Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review
Abstract
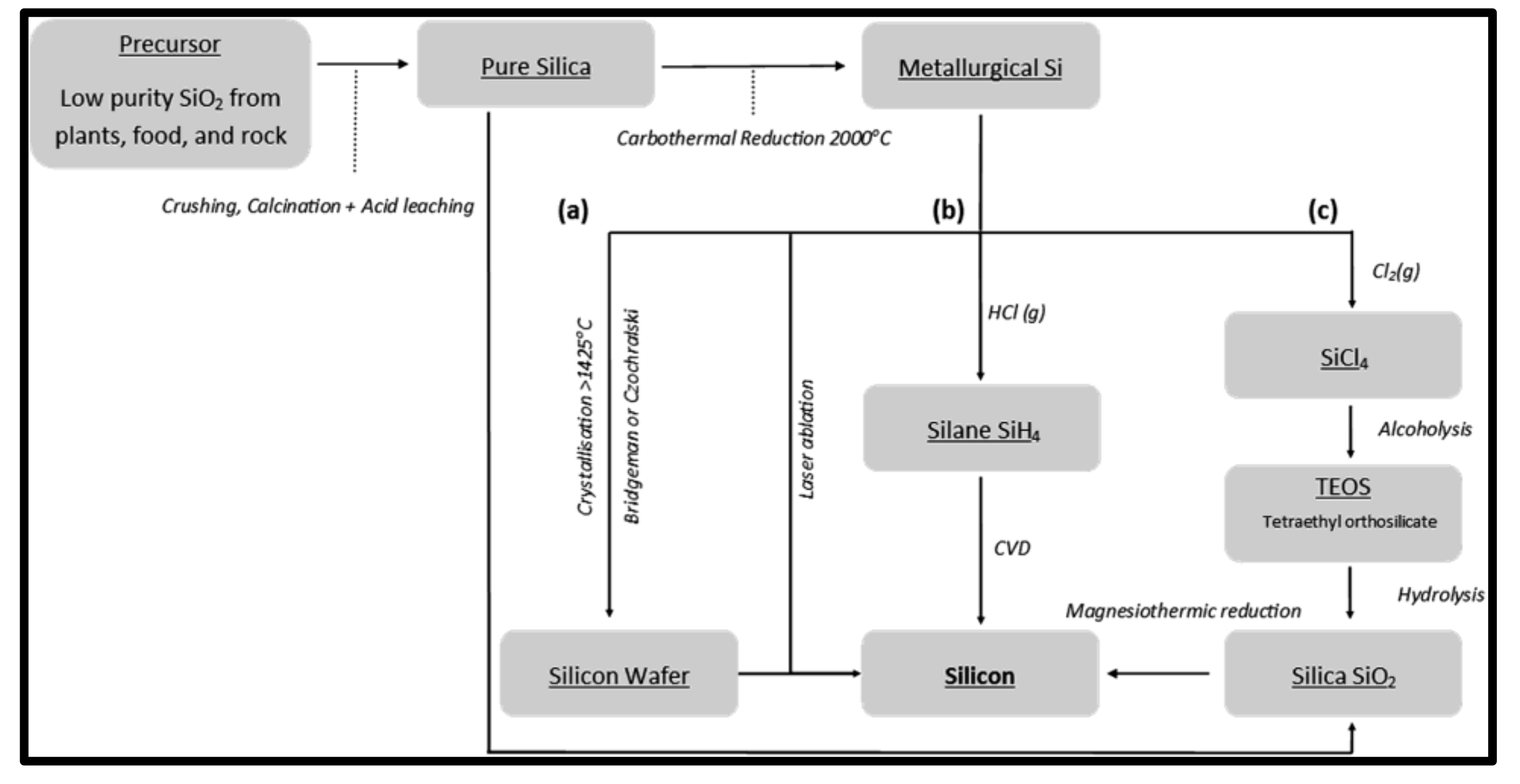
:1. Introduction
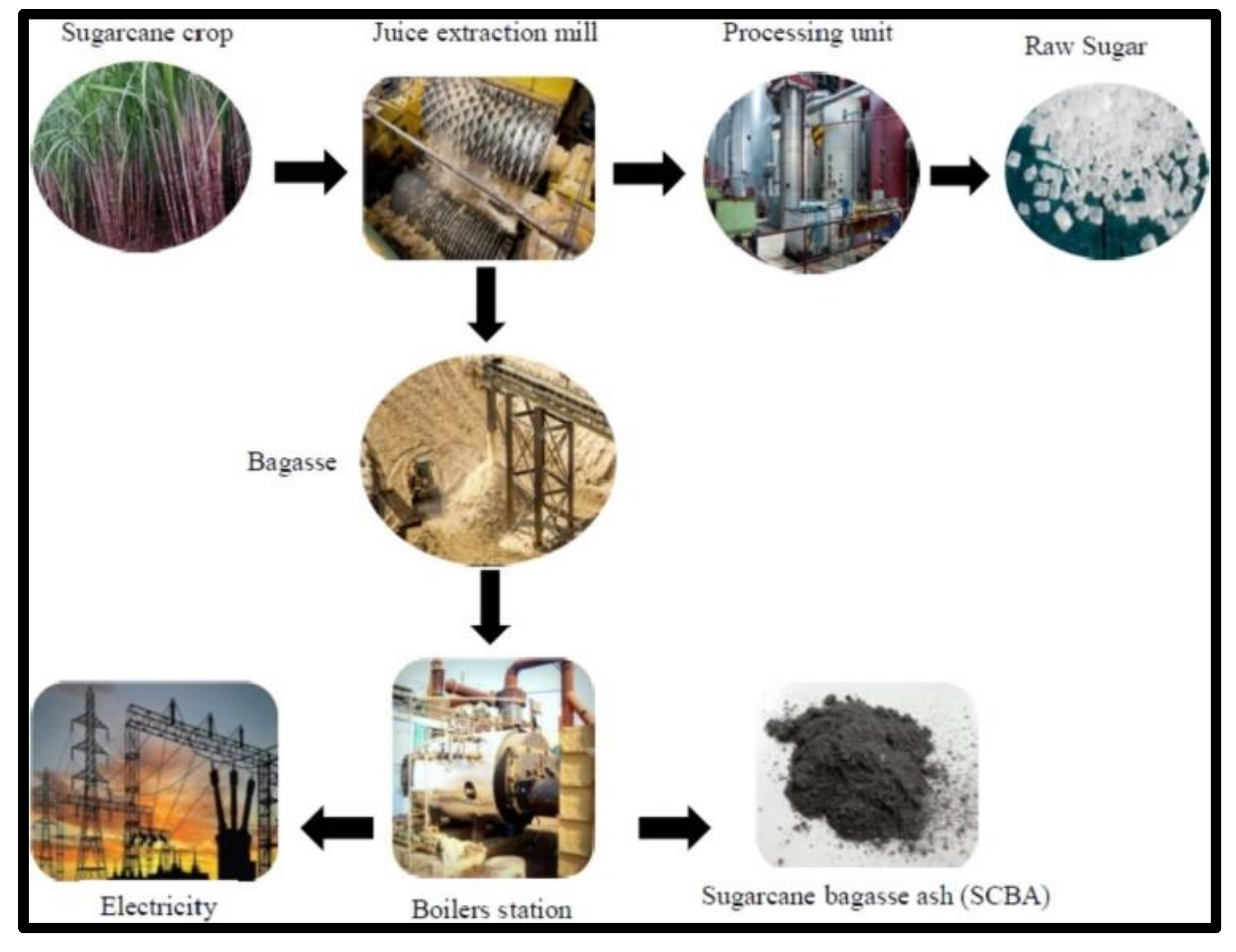
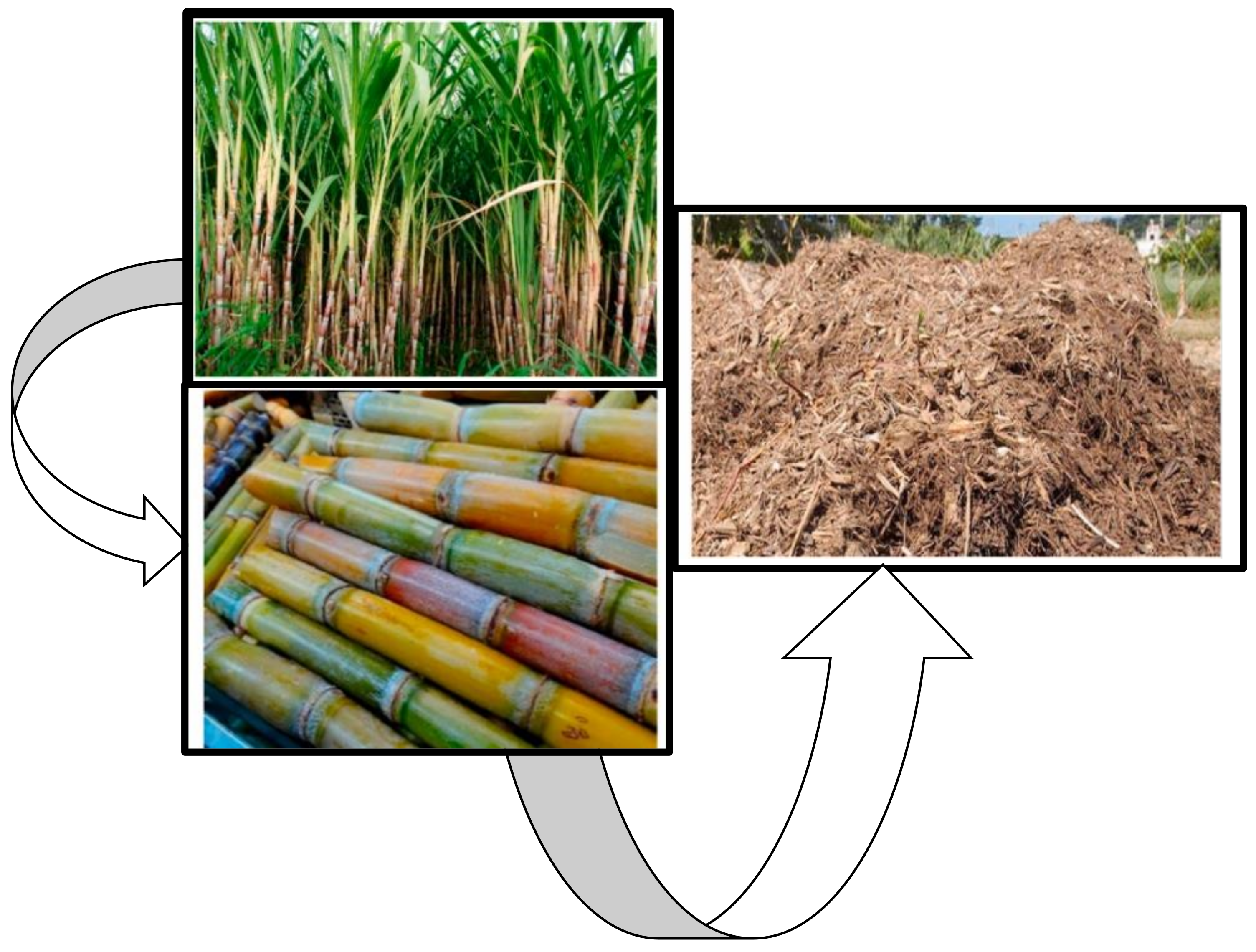
Sugarcane Bagasse in South Africa
2. Extraction of Silica
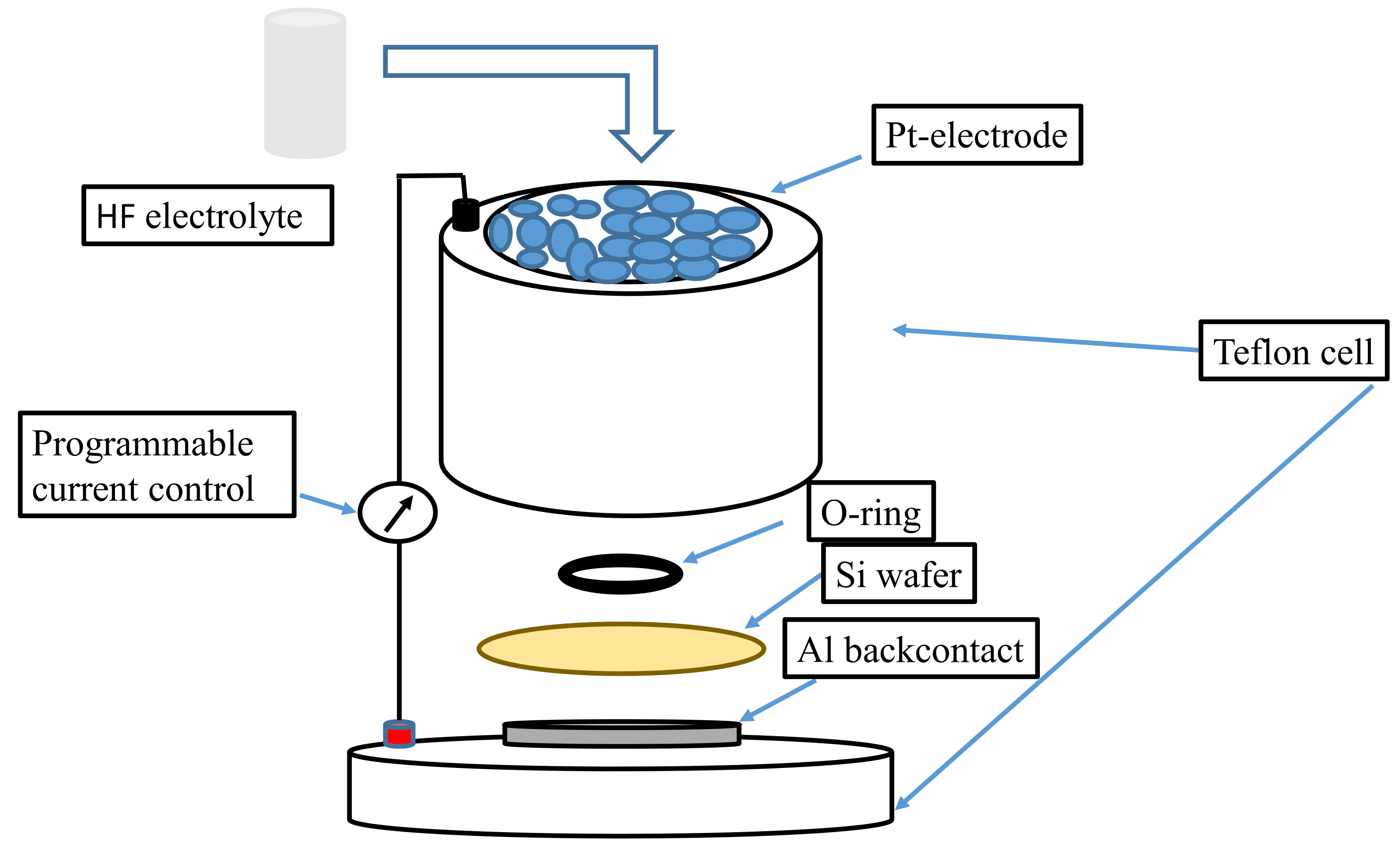
2.1. Electrochemical Process
Triumphs and Challenges
2.2. Ball Milling Process
Triumphs and Challenges
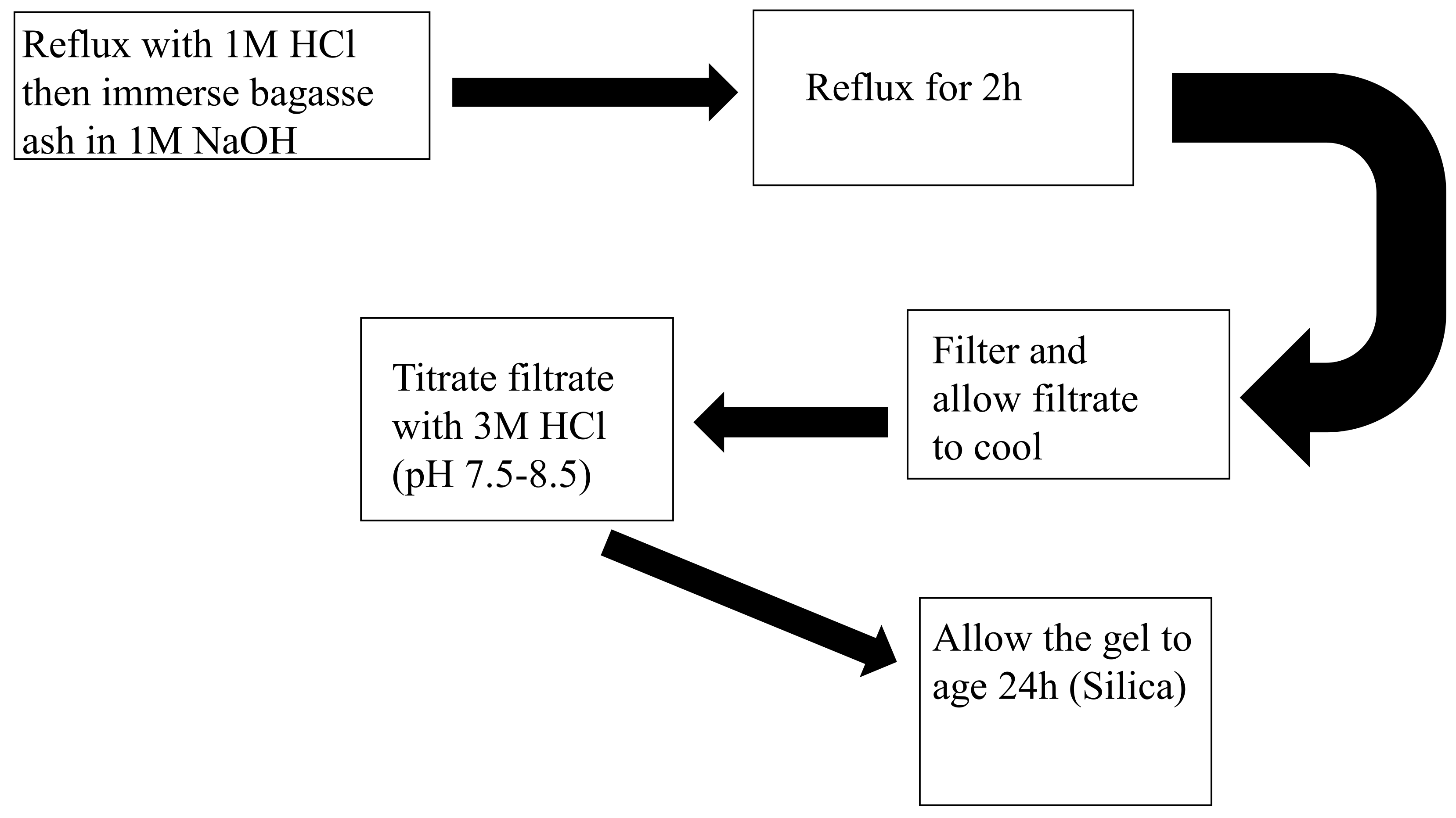
2.3. Sol–Gel Process
Triumphs and Challenges
3. Reduction of Silica into Silicon Nanoparticles
3.1. Carbothermic Reduction of Silica to Silicon
3.2. Magnesiothermic Reduction of Silica to Silicon
4. Purification of Silicon
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohd, N.K.; Wee, N.N.A.N.; Azmi, A.A. Green synthesis of silica nanoparticles using sugarcane bagasse. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2017; Volume 1885, p. 020123. [Google Scholar]
- Faizul, C.P.; Chik, A.; Bari, M.; Noorina, H.J. Extraction of silica from palm ash using organic acid leaching treatment. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2014; Volume 594, pp. 329–333. [Google Scholar]
- Ma, X.; Zhou, B.; Gao, W.; Qu, Y.; Wang, L.; Wang, Z.; Zhu, Y. A recyclable method for production of pure silica from rice hull ash. Powder Technol. 2012, 217, 497–501. [Google Scholar] [CrossRef]
- Das, D.; Yang, Y.; O’Brien, J.S.; Breznan, D.; Nimesh, S.; Bernatchez, S.; Hill, M.; Sayari, A.; Vincent, R.; Kumarathasan, P. Synthesis and Physicochemical Characterization of Mesoporous Nanoparticles. J. Nanomater. 2014, 2014, 62. [Google Scholar] [CrossRef] [Green Version]
- Faizul, C.P.; Abdullah, C.; Fazlul, M. Extraction of silica from palm ashvia citric acid leaching treatment. Adv. Environ. Biol. 2013, 7, 3690–3695. [Google Scholar]
- Yadav, A.L.; Sairam, V.; Muruganandam, L.; Srinivasan, K. An overview of the influences of mechanical and chemical processing on sugarcane bagasse ash characterisation as a supplementary cementitious material. J. Clean. Prod. 2020, 245, 118854. [Google Scholar] [CrossRef]
- Dlamini, M.; Nicholson, R.J.; Kadwa, M. Potential economic benefit of additional transformation initiatives to small scale growers in the South African sugar industry-2018/19. In Proceedings of the Annual Congress-South African Sugar Technologists’ Association, Durban, South Africa, 20–22 August 2019; pp. 22–24. [Google Scholar]
- Singels, A.; McFarlane, S.A.; Basdew, I.; Keeping, M.G.; Nicholson, R.; Pilusa, T.; Sithole, P.; Titshall, L.W. Review of South African sugarcane production in the 2018/19 season: Too much of a good thing? In Proceedings of the Annual Congress-South African Sugar Technologists’ Association, Durban, South Africa, 20–22 August 2019; pp. 1–16. [Google Scholar]
- Farirai, F.; Ozonoh, M.; Aniokete, T.C.; Eterigho-Ikelegbe, O.; Mupa, M.; Zeyi, B.; Daramola, M.O. Methods of extracting silica and silicon from agricultural waste ashes and application of the produced silicon in solar cells: A mini-review. Int. J. Sustain. Eng. 2021, 14, 57–78. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Energy recovery from secondary pulp/paper-mill sludge and sewage sludge with supercritical water treatment. Bioresour. Technol. 2010, 101, 2713–2721. [Google Scholar] [CrossRef]
- Basika, E.; Kigozi, J.; Kiggundu, N. Investigation of Sugar Cane Bagasse Ash as a Binding Material for the Construction Industry. J. Glob. Ecol. Environ. 2015, 2, 205–208. [Google Scholar]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Görgens, J.F. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Torres Agredo, J.; Mejía de Gutiérrez, R.; Escandón Giraldo, C.E.; González Salcedo, L.O. Characterization of sugar cane bagasse ash as supplementary material for Portland cement. Ing. Investig. 2014, 34, 5–10. [Google Scholar] [CrossRef]
- Sales, A.; Lima, S.A. Use of Brazilian sugarcane bagasse ash in concrete as sand replacement. Waste Manag. 2010, 30, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli, V.N.; Akasaki, J.L.; Melges, J.L.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Use of slag/sugar cane bagasse ash (SCBA) blends in the production of alkali-activated materials. Materials 2013, 6, 3108–3127. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, B.; Arbeláez, P.; Girshick, R.; Malik, J. Simultaneous detection and segmentation. In Proceedings of the European Conference on Computer Vision, Zurich, Switzerland, 6–12 September 2014; Springer: Cham, Switzerland; pp. 297–312. [Google Scholar]
- Modani, P.O.; Vyawahare, M.R. Utilization of bagasse ash as a partial replacement of fine aggregate in concrete. Procedia Eng. 2013, 51, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kawade, U.R.; Rathi, V.R.; Girge, V.D. Effect of use of Bagasse Ash on Strength of Concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2997–3000. [Google Scholar]
- El Hussein, M.; Hirst, S.; Salyers, V.; Osuji, J. Using grounded theory as a method of inquiry: Advantages and disadvantages. Qual. Rep. 2014, 19, 777–780. [Google Scholar] [CrossRef]
- Otoko, G.R.; Honest, B.K. Stabilization of Nigerian deltaic laterites with saw dust ash. Int. J. Sci. Res. Manag. 2014, 2, 1287–1292. [Google Scholar]
- Abdulkadir, T.S.; Oyejobi, D.O.; Lawal, A.A. Evaluation of sugarcane bagasse ash as a replacement for cement in concrete works. Acta Tech. Corviniensis-Bull. Eng. 2014, 7, 71. [Google Scholar]
- Abbasi, A.; Zargar, A. Using baggase ash in concrete as pozzolan. Middle East J. Sci. Res. 2013, 13, 716–719. [Google Scholar]
- Ahire, J.H.; Wang, Q.; Coxon, P.R.; Malhotra, G.; Brydson, R.; Chen, R.; Chao, Y. Highly luminescent and nontoxic amine-capped nanoparticles from porous silicon: Synthesis and their use in biomedical imaging. ACS Appl. Mater. Interfaces 2012, 4, 3285–3292. [Google Scholar] [CrossRef]
- Bley, R.A.; Kauzlarich, S.M. A low-temperature solution phase route for the synthesis of silicon nanoclusters. J. Am. Chem. Soc. 1996, 118, 12461–12462. [Google Scholar] [CrossRef]
- Brus, L. Luminescence of silicon materials: Chains, sheets, nanocrystals, nanowires, microcrystals, and porous silicon. J. Phys. Chem. 1994, 98, 3575–3581. [Google Scholar] [CrossRef]
- Plettl, A.; Enderle, F.; Saitner, M.; Manzke, A.; Pfahler, C.; Wiedemann, S.; Ziemann, P. Non-Close-Packed Crystals from Self-Assembled Polystyrene Spheres by Isotropic Plasma Etching: Adding Flexibility to Colloid Lithography. Adv. Funct. Mater. 2009, 19, 3279–3284. [Google Scholar] [CrossRef]
- Lam, C.; Zhang, Y.F.; Tang, Y.H.; Lee, C.S.; Bello, I.; Lee, S.T. Large-scale synthesis of ultrafine Si nanoparticles by ball milling. J. Cryst. Growth 2000, 220, 466–470. [Google Scholar] [CrossRef]
- Gorji, B.; Ghasri, M.A.; Fazaeli, R.; Niksirat, N. Synthesis and characterizations of silica nanoparticles by a new sol-gel method. J. Appl. Chem. Res. 2012, 6, 22–26. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Singh, S.P.; Endley, N. Fabrication of nano-silica from agricultural residue and their application. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–134. [Google Scholar]
- Thuc, C.N.H.; Thuc, H.H. Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar]
- Haile Asmelash, A. Synthesis and Characterization of Nanosilica-zinc Coatings with Silane Coupling Agents on Glass Substrates. Ph.D. Thesis, ASTU, College in Adama, Ethiopia, 2019. [Google Scholar]
- Aramaki, S.; Roy, R. Revised equilibrium diagram for the system Al2O3-SiO2. Nature 1959, 184, 631–632. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. Production and properties of flexible sodium silicate films from rice hull ash silica. Bioresour. Technol. 2000, 72, 99–106. [Google Scholar] [CrossRef]
- Iler, K.R. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Kalapathy, U.; Proctor, A.; Shultz, J. Silica xerogels from rice hull ash: Structure, density and mechanical strength as affected by gelation pH and silica concentration. J. Chem. Technol. Biotechnol. 2000, 75, 464–468. [Google Scholar] [CrossRef]
- Realmuto, L.; Hunting, K.L.; Parkin, R. State Health Agency Workforce Shortages and Implications for Public Health: A Case Study of Restaurant Inspections in Louisian. J. Environ. Health 2013, 76, 32–37. [Google Scholar]
- Zou, J.; Sanelle, P.; Pettigrew, K.A.; Kauzlarich, S.M. Size and spectroscopy of silicon nanoparticles prepared via reduction of SiCl4. J. Clust. Sci. 2006, 17, 565–578. [Google Scholar] [CrossRef]
- Koch, E.C.; Clement, D. Special materials in pyrotechnics: VI. Silicon—An old fuel with new perspectives. Propellants Explos. Pyrotech. 2007, 32, 205–212. [Google Scholar] [CrossRef]
- Nakajima, K.; Usami, N. (Eds.) Crystal Growth of SI for Solar Cells; Springer: Berlin/Heidelberg, Germany, 2009; Volume 97. [Google Scholar]
- Yermekova, Z.; Mansurov, Z.; Mukasyan, A.S. Combustion synthesis of silicon nanopowders. Int. J. Self-Propag. High-Temp. Synth. 2010, 19, 94–101. [Google Scholar] [CrossRef]
- Entwistle, J.; Rennie, A.; Patwardhan, S. A review of magnesiothermic reduction of silica to porous silicon for lithium-ion battery applications and beyond. J. Mater. Chem. A 2018, 6, 18344–18356. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Huang, X.; Zhao, Y.; Hu, X.; Cen, D.; Gao, G.; Bao, Z.; Mei, Y.; Di, Z.; Wu, G. Formation of Si hollow structures as promising anode materials through reduction of silica in AlCl3–NaCl molten salt. ACS Nano 2018, 12, 11481–11490. [Google Scholar] [CrossRef]
- Jing, C.A.I.; Luo, X.T.; Lu, C.H.; Haarberg, G.M.; Laurent, A.; Kongstein, O.E.; Wang, S.L. Purification of metallurgical grade silicon by electrorefining in molten salts. Transac. Nonferrous Metals Soc. China 2012, 22, 3103–3107. [Google Scholar]
- Coletti, G.; Bronsveld, P.C.; Hahn, G.; Warta, W.; Macdonald, D.; Ceccaroli, B.; Wambach, K.; Le Quang, N.; Fernandez, J.M. Impact of metal contamination in silicon solar cells. Adv. Funct. Mater. 2011, 21, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.J.; Paik, U.; Park, J.G. Effects of metallic contaminant type and concentration on photovoltaic performance degradation of p-type silicon solar cells. J. Korean Phys. Soc. 2013, 63, 47–52. [Google Scholar] [CrossRef]
- Morita, K.; Yoshikawa, T. Thermodynamic evaluation of new metallurgical refining processes for SOG-silicon production. Transac. Nonferrous Metals Soc. China 2011, 21, 685–690. [Google Scholar] [CrossRef]
- Delannoy, Y. Purification of silicon for photovoltaic applications. J. Cryst. Growth 2012, 360, 61–67. [Google Scholar] [CrossRef]
- Martorano, M.A.; Neto, J.F.; Oliveira, T.S.; Tsubaki, T.O. Refining of metallurgical silicon by directional solidification. Mater. Sci. Eng. B 2011, 176, 217–226. [Google Scholar] [CrossRef]
- Belomoin, G.; Therrien, J.; Smith, A.; Rao, S.; Twesten, R.; Chaieb, S.; Nayfeh, M.H.; Wagner, L.; Mitas, L. Observation of a magic discrete family of ultrabright Si nanoparticles. Appl. Phys. Lett. 2002, 80, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Won, C.W.; Nersisyan, H.H.; Won, H.I. Solar-grade silicon powder prepared by combining combustion synthesis with hydrometallurgy. Sol. Energy Mater. Sol. Cells 2011, 95, 745–750. [Google Scholar] [CrossRef]
- Su, H.J.; Zhang, J.; Lin, L.I.U.; Fu, H.Z. Preparation, microstructure and dislocation of solar-grade multicrystalline silicon by directional solidification from metallurgical-grade silicon. Transac. Nonferrous Metals Soc. China 2012, 22, 2548–2553. [Google Scholar] [CrossRef]
- Yasuda, K.; Saegusa, K.; Okabe, T.H. Production of solar-grade silicon by halidothermic reduction of silicon tetrachloride. Metall. Mater. Transac. B 2011, 42, 37–49. [Google Scholar] [CrossRef]
- Abdyukhanov, I.M.; Abdyukhanov, M.A.; Kuz’min, Y.A.; Merkushkin, V.M. Production of metallurgical silicon of enhanced quality for land-based solar cells. Metal Sci. Heat Treat. 2000, 42, 246–249. [Google Scholar] [CrossRef]
- He, Z.W.; Liu, X.Q.; Su, Q.; Wang, Y.Y. Improvement of electrical properties of low dielectric constant nanoporous silica films prepared using sol–gel method with catalyst HF. Appl. Phys. A 2006, 82, 349–355. [Google Scholar] [CrossRef]
- Su, Y.; Huang, J. Two consensus problems for discrete-time multi-agent systems with switching network topology. Automatica 2012, 48, 1988–1997. [Google Scholar] [CrossRef]

| Loss of Ignition (LOI) | Country | Authors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 62.1 | 5.42 | 5.54 | 1.0 | 1.12 | - | 2.22 | - | Uganda | (Basika, et al. 2015). [14] |
| 84.0 | 1.7 | 1.1 | 0.5 | 0.6 | - | 0.5 | 3.1 | South Africa | (Aboyade et al. 2011) [15] |
| 72.8 | 5.5 | 6.4 | 3.8 | 2.3 | - | 2.7 | 3.7 | Columbia | (Torres Agredo et al. 2014) [16] |
| 96.2 | 1.7 | 0.2 | 0.1 | 0.1 | 0.1 | 0.3 | 1.04 | Brazil | (Sales and Lima 2010) [17] |
| 31.41 | 6.02 | 7.57 | 16.06 | 1.07 | 0.78 | 1.58 | 32.2 | Brazil | (Castaldelli et al. 2013) [18] |
| 67.82 | 2.56 | 6.33 | 1.54 | 2.03 | - | 2.87 | 2.31 | Thirukovikur | (Hariharan et al. 2014) [19] |
| 87.59 | 0.67 | 0.57 | 2.59 | 1.65 | 0.003 | 3.64 | NA | India | (Modani and Vyawahare 2013) [20] |
| 62.43 | 6.98 | 4.28 | 11.8 | 2.51 | 1.48 | 3.53 | 4.73 | India | (Kawade, Rathi, and Girge 2013) [21] |
| 66.89 | 29.18 | 29.18 | 1.92 | 0.83 | 0.56 | NA | 0.72 | India | (Hussein et al. 2014) [22] |
| 41.15 | 2.70 | 7.00 | 3.20 | 0.12 | 0.03 | 8.75 | 17.7 | Nigeria | (Otoko 2014) [23] |
| 77.25 | 4.21 | 6.37 | 4.05 | 2.61 | 0.11 | 2.34 | 1.40 | Sudan | (Hussein et al. 2014) [24] |
| 72.85 | 6.96 | 1.08 | 9.97 | 6.49 | NA | 6.71 | 4.23 | Nigeria | (Abdulkadir, Oyejobi, and Lawal 2014) [25] |
| 44.70 | 2.90 | 2.40 | 14.9 | 3.50 | NA | 4.40 | 16.7 | Iran | (Abbasi and Zargar 2013) [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seroka, N.S.; Taziwa, R.T.; Khotseng, L. Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Appl. Sci. 2022, 12, 2310. https://doi.org/10.3390/app12052310
Seroka NS, Taziwa RT, Khotseng L. Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Applied Sciences. 2022; 12(5):2310. https://doi.org/10.3390/app12052310
Chicago/Turabian StyleSeroka, Ntalane Sello, Raymond T. Taziwa, and Lindiwe Khotseng. 2022. "Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review" Applied Sciences 12, no. 5: 2310. https://doi.org/10.3390/app12052310
APA StyleSeroka, N. S., Taziwa, R. T., & Khotseng, L. (2022). Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Applied Sciences, 12(5), 2310. https://doi.org/10.3390/app12052310