Effect of Three Chlorhexidine-Based Mouthwashes on Human Gingival Fibroblasts: An In Vitro Study
Abstract
:1. Introduction
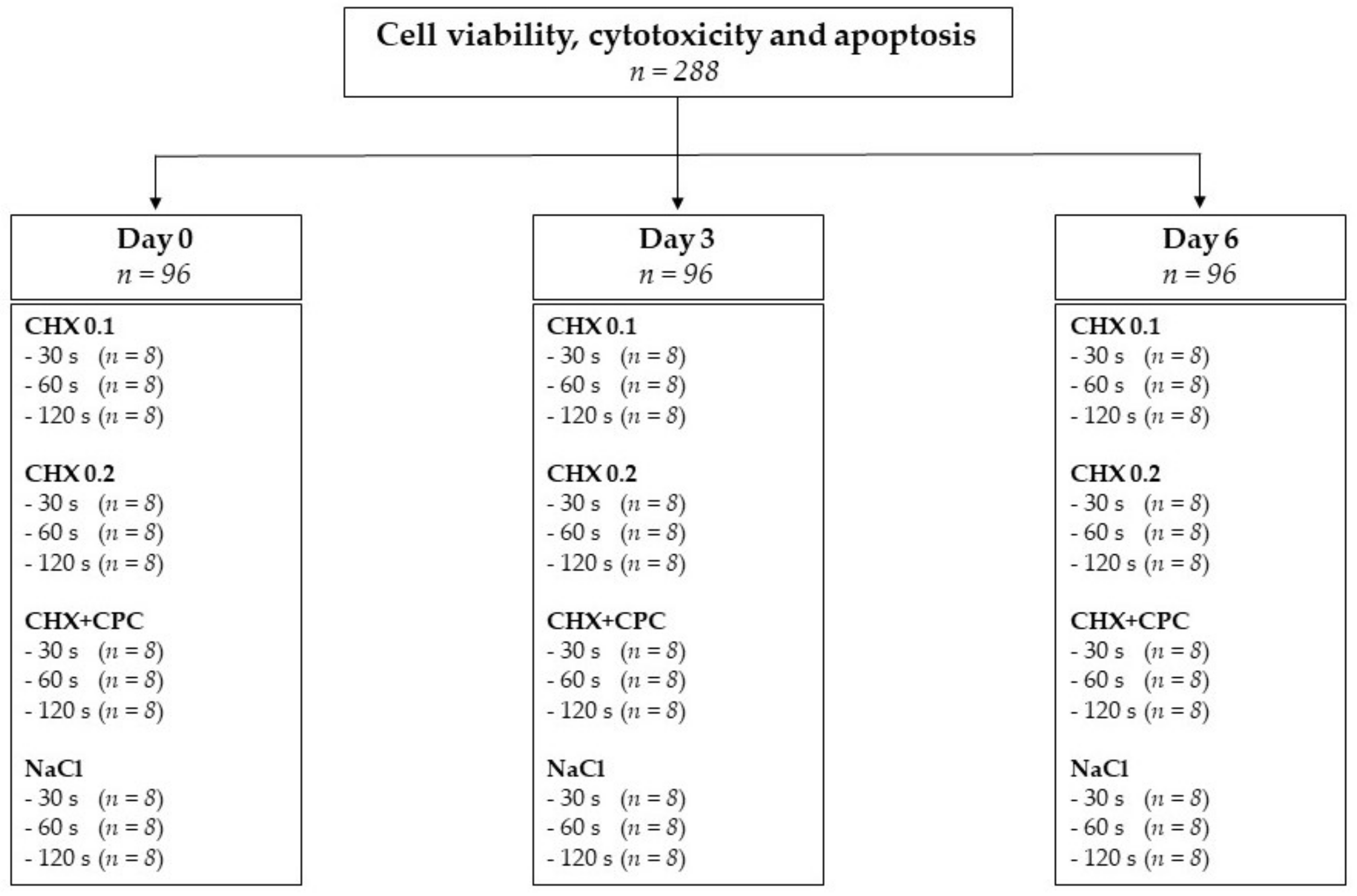
2. Materials and Methods
2.1. Cell Culture
2.2. Treatment Procedure
2.3. In Vitro Tests
2.4. Statistical Analysis
3. Results
3.1. Cell Viability
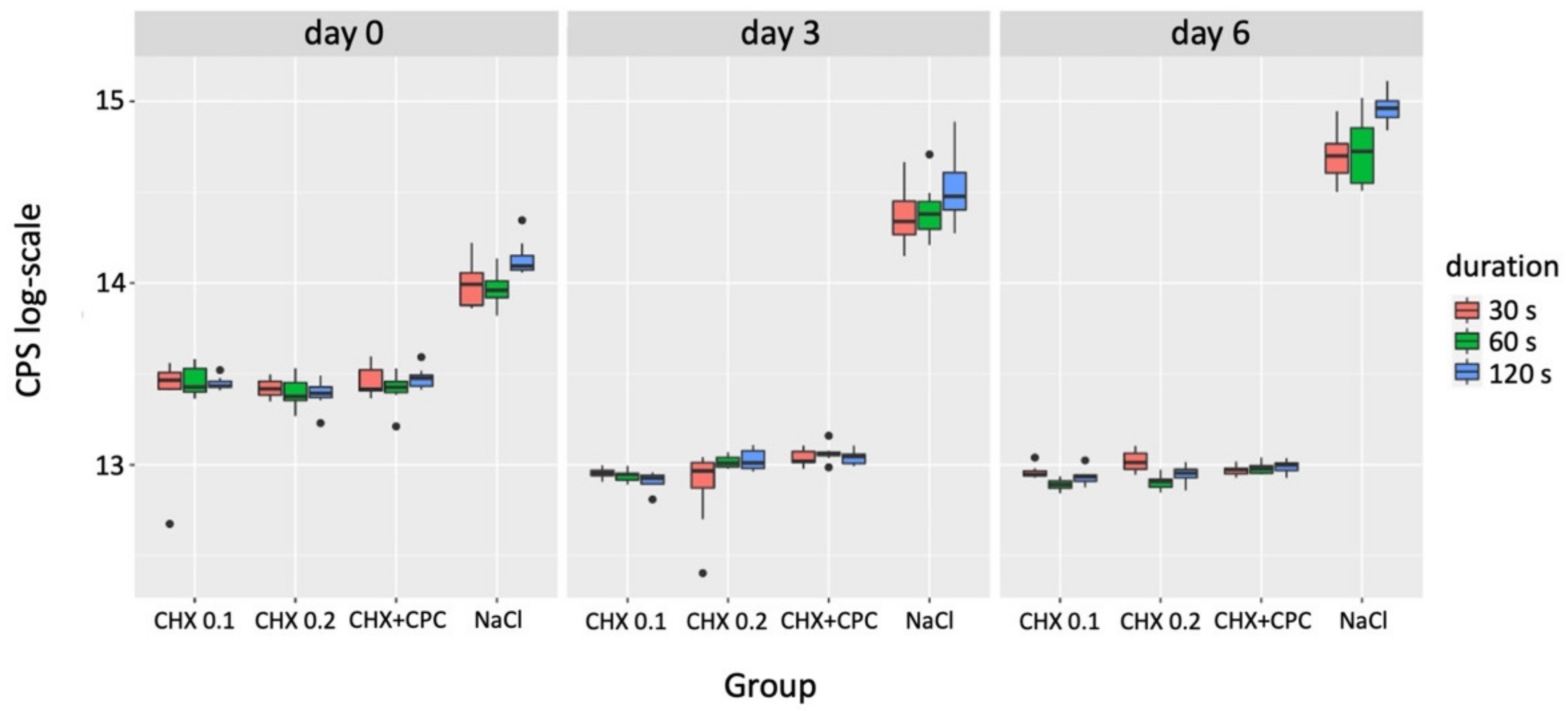
| Grouping Variable | Comparator 1 | Comparator 2 | p-Value (day 0) | p-Value (day 3) | p-Value (day 6) |
|---|---|---|---|---|---|
| CHX 0.1 | 30 s | 60 s | - | - | 0.442 |
| 30 s | 120 s | - | - | 0.143 | |
| 60 s | 120 s | - | - | 0.002 ** | |
| CHX 0.2 | 30 s | 60 s | - | - | 0.002 ** |
| 30 s | 120 s | - | - | 0.143 | |
| 60 s | 120 s | - | - | 0.442 | |
| CHX + CPC | 30 s | 60 s | - | - | - |
| 30 s | 120 s | - | - | - | |
| 60 s | 120 s | - | - | - | |
| NaCl | 30 s | 60 s | 1.000 | - | 1.000 |
| 30 s | 120 s | 0.049 * | - | 0.009 ** | |
| 60 s | 120 s | 0.022 * | - | 0.033 * | |
| 30 s | CHX 0.1 | CHX 0.2 | 1.000 | 1.000 | 0.420 |
| CHX 0.1 | CHX + CPC | 1.000 | 0.151 | 1.000 | |
| CHX 0.1 | NaCl | 0.016 * | 0.000 *** | 0.000 *** | |
| CHX 0.2 | CHX + CPC | 1.000 | 1.000 | 1.000 | |
| CHX 0.2 | NaCl | 0.000 *** | 0.001 ** | 0.106 | |
| CHX + CPC | NaCl | 0.007 ** | 0.226 | 0.001 ** | |
| 60 s | CHX 0.1 | CHX 0.2 | 1.000 | 0.373 | 1.000 |
| CHX 0.1 | CHX + CPC | 1.000 | 0.020 * | 0.046 * | |
| CHX 0.1 | NaCl | 0.022 * | 0.000 *** | 0.000 *** | |
| CHX 0.2 | CHX + CPC | 1.000 | 1.000 | 0.198 | |
| CHX 0.2 | NaCl | 0.000 *** | 0.010* | 0.000 *** | |
| CHX + CPC | NaCl | 0.005 ** | 0.226 | 0.420 | |
| 120 s | CHX 0.1 | CHX 0.2 | 1.000 | 0.092 | 1.000 |
| CHX 0.1 | CHX + CPC | 1.000 | 0.043 * | 0.445 | |
| CHX 0.1 | NaCl | 0.008 ** | 0.000 *** | 0.000 *** | |
| CHX 0.2 | CHX + CPC | 0.420 | 1.000 | 1.000 | |
| CHX 0.2 | NaCl | 0.000 *** | 0.043 * | 0.003 ** | |
| CHX + CPC | NaCl | 0.054 | 0.092 | 0.079 |
3.2. Cytotoxicity
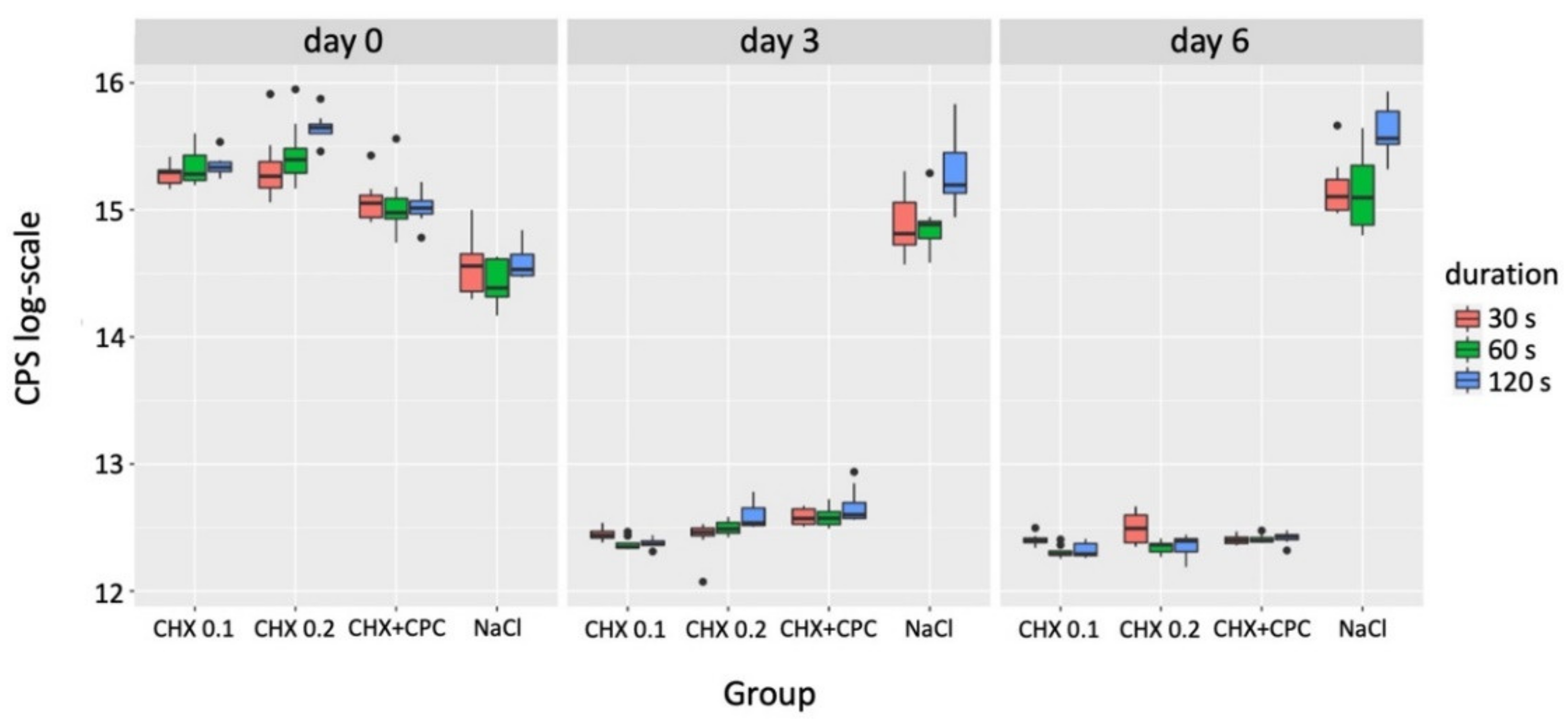
| Grouping Variable | Comparator 1 | Comparator 2 | p-Value (day 0) | p-Value (day 3) | p-Value (day 6) |
|---|---|---|---|---|---|
| CHX 0.1 | 30 s | 60 s | - | 0.014 * | 0.030 * |
| 30 s | 120 s | - | 0.121 | 0.049 * | |
| 60 s | 120 s | - | 1.000 | 1.000 | |
| CHX 0.2 | 30 s | 60 s | 1.000 | 0.609 | 0.040 * |
| 30 s | 120 s | 0.024 * | 0.006 ** | 0.231 | |
| 60 s | 120 s | 0.269 | 0.214 | 1.000 | |
| CHX + CPC | 30 s | 60 s | - | - | - |
| 30 s | 120 s | - | - | - | |
| 60 s | 120 s | - | - | - | |
| NaCl | 30 s | 60 s | - | 1.000 | 1.000 |
| 30 s | 120 s | - | 0.024 * | 0.027 * | |
| 60 s | 120 s | - | 0.024 * | 0.016 * | |
| 30 s | CHX 0.1 | CHX 0.2 | 1.000 | 1.000 | 1.000 |
| CHX 0.1 | CHX + CPC | 0.292 | 0.099 | 1.000 | |
| CHX 0.1 | NaCl | 0.000 *** | 0.000 *** | 0.001 ** | |
| CHX 0.2 | CHX + CPC | 0.373 | 0.185 | 1.000 | |
| CHX 0.2 | NaCl | 0.001 ** | 0.000 *** | 0.033 * | |
| CHX + CPC | NaCl | 0.257 | 0.351 | 0.002 ** | |
| 60 s | CHX 0.1 | CHX 0.2 | 1.000 | 0.420 | 1.000 |
| CHX 0.1 | CHX + CPC | 0.420 | 0.017 * | 0.073 | |
| CHX 0.1 | NaCl | 0.001 ** | 0.000 *** | 0.000 *** | |
| CHX 0.2 | CHX + CPC | 0.173 | 1.000 | 0.814 | |
| CHX 0.2 | NaCl | 0.000 *** | 0.008 ** | 0.002 ** | |
| CHX + CPC | NaCl | 0.226 | 0.257 | 0.226 | |
| 120 s | CHX 0.1 | CHX 0.2 | 0.591 | 0.185 | 1.000 |
| CHX 0.1 | CHX + CPC | 0.472 | 0.019 * | 0.226 | |
| CHX 0.1 | NaCl | 0.004 ** | 0.000 *** | 0.000 *** | |
| CHX 0.2 | CHX + CPC | 0.004 ** | 1.000 | 1.000 | |
| CHX 0.2 | NaCl | 0.000 *** | 0.019 * | 0.002 ** | |
| CHX + CPC | NaCl | 0.591 | 0.185 | 0.141 |
3.3. Apoptosis
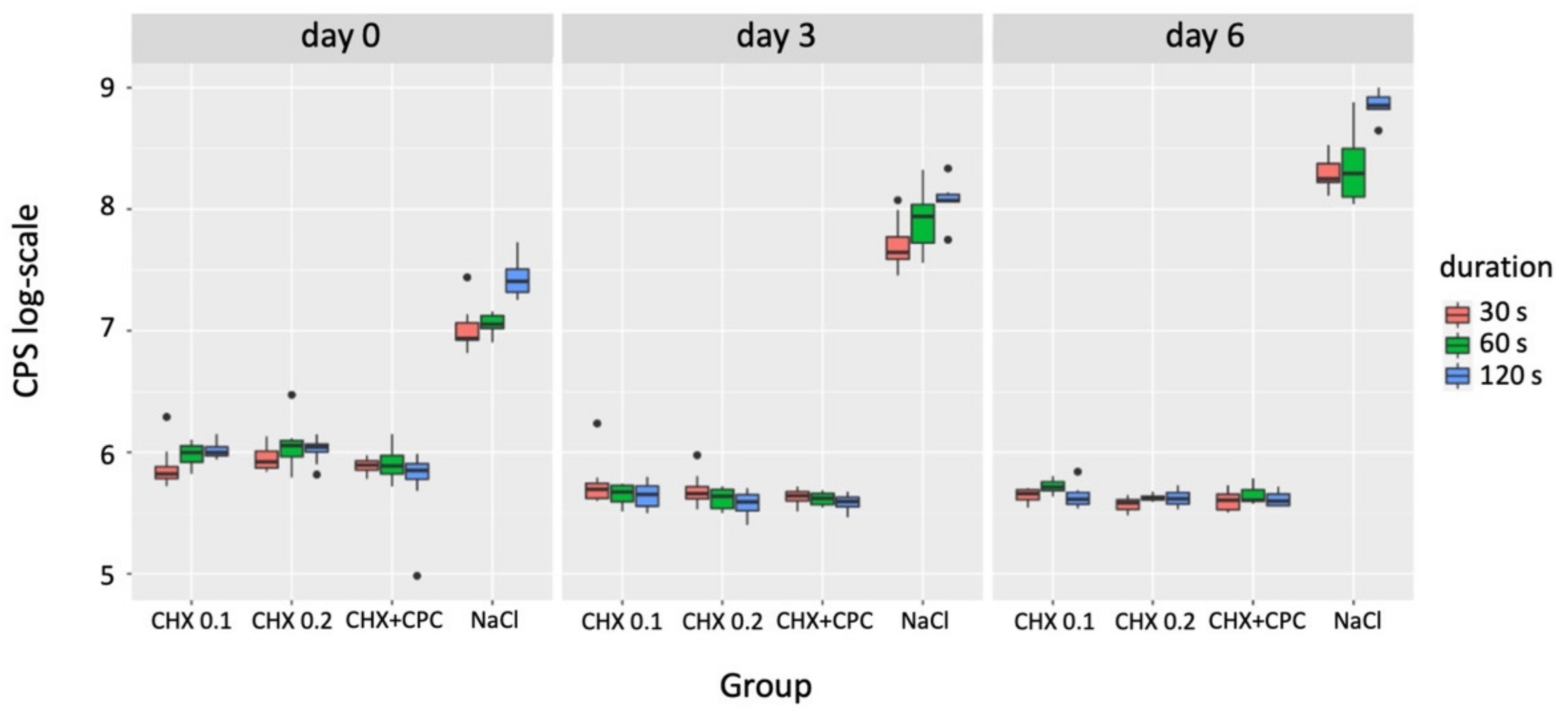
| Grouping Variable | Comparator 1 | Comparator 2 | p-Value (day 0) | p-Value (day 3) | p-Value (day 6) |
|---|---|---|---|---|---|
| CHX 0.1 | 30 s | 60 s | - | - | - |
| 30 s | 120 s | - | - | - | |
| 60 s | 120 s | - | - | - | |
| CHX 0.2 | 30 s | 60 s | - | - | - |
| 30 s | 120 s | - | - | - | |
| 60 s | 120 s | - | - | - | |
| CHX + CPC | 30 s | 60 s | - | - | - |
| 30 s | 120 s | - | - | - | |
| 60 s | 120 s | - | - | - | |
| NaCl | 30 s | 60 s | 1.000 | 0.648 | 1.000 |
| 30 s | 120 s | 0.002 ** | 0.006 ** | 0.004 ** | |
| 60 s | 120 s | 0.011 * | 0.183 | 0.009 ** | |
| 30 s | CHX 0.1 | CHX 0.2 | 0.813 | 1.000 | 0.623 |
| CHX 0.1 | CHX + CPC | 1.000 | 1.000 | 1.000 | |
| CHX 0.1 | NaCl | 0.000 *** | 0.016 * | 0.065 | |
| CHX 0.2 | CHX + CPC | 1.000 | 1.000 | 1.000 | |
| CHX 0.2 | NaCl | 0.048 * | 0.004 ** | 0.000 *** | |
| CHX + CPC | NaCl | 0.004 ** | 0.001 ** | 0.003 ** | |
| 60 s | CHX 0.1 | CHX 0.2 | 1.000 | 1.000 | 0.291 |
| CHX 0.1 | CHX + CPC | 1.000 | 1.000 | 0.444 | |
| CHX 0.1 | NaCl | 0.007 ** | 0.023 * | 0.185 | |
| CHX 0.2 | CHX + CPC | 1.000 | 1.000 | 1.000 | |
| CHX 0.2 | NaCl | 0.028 * | 0.002 ** | 0.000 *** | |
| CHX + CPC | NaCl | 0.000 *** | 0.001 ** | 0.000 *** | |
| 120 s | CHX 0.1 | CHX 0.2 | 1.000 | 1.000 | 1.000 |
| CHX 0.1 | CHX + CPC | 0.248 | 1.000 | 1.000 | |
| CHX 0.1 | NaCl | 0.032 * | 0.024 * | 0.004 ** | |
| CHX 0.2 | CHX + CPC | 0.167 | 1.000 | 1.000 | |
| CHX 0.2 | NaCl | 0.052 | 0.001 ** | 0.005 ** | |
| CHX + CPC | NaCl | 0.000 *** | 0.002 ** | 0.003 ** |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), 74–84. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), 267–290. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, A.; Toekan, S.; Saure, D.; Körner, G. Survival and success of implants in a private periodontal practice: A 10 year retrospective study. BMC Oral Health 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Müller Campanile, V.; Megally, A.; Campanile, G.; Gayet-Ageron, A.; Giannopoulou, C.; Mombelli, A. Risk factors for recurrence of periodontal disease in patients in maintenance care in a private practice. J. Clin. Periodontol. 2019, 46, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Muller-Campanile, V.; Lang, N.P. Changes in the prevalence of residual pockets and tooth loss in treated periodontal patients during a supportive maintenance care program. J. Clin. Periodontol. 1998, 25, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Quirynen, M. Risk indicators for peri-implantitis. A narrative review. Clin. Oral Implant. Res. 2015, 26 (Suppl. S11), 15–44. [Google Scholar] [CrossRef]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: A systematic review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Mombelli, A. Maintenance therapy for teeth and implants. Periodontol. 2000 2019, 79, 190–199. [Google Scholar] [CrossRef]
- Costa, F.O.; Takenaka-Martinez, S.; Cota, L.O.; Ferreira, S.D.; Silva, G.L.; Costa, J.E. Peri-implant disease in subjects with and without preventive maintenance: A 5-year follow-up. J. Clin. Periodontol. 2012, 39, 173–181. [Google Scholar] [CrossRef]
- Monje, A.; Aranda, L.; Diaz, K.T.; Alarcón, M.A.; Bagramian, R.A.; Wang, H.L.; Catena, A. Impact of Maintenance Therapy for the Prevention of Peri-implant Diseases: A Systematic Review and Meta-analysis. J. Dent. Res 2016, 95, 372–379. [Google Scholar] [CrossRef]
- Müller, H.D.; Eick, S.; Moritz, A.; Lussi, A.; Gruber, R. Cytotoxicity and Antimicrobial Activity of Oral Rinses In Vitro. BioMed Res. Int. 2017, 2017, 4019723. [Google Scholar] [CrossRef]
- Schmidt, J.; Zyba, V.; Jung, K.; Rinke, S.; Haak, R.; Mausberg, R.F.; Ziebolz, D. Cytotoxic effects of octenidine mouth rinse on human fibroblasts and epithelial cells-an in vitro study. Drug. Chem. Toxicol. 2016, 39, 322–330. [Google Scholar] [CrossRef]
- Faria, G.; Cardoso, C.R.; Larson, R.E.; Silva, J.S.; Rossi, M.A. Chlorhexidine-induced apoptosis or necrosis in L929 fibroblasts: A role for endoplasmic reticulum stress. Toxicol. Appl. Pharmacol. 2009, 234, 256–265. [Google Scholar] [CrossRef]
- Ausiello, P.; Cassese, A.; Miele, C.; Beguinot, F.; Garcia-Godoy, F.; Di Jeso, B.; Ulianich, L. Cytotoxicity of dental resin composites: An in vitro evaluation. J. Appl. Toxicol. 2013, 33, 451–457. [Google Scholar] [CrossRef]
- Alpaslan Yayli, N.Z.; Tunc, S.K.; Degirmenci, B.U.; Dikilitas, A.; Taspinar, M. Comparative evaluation of the cytotoxic effects of different oral antiseptics: A primary culture study. Niger. J. Clin. Pract. 2021, 24, 313–320. [Google Scholar] [CrossRef]
- John, G.; Becker, J.; Schwarz, F. Effects of taurolidine and chlorhexidine on SaOS-2 cells and human gingival fibroblasts grown on implant surfaces. Int. J. Oral Maxillofac. Implant. 2014, 29, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Balloni, S.; Locci, P.; Lumare, A.; Marinucci, L. Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviour. Toxicol. In Vitro 2016, 34, 88–96. [Google Scholar] [CrossRef]
- Coelho, A.S.; Laranjo, M.; Gonçalves, A.C.; Paula, A.; Paulo, S.; Abrantes, A.M.; Caramelo, F.; Ferreira, M.M.; Silva, M.J.; Carrilho, E.; et al. Cytotoxic effects of a chlorhexidine mouthwash and of an enzymatic mouthwash on human gingival fibroblasts. Odontology 2020, 108, 260–270. [Google Scholar] [CrossRef]
- Treglia, A.S.; Turco, S.; Ulianich, L.; Ausiello, P.; Lofrumento, D.D.; Nicolardi, G.; Miele, C.; Garbi, C.; Beguinot, F.; Di Jeso, B. Cell fate following ER stress: Just a matter of "quo ante" recovery or death? Histol. Histopathol. 2012, 27, 1–12. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database. Syst. Rev. 2017, 3, Cd008676. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Graziani, F.; Gabriele, M.; D’Aiuto, F.; Suvan, J.; Tonelli, M.; Cei, S. Dental plaque, gingival inflammation and tooth -discolouration with different commercial -formulations of 0.2% chlorhexidine rinse: A double-blind randomised controlled clinical trial. Oral Health Prev. Dent. 2015, 13, 101–111. [Google Scholar] [CrossRef]
- Smith, R.G.; Moran, J.; Addy, M.; Doherty, F.; Newcombe, R.G. Comparative staining in vitro and plaque inhibitory properties in vivo of 0.12% and 0.2% chlorhexidine mouthrinses. J. Clin. Periodontol. 1995, 22, 613–617. [Google Scholar] [CrossRef]
- Pulcini, A.; Bollaín, J.; Sanz-Sánchez, I.; Figuero, E.; Alonso, B.; Sanz, M.; Herrera, D. Clinical effects of the adjunctive use of a 0.03% chlorhexidine and 0.05% cetylpyridinium chloride mouth rinse in the management of peri-implant diseases: A randomized clinical trial. J. Clin. Periodontol. 2019, 46, 342–353. [Google Scholar] [CrossRef]
- Bollain, J.; Pulcini, A.; Sanz-Sánchez, I.; Figuero, E.; Alonso, B.; Sanz, M.; Herrera, D. Efficacy of a 0.03% chlorhexidine and 0.05% cetylpyridinium chloride mouth rinse in reducing inflammation around the teeth and implants: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 1729–1741. [Google Scholar] [CrossRef]
- Quirynen, M.; Soers, C.; Desnyder, M.; Dekeyser, C.; Pauwels, M.; van Steenberghe, D. A 0.05% cetyl pyridinium chloride/0.05% chlorhexidine mouth rinse during maintenance phase after initial periodontal therapy. J. Clin. Periodontol. 2005, 32, 390–400. [Google Scholar] [CrossRef]
- García-Gargallo, M.; Zurlohe, M.; Montero, E.; Alonso, B.; Serrano, J.; Sanz, M.; Herrera, D. Evaluation of new chlorhexidine- and cetylpyridinium chloride-based mouthrinse formulations adjunctive to scaling and root planing: Pilot study. Int. J. Dent. Hyg. 2017, 15, 269–279. [Google Scholar] [CrossRef]
- Mor-Reinoso, C.; Pascual, A.; Nart, J.; Quirynen, M. Inhibition of de novo plaque growth by a new 0.03% chlorhexidine mouth rinse formulation applying a non-brushing model: A randomized, double blind clinical trial. Clin. Oral Investig. 2016, 20, 1459–1467. [Google Scholar] [CrossRef]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Becker, K.; Brunello, G.; Scotti, L.; Drescher, D.; John, G. Efficacy of 0.05% Chlorhexidine and 0.05% Cetylpyridinium Chloride Mouthwash to Eliminate Living Bacteria on In Situ Collected Biofilms: An In Vitro Study. Antibiotics 2021, 10, 730. [Google Scholar] [CrossRef]
- Brunello, G.; Becker, K.; Scotti, L.; Drescher, D.; Becker, J.; John, G. The Effects of Three Chlorhexidine-Based Mouthwashes on Human Osteoblast-Like SaOS-2 Cells. An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 9986. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Pasqualotto, D.; Rinaldo, F.; Mazur, M.; Corridore, D.; Nofroni, I.; Ottolenghi, L.; Nardi, G.M. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with Anti-Discoloration System and cetylpyridinium chloride. Int. J. Dent. Hyg. 2019, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr. Guidelines for reporting pre-clinical in vitro studies on dental materials. J. Evid. -Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- R. Core Team. R, A Language and Environment for Statistical Computing; R. Core Team: Vienna, Austria, 2018. [Google Scholar]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S.; Wills, L.P.; Schnellmann, R.G. Measurement of cell death in Mammalian cells. Curr. Protoc. Pharmacol. 2012, 56, 12.8.1–12.8.24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krampe, B.; Al-Rubeai, M. Cell death in mammalian cell culture: Molecular mechanisms and cell line engineering strategies. Cytotechnology 2010, 62, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Gelles, J.D.; Chipuk, J.E. Robust high-throughput kinetic analysis of apoptosis with real-time high-content live-cell imaging. Cell Death Dis. 2016, 7, e2493. [Google Scholar] [CrossRef]
- Isherwood, B.; Timpson, P.; McGhee, E.J.; Anderson, K.I.; Canel, M.; Serrels, A.; Brunton, V.G.; Carragher, N.O. Live cell in vitro and in vivo imaging applications: Accelerating drug discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar] [CrossRef] [Green Version]
- Arweiler, N.B.; Auschill, T.M.; Sculean, A. Patient self-care of periodontal pocket infections. Periodontology 2000 2018, 76, 164–179. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Mombelli, A.; Loup, P.J.; Heitz, F.; Kruger, E.; Lang, N.P. Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin. Oral Implant. Res. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), 173–182. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Zyba, V.; Jung, K.; Rinke, S.; Haak, R.; Mausberg, R.F.; Ziebolz, D. Effects of octenidine mouth rinse on apoptosis and necrosis of human fibroblasts and epithelial cells-an in vitro study. Drug Chem. Toxicol. 2018, 41, 182–187. [Google Scholar] [CrossRef]
- Ülker, M.; Çelik, A.C.T.; Yavuz, E.; Kahvecioğlu, F.; Ülker, H.E. Real-Time Analysis of Antiproliferative Effects of Mouthwashes Containing Alcohol, Sodium Fluoride, Cetylpyridinium Chloride, and Chlorhexidine In Vitro. BioMed Res. Int. 2021, 2021, 2610122. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro three-dimensional organotypic culture models of the oral mucosa. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Franklin, K.L.; Brook, I.M.; van Noort, R. Biologic assessment of antiseptic mouthwashes using a three-dimensional human oral mucosal model. J. Periodontol. 2009, 80, 769–775. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Durbakula, K.; Prabhu, V.; Jose, M. Genotoxicity of non-alcoholic mouth rinses: A micronucleus and nuclear abnormalities study with fluorescent microscopy. J. Investig. Clin. Dent. 2018, 9, e12309. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunello, G.; Becker, K.; Scotti, L.; Drescher, D.; Becker, J.; John, G. Effect of Three Chlorhexidine-Based Mouthwashes on Human Gingival Fibroblasts: An In Vitro Study. Appl. Sci. 2022, 12, 2417. https://doi.org/10.3390/app12052417
Brunello G, Becker K, Scotti L, Drescher D, Becker J, John G. Effect of Three Chlorhexidine-Based Mouthwashes on Human Gingival Fibroblasts: An In Vitro Study. Applied Sciences. 2022; 12(5):2417. https://doi.org/10.3390/app12052417
Chicago/Turabian StyleBrunello, Giulia, Kathrin Becker, Luisa Scotti, Dieter Drescher, Jürgen Becker, and Gordon John. 2022. "Effect of Three Chlorhexidine-Based Mouthwashes on Human Gingival Fibroblasts: An In Vitro Study" Applied Sciences 12, no. 5: 2417. https://doi.org/10.3390/app12052417
APA StyleBrunello, G., Becker, K., Scotti, L., Drescher, D., Becker, J., & John, G. (2022). Effect of Three Chlorhexidine-Based Mouthwashes on Human Gingival Fibroblasts: An In Vitro Study. Applied Sciences, 12(5), 2417. https://doi.org/10.3390/app12052417








