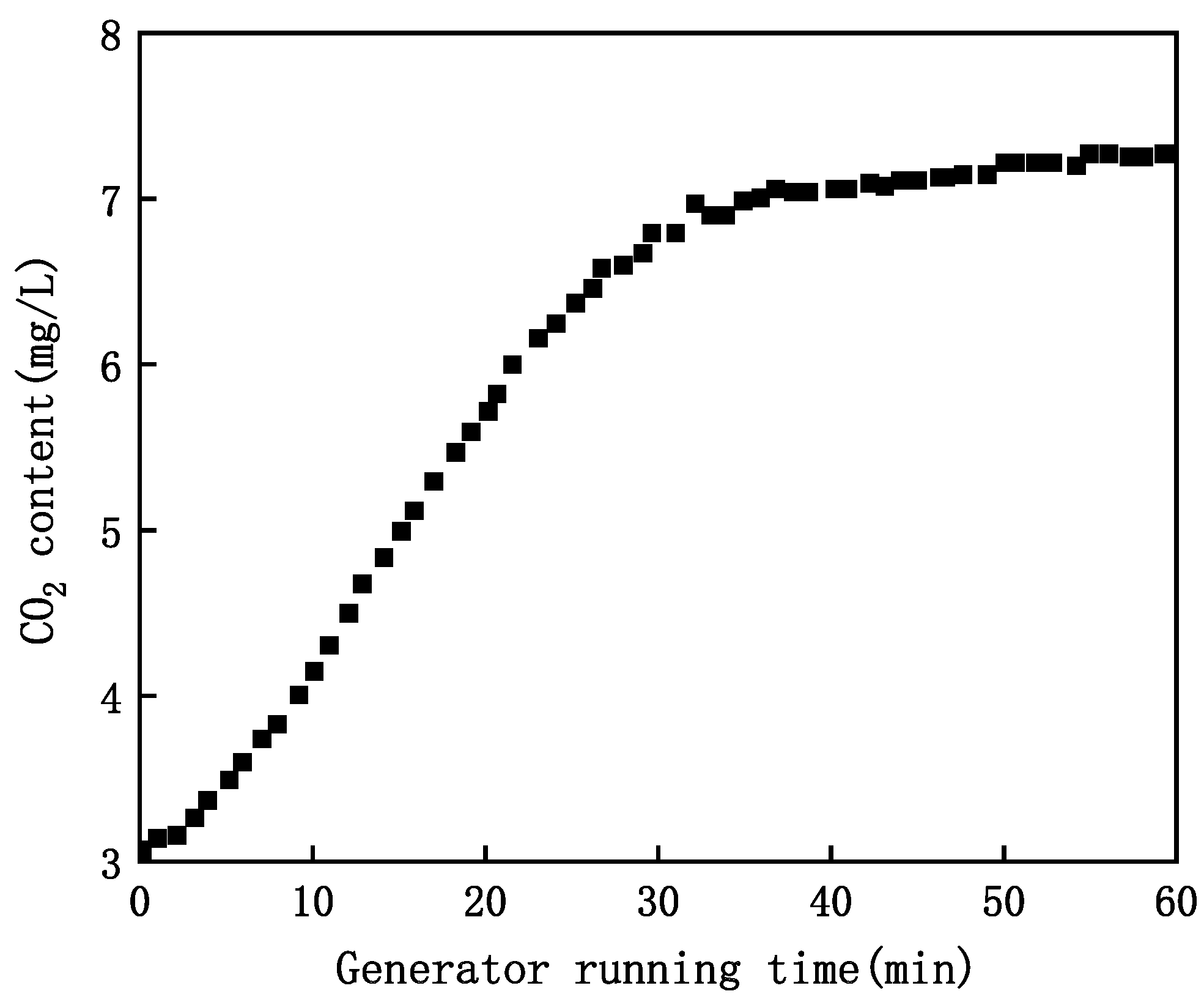
- Impact of Generator Running Time on CO2 content in MNB Water
- (3)
- Impact of Inlet Water Temperature on CO2 MNB content
3.2. Response Surface Methodology Results and Analysis
- (1)
- Orthogonal Experimental Design and Results
- (2)
- Response Surface Model Fitting and Analysis
- (3)
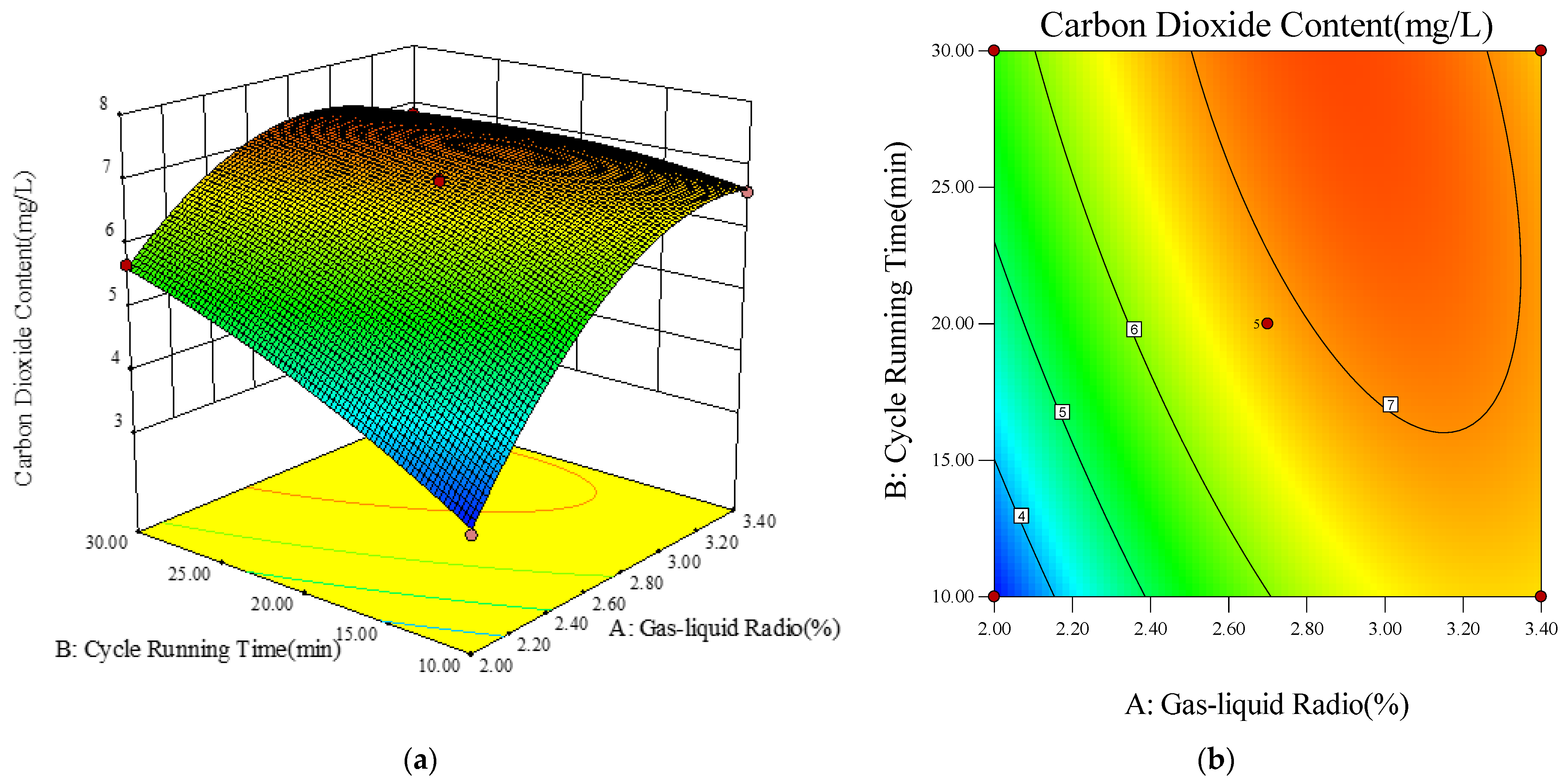
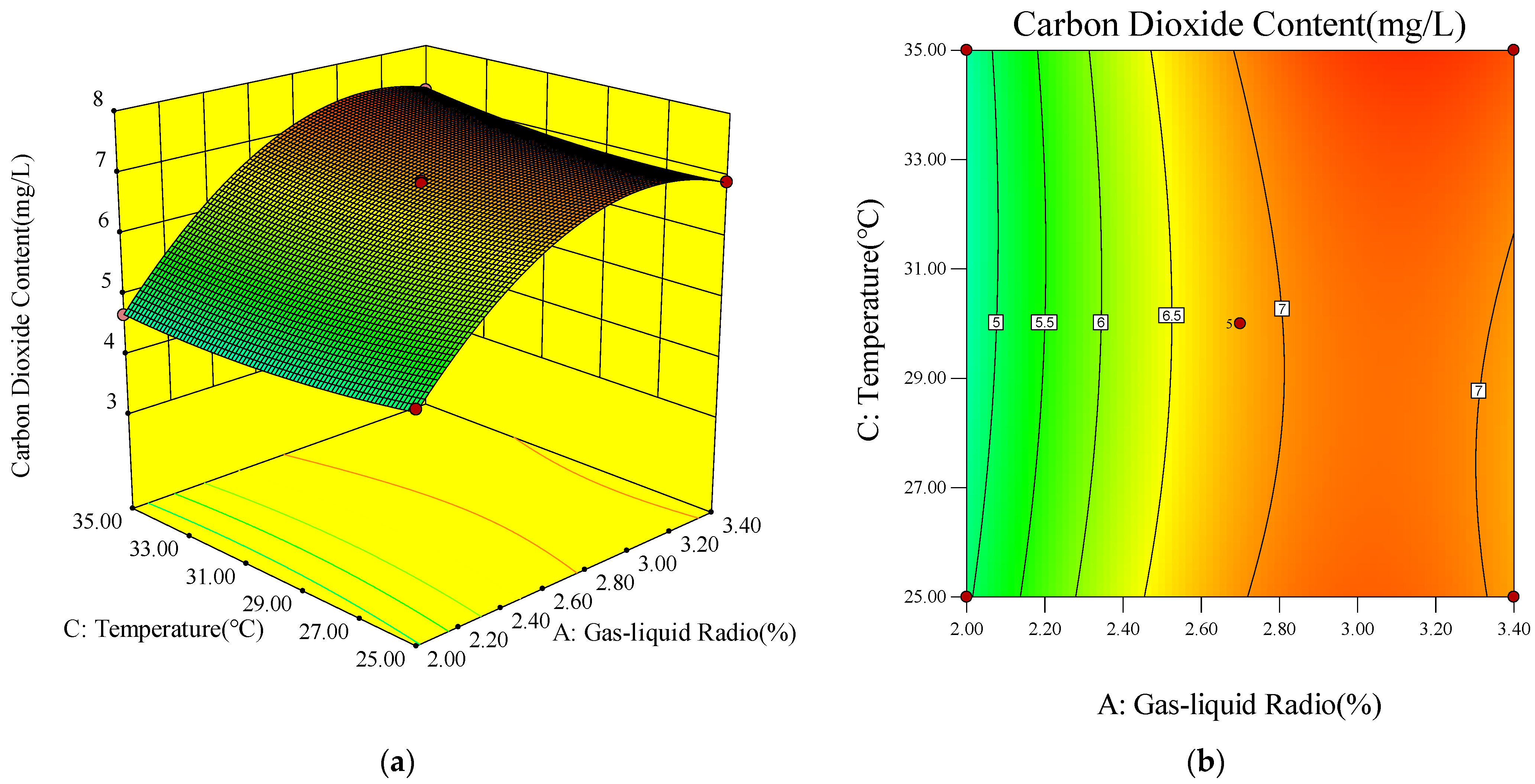
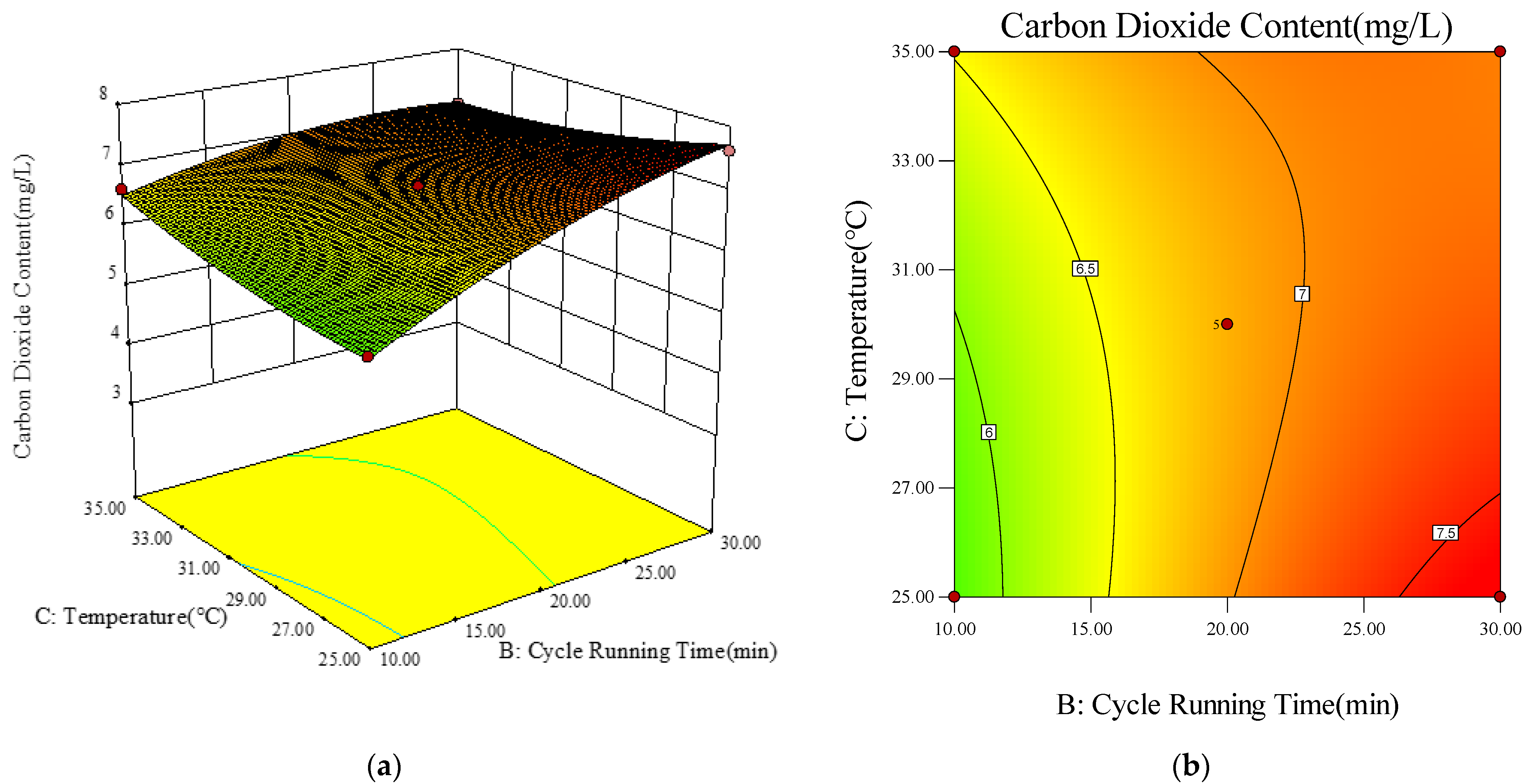
- Response Surface and Contour Diagram Analysis
- (4)
- Model Optimization and Validation Results
3.3. Particle Size and Distribution Properties of CO2 MNBs from Dissolved Gas Release Method
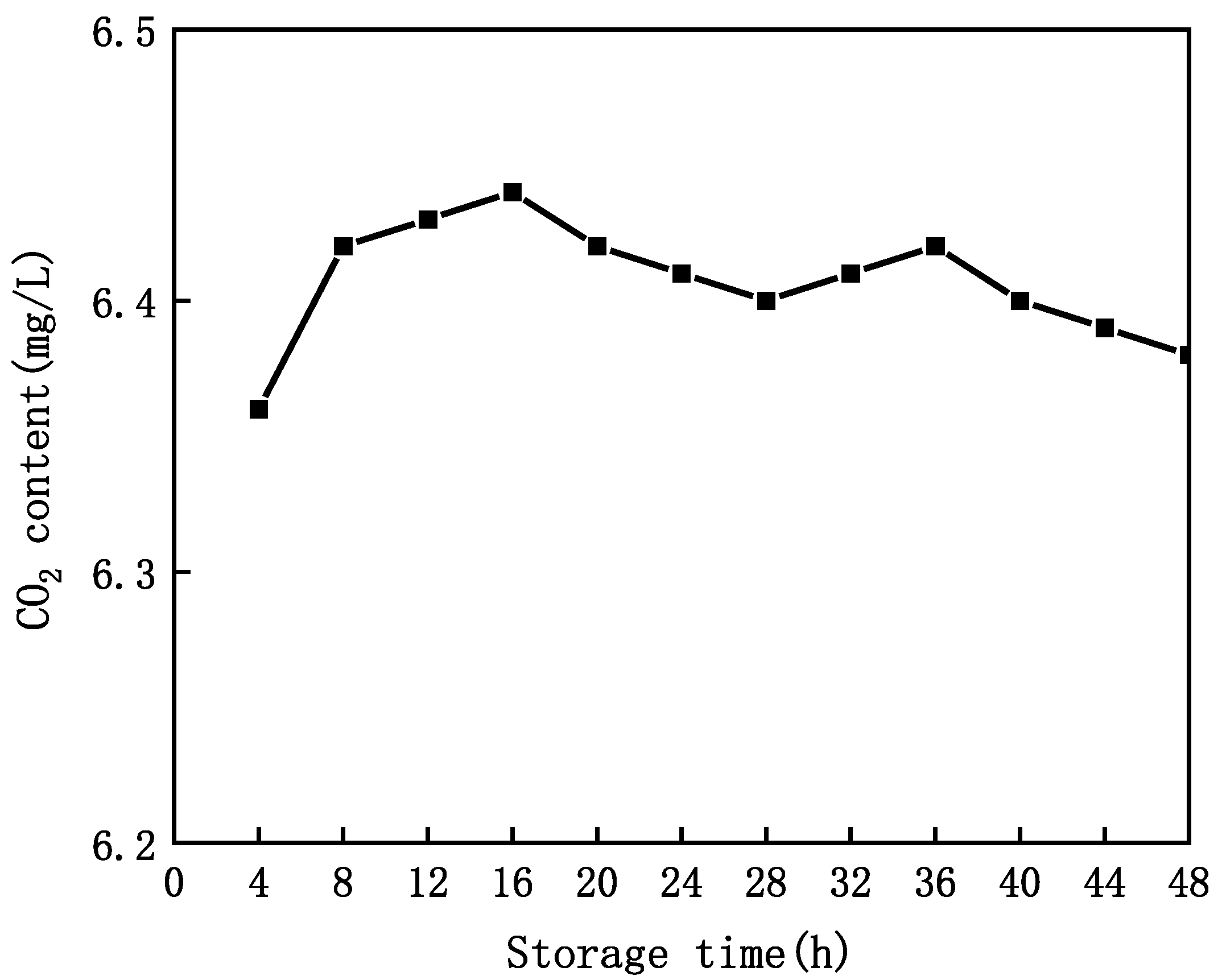
3.4. Properties of CO2 Content and Lifetime in MNB Water
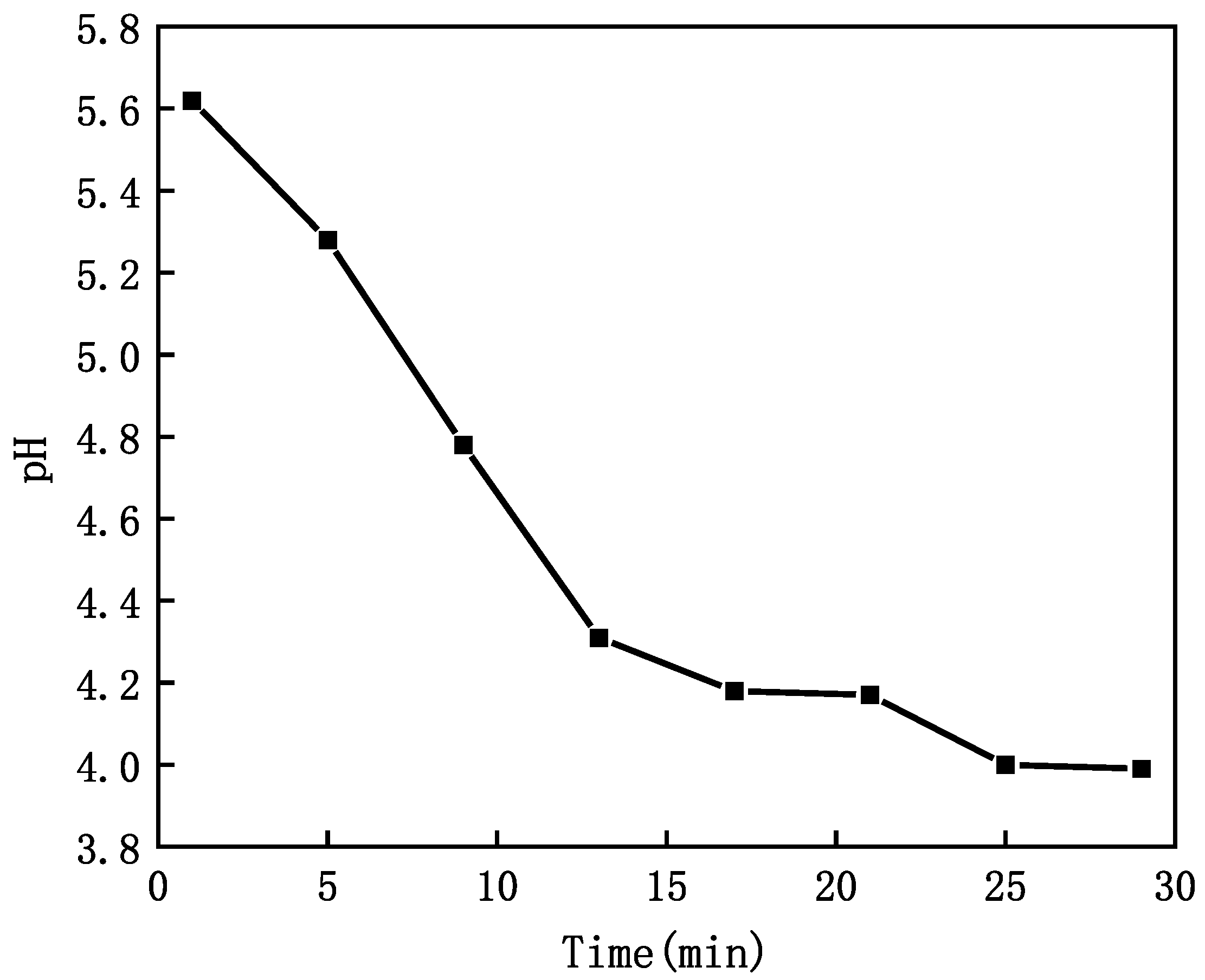
3.5. pH Property of CO2 MNB Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donohue, R.J.; Roderick, M.L.; McVicar, T.R.; Farquhar, G.D. Impact of CO2 fertilization on maximum foliage cover across the globe’s warm, arid environments. Geophys. Res. Lett. 2013, 40, 3031–3035. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ding, Y.; Li, D.; Miao, Z. Automatic carbon dioxide enrichment strategies in the greenhouse: A review. Biosyst. Eng. 2018, 171, 101–119. [Google Scholar] [CrossRef]
- Jin, C.; Du, S.; Wang, Y.; Condon, J.; Lin, X.; Zhang, Y. Carbon dioxide enrichment by composting in greenhouses and its effect on vegetable production. J. Plant Nutr. Soil Sci. 2009, 172, 418–424. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Liang, H. Experimental study of the effects of CO2 enrichment on growth, development and yield of C3 and C4 crops. Sci. Agric. Sin. 1998, 31, 55–61. [Google Scholar]
- Martzopoulou, A.; Vafiadis, D.; Fragos, V. Energy Gain in Passive Solar Greenhouses Due to CO2 Enrichment. Energies 2020, 13, 1242. [Google Scholar] [CrossRef] [Green Version]
- Pingale, B.N.; Singh, S.D.; Yadav, A. Potential impacts of increasing atmospheric carbon dioxide on yield and plant growth of rice (Oryza sativa) and maize (Zea mays) crops. Indian J. Agric. Sci. 2017, 87, 1041–1044. [Google Scholar]
- Ksiksi, T.S.; Thruppoyil, S.B.; Palakkott, A.R. CO2 enrichment affects eco-physiological growth of maize and alfalfa under different water stress regimes in the UAE. Physiol. Mol. Biol. Plants 2018, 24, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Ha, B.; Ko, J. Simulation of CO2 enrichment and climate change impacts on soybean production. Int. Agrophys. 2016, 30, 25–37. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Haris, S.; Qiu, X.; Klammler, H.; Mohamed, M.M. The use of micro-nano bubbles in groundwater remediation: A comprehensive review. Groundw. Sustain. Dev. 2020, 11, 100463. [Google Scholar] [CrossRef]
- Grzegorczyk-Frańczak, M.; Barnat-Hunek, D.; Andrzejuk, W.; Zaburko, J.; Zalewska, M.; Łagód, G. Physical Properties and Durability of Lime-Cement Mortars Prepared with Water Containing Micro-Nano Bubbles of Various Gases. Materials 2021, 14, 1902. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Dong, C.D. Advances in micro- and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, H. Micro- and Nanobubbles: Fundamentals and Applications; Pan Stanford: Singapore, 2019; p. 219. [Google Scholar]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Wang, T.; Pan, J.; Zhou, B.; Muhammad, T.; Zhou, C.; Li, Y. Micro-nano bubble water oxygation: Synergis-tically improving irrigation water use efficiency, crop yield and quality. J. Clean Prod. 2019, 222, 835–843. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Xu, F.; Muhammad, T.; Li, Y. Appropriate dissolved oxygen concentration and application stage of micro-nano bubble water oxygation in greenhouse crop plantation. Agric. Water Manag. 2019, 223, 105713. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, L.; Liu, G. Basic characteristics and application of micro-nano bubbles in water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 510, 42050. [Google Scholar] [CrossRef]
- Mäkelä, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Qi, N.; Qin, Y.; Zhang, X.; Li, Y. Generation and Stability of Size-Adjustable Bulk Nanobubbles Based on Periodic Pressure Change. Sci. Rep. 2019, 9, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Coll. Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640, 772–797. [Google Scholar] [CrossRef]
- Cunico, L.P.; Acosta, M.C.; Turner, C. Experimental measurements and modeling of curcumin solubility in CO2-expanded ethanol. J. Supercrit. Fluids 2017, 130, 381–388. [Google Scholar] [CrossRef]
- Zubeir, L.F.; van Osch, D.J.; Rocha, M.A.; Banat, F.; Kroon, M.C. Carbon dioxide solubilities in decanoic acid-based hydro-phobic deep eutectic solvents. J. Chem. Eng. Data 2018, 63, 913–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Li, Y.; Liu, X.; Wang, K.; Muhammad, T. Synergistic improvement in spring maize yield and quality with mi-cro/nanobubbles water oxygation. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Khan, P.; Khan, P.; Zhu, W.; Huang, F.; Gao, W.; Khan, N.A. Micro-nanobubble technology and water-related application. Water Supply 2020, 20, 2021–2035. [Google Scholar] [CrossRef]
- Yu, D.S.; Sun, X.R.; Gao, X.H.; Li, Y.; Xing, K. Measurement of CO2 solubility in water based on conductivity method. Chem. Reag. 2021, 43, 954–958. [Google Scholar] [CrossRef]
- Maghami, S.; Mehrabani-Zeinabad, A.; Sadeghi, M.; Sánchez-Laínez, J.; Zornoza, B.; Téllez, C.; Coronas, J. Mathematical modeling of temperature and pressure effects on permeability, diffusivity and solubility in polymeric and mixed matrix membranes. Chem. Eng. Sci. 2019, 205, 58–73. [Google Scholar] [CrossRef]



| Material | Specification | Manufacturer |
|---|---|---|
| Carbon dioxide gas | Purity of 99.9% | Beijing Aokang Shuangquan Energy Technologies Co., Ltd. (Beijing, China) |
| Deionized water | Analytical reagent | Beijing Kefeng Zhengye Business Center (Beijing, China) |
| NaCl | Analytical reagent | Shanghai Maclin Biochemical Technology Co., Ltd. (Shanghai, China) |
| NaHCO3 | Analytical reagent | Shanghai Maclin Biochemical Technology Co., Ltd. |
| H3PO4 | Analytical reagent | Shanghai Maclin Biochemical Technology Co., Ltd. |
| NaOH | Analytical reagent | Shanghai Maclin Biochemical Technology Co., Ltd. |
| Instrument Name and Model | Manufacturer |
|---|---|
| Carbon dioxide meter (multifunctional analytical FC-100 model + pCO2-1 carbon dioxide gas sending electrode) | Beijing Zhongxihuada Technology Co., Ltd. (Beijing, China) |
| pH meter | Mettler Toledo International Trading (Shanghai) Co., Ltd. (Shanghai, China) |
| Magnetic stirrer | Scilogex (Connecticut, CT, USA) |
| Electronic balance | Ohaus Instrument (Shanghai) Co., Ltd. (Shanghai, China) |
| Factor | Encoded Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A Gas-liquid ratio (v/v, %) | 2.0 | 2.7 | 3.4 |
| B Running time (min) | 10 | 20 | 30 |
| C Water temperature (°C) | 25 | 30 | 35 |
| Item | Value |
|---|---|
| Std. Dev. | 0.090 |
| Mean | 6.31 |
| C.V.% | 1.42 |
| PRESS | 0.80 |
| R2 | 0.9973 |
| Adj R2 | 0.9938 |
| Pred R2 | 0.9608 |
| Adeq Precision | 65.718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Lu, X.; Tao, S.; Ren, Y.; Gao, W.; Liu, X.; Yang, B. Preparation and Properties of CO2 Micro-Nanobubble Water Based on Response Surface Methodology. Appl. Sci. 2021, 11, 11638. https://doi.org/10.3390/app112411638
Wang B, Lu X, Tao S, Ren Y, Gao W, Liu X, Yang B. Preparation and Properties of CO2 Micro-Nanobubble Water Based on Response Surface Methodology. Applied Sciences. 2021; 11(24):11638. https://doi.org/10.3390/app112411638
Chicago/Turabian StyleWang, Bingbing, Xiangjie Lu, Sha Tao, Yanzhao Ren, Wanlin Gao, Xinliang Liu, and Bangjie Yang. 2021. "Preparation and Properties of CO2 Micro-Nanobubble Water Based on Response Surface Methodology" Applied Sciences 11, no. 24: 11638. https://doi.org/10.3390/app112411638
APA StyleWang, B., Lu, X., Tao, S., Ren, Y., Gao, W., Liu, X., & Yang, B. (2021). Preparation and Properties of CO2 Micro-Nanobubble Water Based on Response Surface Methodology. Applied Sciences, 11(24), 11638. https://doi.org/10.3390/app112411638






