The Study of Metal Corrosion Resistance near Weld Joints When Erecting Building and Structures Composed of Precast Structures
Abstract
:1. Introduction
2. Materials
3. Methods
- υ—corrosion rate;
- ∆m—sample mass variation;
- S—sample area;
- τ—exposure time.
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramaniam, R. On the corrosion resistance of the Delhi iron pillar. Corros. Sci. 2000, 42, 2103–2129. [Google Scholar] [CrossRef]
- Dwivedi, D.; Mata, J.P.; Salvemini, F.; Rowles, M.R.; Becker, T.; Lepková, K. Uncovering the superior corrosion resistance of iron made via ancient Indian iron-making practice. Sci. Rep. 2021, 11, 4221. [Google Scholar] [CrossRef] [PubMed]
- Goldobina, L.A.; Orlov, P.S. Analysis of the corrosion destruction causes in underground pipelines and new solutions for increasing corrosion steel’s resistance. J. Min. Inst. 2016, 219, 459. [Google Scholar] [CrossRef]
- Ponomarev, A.I.; Yusupov, A.D. Effect of shear stress on the wall of technological pipelines at a gas condensate field on the intensity of carbon dioxide corrosion. J. Min. Inst. 2020, 244, 439–447. [Google Scholar] [CrossRef]
- Sivenkov, A.V. Chemical-thermal treatment of steel in an environment of low-melting solutions. J. Min. Inst. 2014, 209, 244–249. [Google Scholar]
- Dzhabbarov, S.N.; Pryakhin, E.I. Using the Diffusion Saturation Technology for the Surface of Different Steel Products from Melts of Low-Melting Metals. Key Eng. Mater. 2020, 836, 36–40. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Nikolaeva, L.A.; Igonin, T.N. Estimating the First-year Corrosion Losses of Structural Metals for Continental Regions of the World. Siv. Eng. J. 2020, 6, 1503–1518. [Google Scholar] [CrossRef]
- Shannag, M.J.; Higazey, M. Strengthening and Repair of a Precast Reinforced Concrete Residential Building. Civ. Eng. J. 2020, 12, 2457–2473. [Google Scholar] [CrossRef]
- Dvorkin, L.I. (Ed.) Betonovedenie (Concrete study). Monograph. In Osnovnye Raznovidnosti Betonov (Major Concrete Varieties); Infra-Inzheneriya Publ.: Vologda, Russia, 2021; Volume 2, pp. 152–608. (In Russian) [Google Scholar]
- Moskvin, V.M.; Ivanov, F.M.; Alekseev, S.N.; Guzeev, E.A. Korroziya Betona i Zhelezobetona, Metody Ikh Zashchity (Corrosion of Concrete and Reinforced Concrete, the Methods of Their Protection); Stroyizdat Publ.: Moscow, Russia, 1980; pp. 533–536. (In Russian) [Google Scholar]
- Belentsov, Y.A.; Smirnova, O.M.; Kazanskaya, L.F. Improvement of competitive edge of precast reinforced concrete by increasing the reliability level and quality control. IOP Conf. Ser. Mater. Sci. Eng. 2019, 666, 012037. [Google Scholar] [CrossRef]
- Piskarev, P.Y.; Gervash, A.A.; Vologzhanina, S.A.; Ermakov, B.S.; Kudryavceva, A.M. Study of the Bimetallic Joint CuCrZr/316L(N). Mater. Sci. Forum 2021, 1040, 8–14. [Google Scholar] [CrossRef]
- Sharapova, D.M.; Sharapov, M.G.; Sharonov, N.I. Structure Formation of Butt Joints Made of Aluminum Alloys to Ensure the Quality of Mechanical Engineering Products. Mater. Sci. Forum 2021, 1022, 119–126. [Google Scholar] [CrossRef]
- Nosov, V.V.; Grigoriev, E.V.; Peretyatko, S.A.; Artyushchenko, A.P. Nanotechnologies of Strength Control of Materials. Mater. Sci. Forum 2021, 1040, 101–108. [Google Scholar] [CrossRef]
- Nosov, V.V.; Zelenskii, N.A. Estimating the strength of welded hull elements of a submersible based on the micromechanical model of temporal dependences of acoustic-emission parameters. Mater. Sci. Forum 2017, 53, 89–95. [Google Scholar] [CrossRef]
- Nosov, V.V. On the principles of optimizing the technologies of acoustic-emission strength control of industrial objects. Mater. Sci. Forum Russ. 2016, 52, 386–399. [Google Scholar] [CrossRef]
- Pecherskaya, E.A.; Golubkov, P.E.; Artamonov, D.V.; Pecherskiy, A.V.; Mel’Nikov, O.A.; Shepeleva, A.E. The Model of the Relationship of the of Micro-arc Oxidation Process Parameters Based on Graph Theory. In Moscow Workshop on Electronic and Networking Technologies (MWENT-2020); Publishing House of the National Research University Higher School of Economics: Moscow, Russia, 2020. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, R.; Chen, F.; Yan, G.; Tian, W.; Zhang, J.; Zhang, J. Electrochemical process of early-stage corrosion detection based on N-doped carbon dots with superior Fe3+ responsiveness. J. Colloid Interface Sci. 2021, 606 Pt 1, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Potapov, I.A.; Kondrat’ev, A.V. Remote monitoring of pipelines usingtele communications technology. J. Min. Inst. 2014, 209, 138–143. [Google Scholar]
- Issa, B.; Bazhin, V.Y.; Telyakov, N.M.; Telyakov, A. Increasing of corrosion resistance of welded radiant and convection coiled-pipes in tubular furnaces at kinef crude oil refinery, Technical Sessions Proceedings. In Proceedings of the 6th Youth Forum of the World Petroleum Council—Future Leaders Forum, Saint Petersburg, Russia, 23–28 June 2019; pp. 243–249. [Google Scholar] [CrossRef]
- Syzrantseva, K.V.; Syzrantsev, V.N.; Dvoynikov, M.V. The application of finite element analysis during development of the Integral Strain Gauges calibration method for the study of the welded construction. Mater. Sci. Forum 2017, 177, 12133. [Google Scholar] [CrossRef] [Green Version]
- Maksarov, V.V.; Vasin, S.A.; Efimov, A.E. Dynamic Stabilization in Reaming Internal Surfaces of Welded Components. Russ. Eng. Res. 2021, 41, 939–943. [Google Scholar] [CrossRef]
- Maksarov, V.V.; Efimov, A.E.; Olt, J.J. Improving the quality of hole processing in welded products made of dissimilar materials with a new boring tool. Int. J. Adv. Manuf. Technol. 2021, 118, 1027–1042. [Google Scholar] [CrossRef]
- Maksarov, V.; Khalimonenko, A.; Olt, J. Managing the process of machining on machines on the basis of dynamic modelling for a technological system. J. Silic. Based Compos. Mater. 2017, 69, 66–71. [Google Scholar] [CrossRef]
- Maksarov, V.V.; Vasin, S.A.; Efimov, A.E.; Keksin, A.I. Influence of the Microstructure on the Damping Properties of Stress-Strain Tool Systems in the Processing of Welded Structures from Dissimilar Steels. Mater. Sci. Forum 2021, 1022, 7–16. [Google Scholar] [CrossRef]
- Popova, K.; Prošek, T. Corrosion Monitoring in Atmospheric Conditions: A Review. Metals 2022, 12, 171. [Google Scholar] [CrossRef]
- Khaled, K.F.; Abdel-Rehim, S.S. Electrochemical investigation of corrosion and corrosion inhibition of iron in hydrochloric acid solutions. Arab. J. Chem. 2011, 4, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Souriou, D.; Bosc, F.; Guilbert, D.; Kadkhodazadeh, S.; Ihamouten, A.; Fauchard, C.; Dérobert, X. Dielectric relaxation of iron corrosion products in limestone under saline environment. In Proceedings of the NSG2021 2nd Conference on Geophysics for Infrastructure Planning, Monitoring and BIM, Bordeaux, France, 9 August–2 September 2021; European Association of Geoscientists & Engineers: EAGE Publications BV: Houten, The Netherlands, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Molodin, V.V.; Anufrieva, A.E.; Leonovich, S.N. An impact of concrete surfaces carbonization on their cohesion with newly-laid concrete. Nauka Tekhnika Sci. Eng. 2021, 20, 320–328. (In Russian) [Google Scholar]
- Merzlyakov, M.Y.; Hernandez, J.R.R.; Zhapkhandayev, C.A. Development of cement slurries for oil and gas wells lining in aggressive environment. In Youth Technical Sessions Proceedings; Taylor and Francis Group Publishers: London, UK, 2019; pp. 387–393. [Google Scholar] [CrossRef]
- Kreshkov, A.P. Osnovy Analiticheskoy Khimii (The Fundamentals of Analytical Chemistry), 3rd ed.; Cengage Learning: Moscow, Russia, 1971; Volume 2, pp. 424–425. (In Russian) [Google Scholar]
- Ivanov, I.I.; Dubovikov, O.A.; Grigor’Eva, L.V.; Kuzhaeva, A.A.; Zgonnik, P.V. Study of stability of constructional materials in melts containing lower titanium chlorides. Russ. J. Appl. Chem. 2011, 84, 1529–1531. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Dubovikov, O.A.; Grigor’Eva, L.V.; Kuzhaeva, A.A.; Zgonnik, P.V. Study of the corrosion stability of some materials in the titanium (II) and (III) chloride melts. J. Min. Inst. 2013, 202, 220–225. [Google Scholar]
- Noubactep, C. Should the term ′metallic iron′ appear in the title of a research paper. Chemosphere 2022, 287 Pt 4, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Roberts, K.P. Mechanistic modeling of erosion–corrosion for carbon steel. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Elsevier & Woodhead Publishing: Cambridge, UK, 2017; pp. 756–759. [Google Scholar] [CrossRef]
- Hernandez, S.; Nassef, A.S. Erosion–corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Elsevier & Woodhead Publishing: Cambridge, UK, 2017; pp. 341–352. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.; Zhang, D.; Zhu, X. Stability and corrosion property of oil-infused hydrophobic silica nanoparticle coating. Surf. Eng. 2021, 37, 206–211. [Google Scholar] [CrossRef]
- Smirnova, O.; Kazanskaya, L.; Koplík, J.; Tan, H.; Gu, X. Concrete Based on Clinker-Free Cement: Selecting the Functional Unit for Environmental Assessment. Sustainability 2021, 13, 135. [Google Scholar] [CrossRef]
- Smirnova, O.M. Low-Clinker Cements with Low Water Demand. J. Mater. Civ. Eng. 2020, 32, 06020008. [Google Scholar] [CrossRef]
- Yakovlev, G.; Polyanskikh, I.; Gordina, A.; Pudov, I.; Černý, V.; Gumenyuk, A.; Smirnova, O. Influence of Sulphate Attack on Properties of Modified Cement Composites. Appl. Sci. 2021, 11, 8509. [Google Scholar] [CrossRef]

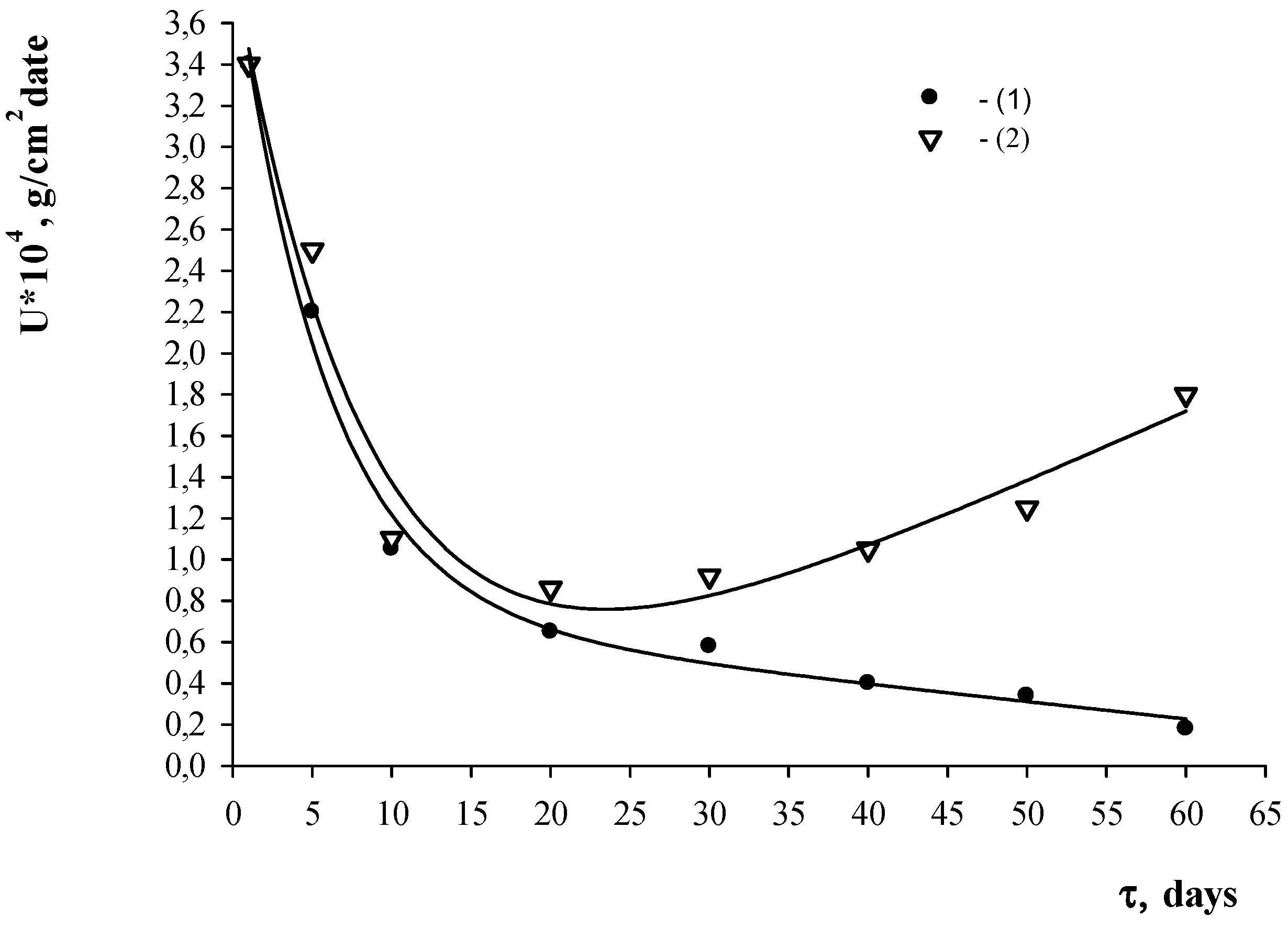
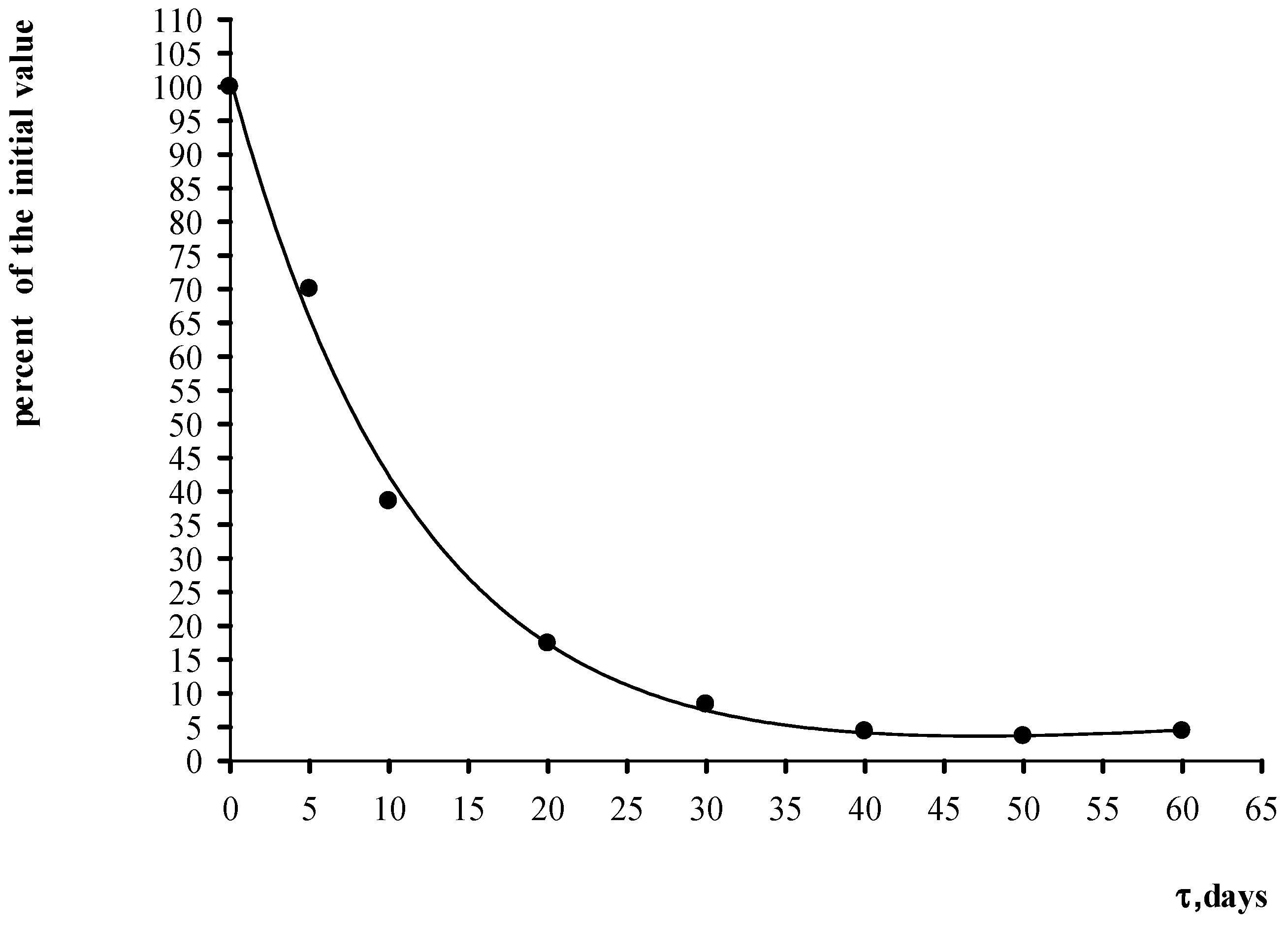



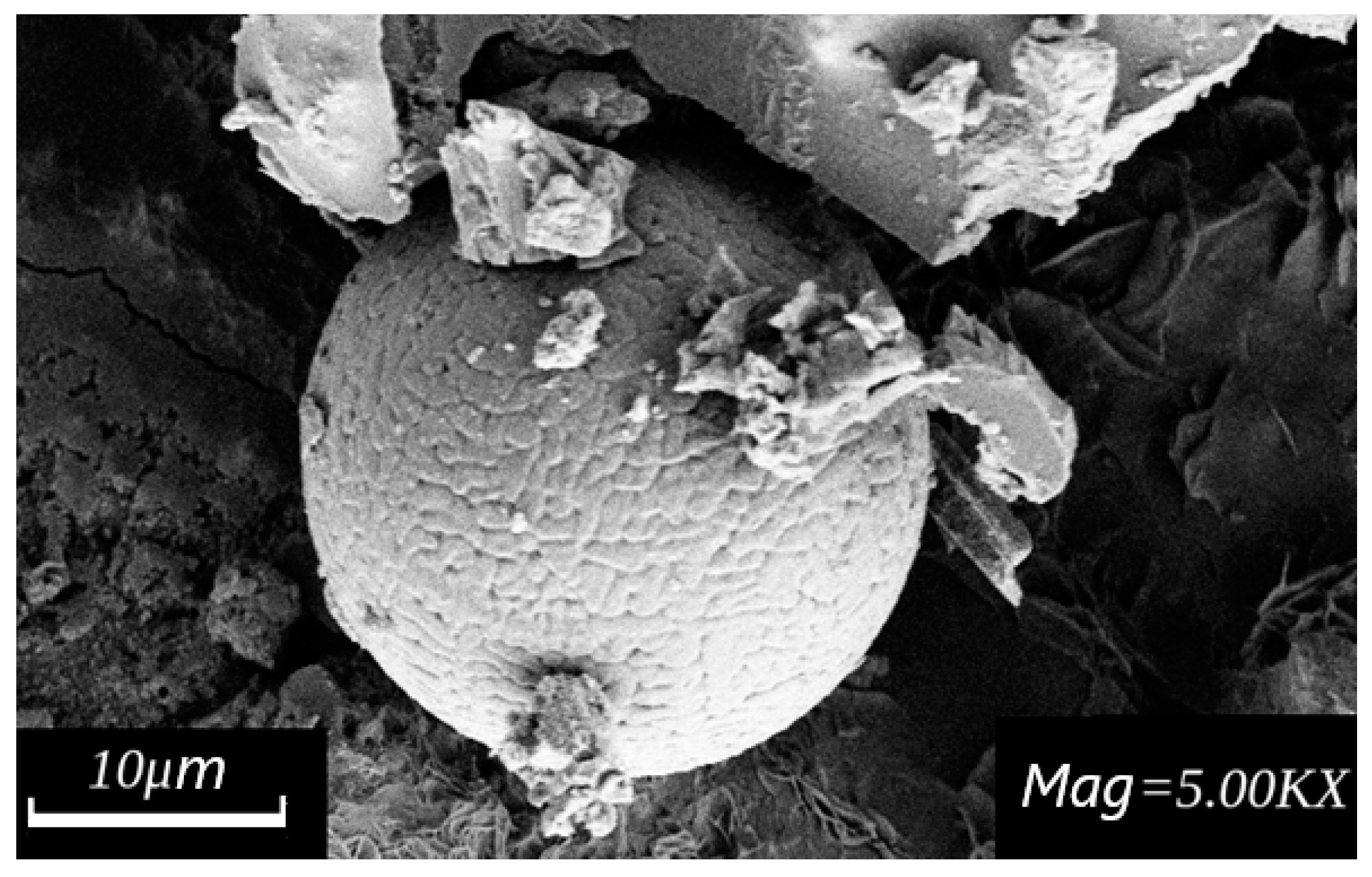
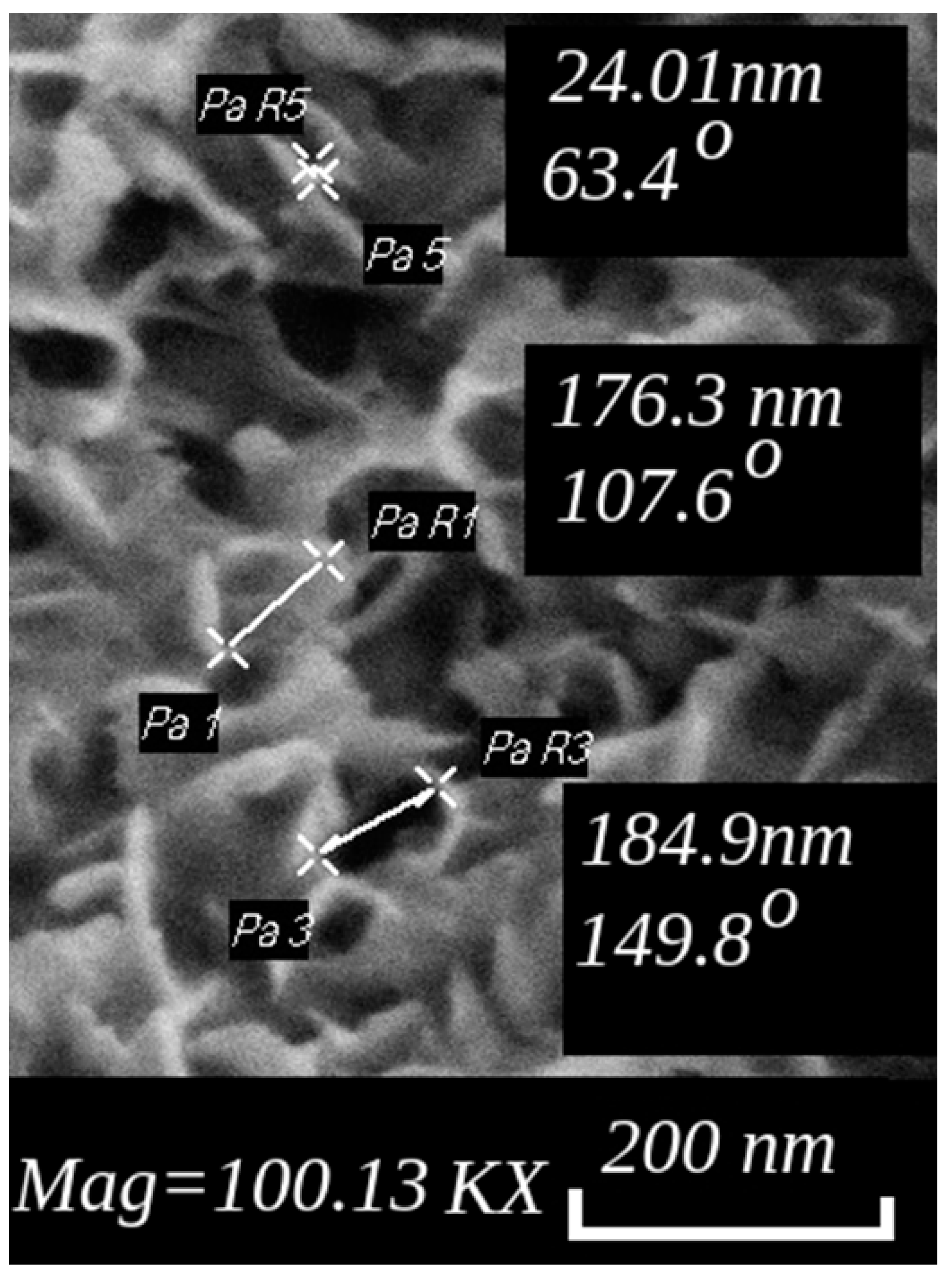
| Control Parameters | Material Grade | ||
|---|---|---|---|
| Components | U7 | St.3 | ANO-4 |
| GOST 1435–99 | GOST 380–2005 | GOST 5.1215–72 | |
| As | - | up to 0.08 | - |
| C | 0.66–0.73 | 0.14–0.22 | up to 0.10 |
| Cr | up to 0.2 | up to 0.3 | - |
| Cu | up to 0.25 | up to 0.3 | - |
| Fe | base | base | base |
| Mn | 0.17–0.33 | 0.4–0.65 | 0.6–0.8 |
| Ni | up to 0.25 | up to 0.3 | - |
| N | - | up to 0.008 | - |
| P | up to 0.03 | up to 0.04 | up to 0.035 |
| S | up to 0.028 | up to 0.05 | up to 0.035 |
| Si | 0.17–0.33 | 0.05–0.17 | up to 0.18 |
| Sample No. | Sample Material | Saline Media Composition, wt. % | Initial Corrosion Rate Per First Day, g/cm3 Day ∙104 | ||
|---|---|---|---|---|---|
| Na2CO3 | NaHCO3 | NaCl | |||
| 1 | St3 | 4.2 4.2 | 33.6 33.6 | 3.5 - | 3.4 1.8 |
| 2 | U7 | 4.2 4.2 | 33.6 33.6 | 3.5 - | 1.2 0.95 |
| 3 | Weld joint St3-St3 | 4.2 4.2 | 33.6 33.6 | 3.5 - | 3.2 2.13 |
| 4 | Weld joint St3-U7 | 4.2 4.2 | 33.6 33.6 | 3.5 - | 3.42 2.4 |
| 5 | Weld joint U7-U7 | 4.2 4.2 | 33.6 33.6 | 3.5 - | 3.3 2.10 |
| Spectrum No | Contents at the Surface of the Sample Material, wt. % | |||||||
|---|---|---|---|---|---|---|---|---|
| B Stat. | C | O | Na | S | Cl | Fe | Total | |
| 1 | Y | 6.95 | 34.19 | 0.14 | 0.49 | 0.31 | 57.93 | 100.00 |
| 2 | Y | 8.14 | 31.65 | 0.13 | 0.56 | 0.27 | 59.24 | 100.00 |
| 3 | Y | 7.44 | 35.01 | 0.00 | 0.50 | 0.31 | 56.74 | 100.00 |
| 4 | Y | 8.62 | 34.13 | 0.20 | 0.46 | 0.27 | 56.32 | 100.00 |
| 5 | Y | 5.15 | 32.87 | 0.21 | 0.98 | 0.47 | 60.32 | 100.00 |
| 6 | Y | 5.44 | 34.00 | 0.15 | 0.86 | 0.25 | 59.30 | 100.00 |
| 7 | Y | 5.15 | 33.58 | 0.08 | 0.30 | 0.26 | 60.63 | 100.00 |
| 8 | Y | 7.74 | 31.19 | 0.13 | 0.36 | 0.22 | 60.36 | 100.00 |
| 9 | Y | 7.43 | 31.63 | 0.15 | 0.37 | 0.22 | 60.21 | 100.00 |
| 10 | Y | 6.04 | 31.06 | 0.15 | 0.70 | 0.41 | 61.65 | 100.00 |
| Average | 6.81 | 32.93 | 0.13 | 0.56 | 0.30 | 59.27 | 100.00 | |
| Standard deviation | 1.28 | 1.44 | 0.06 | 0.22 | 0.08 | 1.75 | ||
| max. | 8.62 | 35.01 | 0.21 | 0.98 | 0.47 | 61.65 | ||
| min. | 5.15 | 31.06 | 0.00 | 0.30 | 0.22 | 56.32 | ||
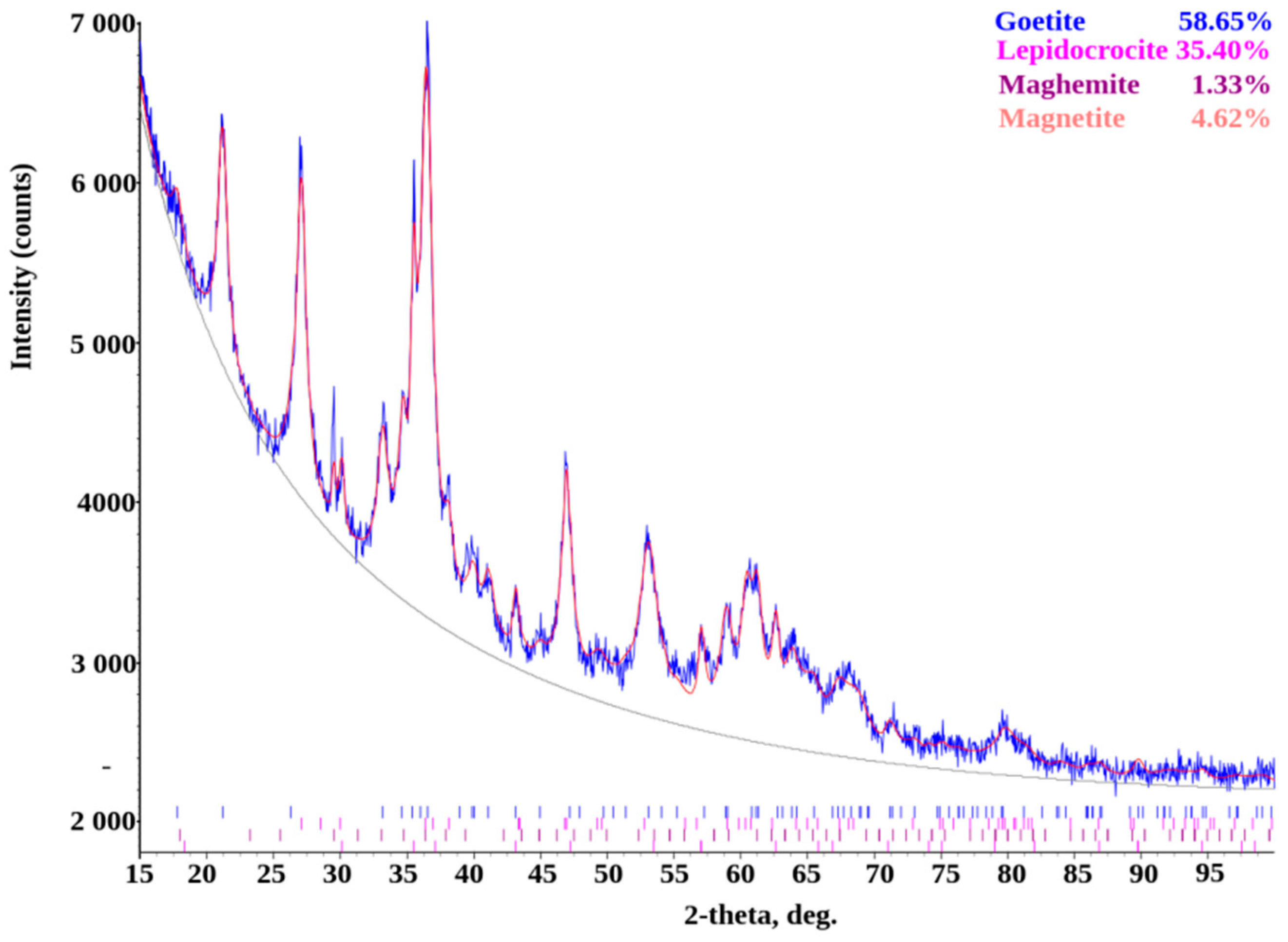
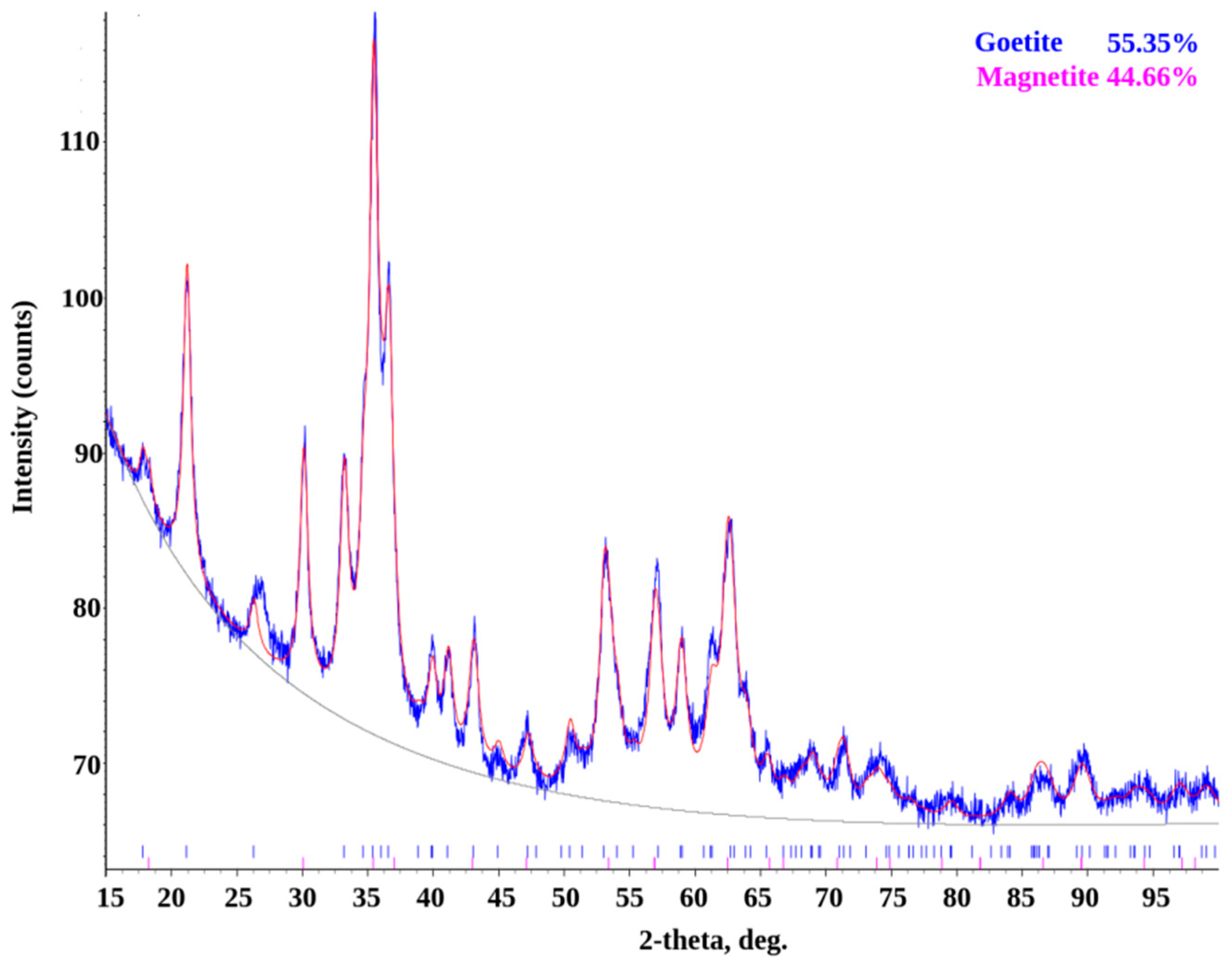
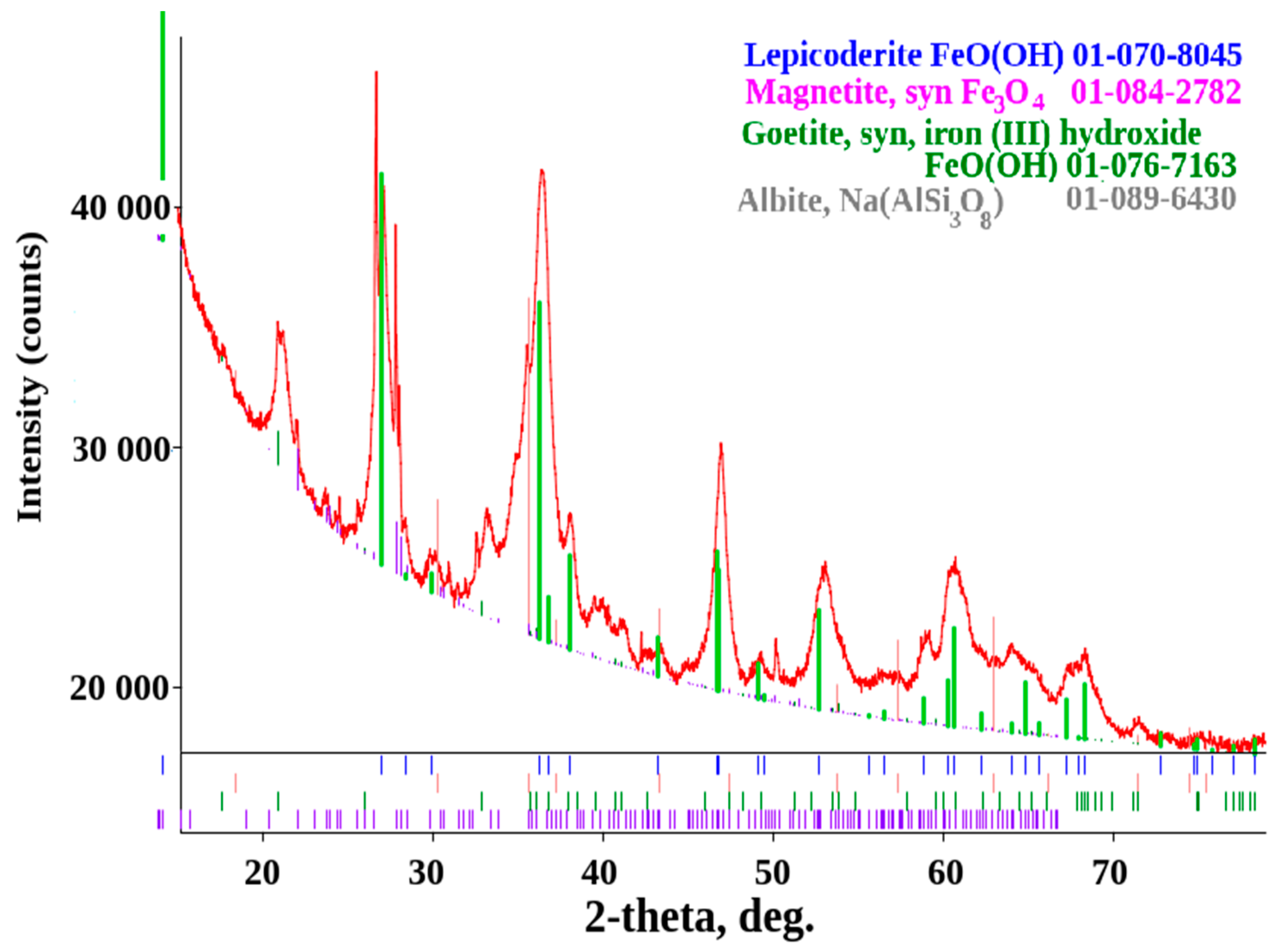
| Phase | Contains at the Surface of the Sample Material, % | ||||
|---|---|---|---|---|---|
| No | Name | Formula | St3 | U7 | Weld Joint St3-U7 |
| 1 | Iron(III) oxide hydroxide, goethite | FeO(OH) | 58.65 | 55.35 | 44.51 |
| 2 | Lepidocrocite | FeO(OH) | 35.40 | - | 38.29 |
| 4 | Magnetite | Fe3O4 | 4.62 | 44.65 | 4.16 |
| 5 | Maghemite | Fe3O4 | 1.33 | - | - |
| 6 | Albite | Na[AlSi3O8] | - | - | 13.03 |
| 7 | Muscovite | KAl2[AlSi3O10](OH)2 | - | - | traces |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgonnik, P.V.; Kuzhaeva, A.A.; Berlinskiy, I.V. The Study of Metal Corrosion Resistance near Weld Joints When Erecting Building and Structures Composed of Precast Structures. Appl. Sci. 2022, 12, 2518. https://doi.org/10.3390/app12052518
Zgonnik PV, Kuzhaeva AA, Berlinskiy IV. The Study of Metal Corrosion Resistance near Weld Joints When Erecting Building and Structures Composed of Precast Structures. Applied Sciences. 2022; 12(5):2518. https://doi.org/10.3390/app12052518
Chicago/Turabian StyleZgonnik, Pyotr V., Alyona A. Kuzhaeva, and Igor V. Berlinskiy. 2022. "The Study of Metal Corrosion Resistance near Weld Joints When Erecting Building and Structures Composed of Precast Structures" Applied Sciences 12, no. 5: 2518. https://doi.org/10.3390/app12052518
APA StyleZgonnik, P. V., Kuzhaeva, A. A., & Berlinskiy, I. V. (2022). The Study of Metal Corrosion Resistance near Weld Joints When Erecting Building and Structures Composed of Precast Structures. Applied Sciences, 12(5), 2518. https://doi.org/10.3390/app12052518





