The Effects of Spray Volume on the Management of Bemisia tabaci (Hemiptera: Aleyrodidae) in the Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant
2.2. Insect
2.3. Insecticide
2.4. Experimental Design
2.5. Evaluation
2.6. Statistical Analyses
3. Results
3.1. Young Instars
3.2. Late Instars
3.3. All Instars Combined
3.4. Treatment Comparisons and Efficacy Scores
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osborne, L.S.; Oetting, R.D. Biological control of pests attacking greenhouse grown ornamentals. Fla. Entomol. 1989, 3, 408–413. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, National Agricultural Statistics Service. Census of Horticultural Specialties Potted Flowering Plants Sold for Indoor or Patio Uses Sold: Table 9. 2019. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/hortic_1_0009_0010.pdf (accessed on 27 December 2021).
- Byrne, D.N.; Bellows, T.S. Whitefly biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- Sani, I.; Ismail, S.I.; Abdullah, S.; Jalinas, J.; Jamian, S.; Saad, N. A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 2020, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Hoddle, M.S.; Van Driesche, R. Evaluation of Encarsia formosa (Hymenoptera: Aphelinidae) to control Bemisia argentifolii (Homoptera: Aleyrodidae) on poinsettia (Euphorbia pulcherrima): A lifetable analysis. Fla. Entomol. 1996, 79, 1–12. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Lyon, S. Commercial adoption of biological control-based IPM for whiteflies in poinsettia. Fla. Entomol. 2003, 86, 481–483. [Google Scholar] [CrossRef]
- Vafaie, E.K.; Pemberton, H.B.; Gu, M.; Kerns, D.; Eubanks, M.D.; Heinz, K.M. Using multiple natural enemies to management sweetpotato whiteflies (Hemiptera: Aleyrodidae) in commercial poinsettia (Malpighiales: Euphorbiaceae) production. J. Integr. Pest Manag. 2021, 12, 18. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Lyon, S.M.; Hoddle, M.S.; Roy, S.; Sanderson, J.P. Assessment of cost and performance of Eretmocerus eremicus (Hymenoptera: Aphelinidae) for whitefly (Homoptera: Aleyrodidae) control in commercial poinsettias crops. Fla. Entomol. 1999, 82, 570. [Google Scholar] [CrossRef]
- Vafaie, E.K.; Pemberton, H.; Gu, M.; Kerns, D.; Eubanks, M.D.; Heinz, K.M. Whitefly abundance on rooted poinsettia cuttings and finished poinsettias. HortTechnology 2020, 30, 486–491. [Google Scholar] [CrossRef]
- Hudson, W.; Joseph, S.V. Ornamentals: Commercial plant production insect control. In Georgia Pest Management Handbook; University of Georgia Extension: Athens, GA, USA, 2022; pp. 190–195. [Google Scholar]
- Gill, G.S.; Chong, J.H. Efficacy of selected insecticides as replacement for neonicotinoids in managing sweetpotato whitefly on poinsettia. HortTechnology 2021, 31, 745–752. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Getter, K.L.; Behe, B.K. Consumer preferences for traditional, neonicotinoid-free, bee-friendly, or biological control pest management practices on floriculture crops. HortScience 2015, 50, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Rihn, A.; Khachatryan, H. Does consumer awareness of neonicotinoid insecticides influence their preferences for plants? HortScience 2016, 51, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Getter, K.L.; Behe, B.K.; Wollaeger, H. Comparative consumer perspectives on eco-friendly and insect management practices on floriculture crops. HortTechnology 2016, 26, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Khachatryan, H.; Rihn, A. Consumer preferences for labels disclosing the use of neonicotinoid pesticides: Evidence from experimental auctions. J. Agr. Resour. Econ. 2020, 45, 496–517. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [Green Version]
- [IRAC] Insecticide Resistance Action Committee. The IRAC Mode of Action Classification Online. 2022. Available online: https://irac-online.org/modes-of-action/ (accessed on 9 January 2022).
- Palumbo, J.C.; Horowitz, A.R.; Prabhaker, N. Insecticidal control and resistance management for Bemisia tabaci. Crop Prot. 2001, 20, 739–765. [Google Scholar] [CrossRef]
- Gorman, K.; Devine, G.; Bennison, J.; Coussons, P.; Punchard, N.; Denholm, I. Report of resistance to the neonicotinoid insecticide imidacloprid in Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2007, 63, 555–558. [Google Scholar] [CrossRef]
- Brück, E.; Elbert, A.; Fischer, R.; Krueger, S.; Kühnhold, J.; Klueken, A.M.; Nauen, R.; Niebes, J.-F.; Reckmann, U.; Schnorbach, H.-J.; et al. Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: Biological profile and field performance. Crop Prot. 2009, 28, 838–844. [Google Scholar] [CrossRef]
- Schuster, D.J.; Mann, R.S.; Toapanta, M.; Cordero, R.; Thompson, S.; Cyman, S.; Morris, R.F. Monitoring neonicotinoid resistance in biotype B of Bemisia tabaci in Florida. Pest Manag. Sci. 2010, 66, 186–195. [Google Scholar] [CrossRef]
- Kontsedalov, S.; Gottlieb, Y.; Ishaaya, I.; Nauen, R.; Horowitz, R.; Ghanim, M. Toxicity of spiromesifen to the developmental stages of Bemisia tabaci biotype B. Pest Manag. Sci. 2009, 65, 5–13. [Google Scholar] [CrossRef]
- Buitenhuis, R.; Brownbridge, M.; Brommit, A.; Saito, T.; Murphy, G. How to start with a clean crop: Biopesticide dips reduce populations of Bemisia tabaci (Hemiptera: Aleyrodidae) on greenhouse poinsettia propagative cuttings. Insects 2016, 7, 48. [Google Scholar] [CrossRef]
- Dibble, J. Insecticide application and coverage. Calif. Agric. 1962, 16, 8–9. [Google Scholar]
- Martini, X.; Kincy, N.; Nansen, C. Quantitative impact assessment of spray coverage and pest behavior on contact pesticide performance. Pest Manag. Sci. 2012, 68, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, C.L.; Babcock, J.M.; Denholm, I.; Gorman, K.; Thomas, J.D.; Sparks, T.C. Cross resistance relationships of the sulfoximine insecticide sulfoxaflor with neonicotinoid and other insecticides in the whiteflies Bemisia tabaci and Trialeurodes vaporariorum. Pest Manag. Sci. 2012, 69, 809–813. [Google Scholar] [CrossRef]
- Joseph, S.V.; Bolda, M. Efficacy of insecticides against Lygus hesperus Knight (Hemiptera: Miridae) in the California’s Central Coast strawberry. Int. J. Fruit Sci. 2016, 16, 178–187. [Google Scholar] [CrossRef]
- Joseph, S.V.; Bolda, M. Evaluating the potential utility of an electrostatic sprayer and a tractor-mounted vacuum machine for Lygus hesperus (Hemiptera: Miridae) management in California’s coastal strawberry. Crop Prot. 2018, 113, 104–111. [Google Scholar] [CrossRef]
- SAS Institute. SAS, Version 9.4.; SAS Institute Inc.: Cary, NC, USA, 2012.
- Radosevich, D.L.; Cloyd, R.A. Spray volume and frequency impacts on insecticide efficacy against the citrus mealybug (Hemiptera: Pseudococcidae) on coleus under greenhouse conditions. J. Entomol. Sci. 2021, 56, 305–320. [Google Scholar] [CrossRef]
- Wang, G.; Lan, Y.; Qi, H.; Chen, P.; Hewitt, A.; Han, Y. Field evaluation of an unmanned aerial vehicle (UAV) sprayer: Effect of spray volume on deposition and the control of pests and disease in wheat. Pest Manag. Sci. 2019, 75, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Stansly, P.A.; Qureshi, J.A.; Kostyk, B.C. Effect of spray volume and sprayer type on efficacy of insecticides for control of Asian citrus psyllid and citrus leafminer on oranges, 2010. Arthropod Manag. Tests 2011, 36, D16. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.; Henneberry, T.J.; Toscano, N.C. Assessment of cross-resistance potential to neonicotinoid insecticides in Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 2005, 95, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.A.; Nagle, C.A.; MacVean, C.A.; McKenzie, C.L. Susceptibility of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) to imidacloprid, thiamethoxam, dinotefuran, and flupyradifurone in South Florida. Insects 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Braman, S.K.; Hanula, J.L. The range and response of neonicotinoids on hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae). J. Environ. Hort. 2011, 29, 197–204. [Google Scholar] [CrossRef]
- Joseph, S.V.; Grettenberger, I.; Godfrey, L. Insecticides applied to soil of transplant plugs for Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae) management in broccoli. Crop Prot. 2016, 87, 68–77. [Google Scholar] [CrossRef]
- Dripps, J.E.; Boucher, R.E.; Chloridis, A.; Cleveland, C.B.; DeAmicis, C.V.; Gomez, L.E.; Paroonagian, D.L.; Pavan, L.A.; Sparks, T.C.; Watson, G.B. The spinosyn insecticides. In Green Trends in Insect Control; Lopez, O., Fernandez-Bolanos, J.G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 163–212. [Google Scholar]
- Babcock, J.M.; Gerwick, C.B.; Huang, J.X.; Loso, M.R.; Nakamura, G.; Nolting, S.P.; Rogers, R.B.; Sparks, T.C.; Thomas, J.; Watson, G.B.; et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest. Manag. Sci. 2011, 67, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Whitefly Efficacy. Environmental Horticulture Program Research Project Sheet. The IR4 Project. 2021. Available online: https://www.ir4project.org/ehc/ehc-registration-support-research/env-hort-extension-resources/ (accessed on 1 January 2022).
- Xxpire Label. Corteva AgriScience, Indianapolis, Indiana, USA. 2022. Available online: https://www.corteva.us/products-and-solutions/turf-and-ornamental/xxpire.html (accessed on 1 January 2022).
- Nansen, C.; Ridsdill-Smith, J.T. The performance of insecticides—A critical review. In Insecticides—Development of Safer and More Effective Technologies; Trdan, S., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]

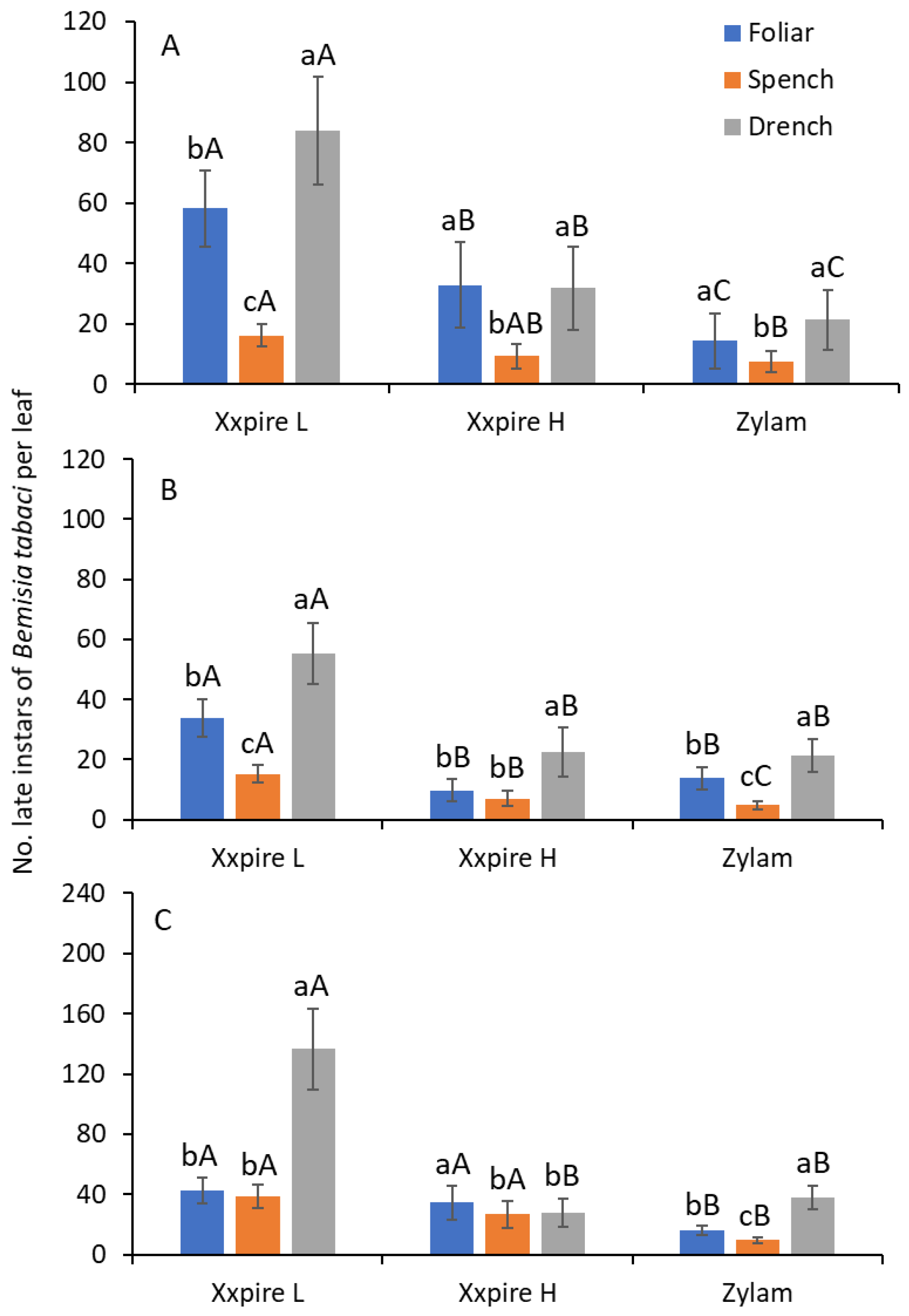
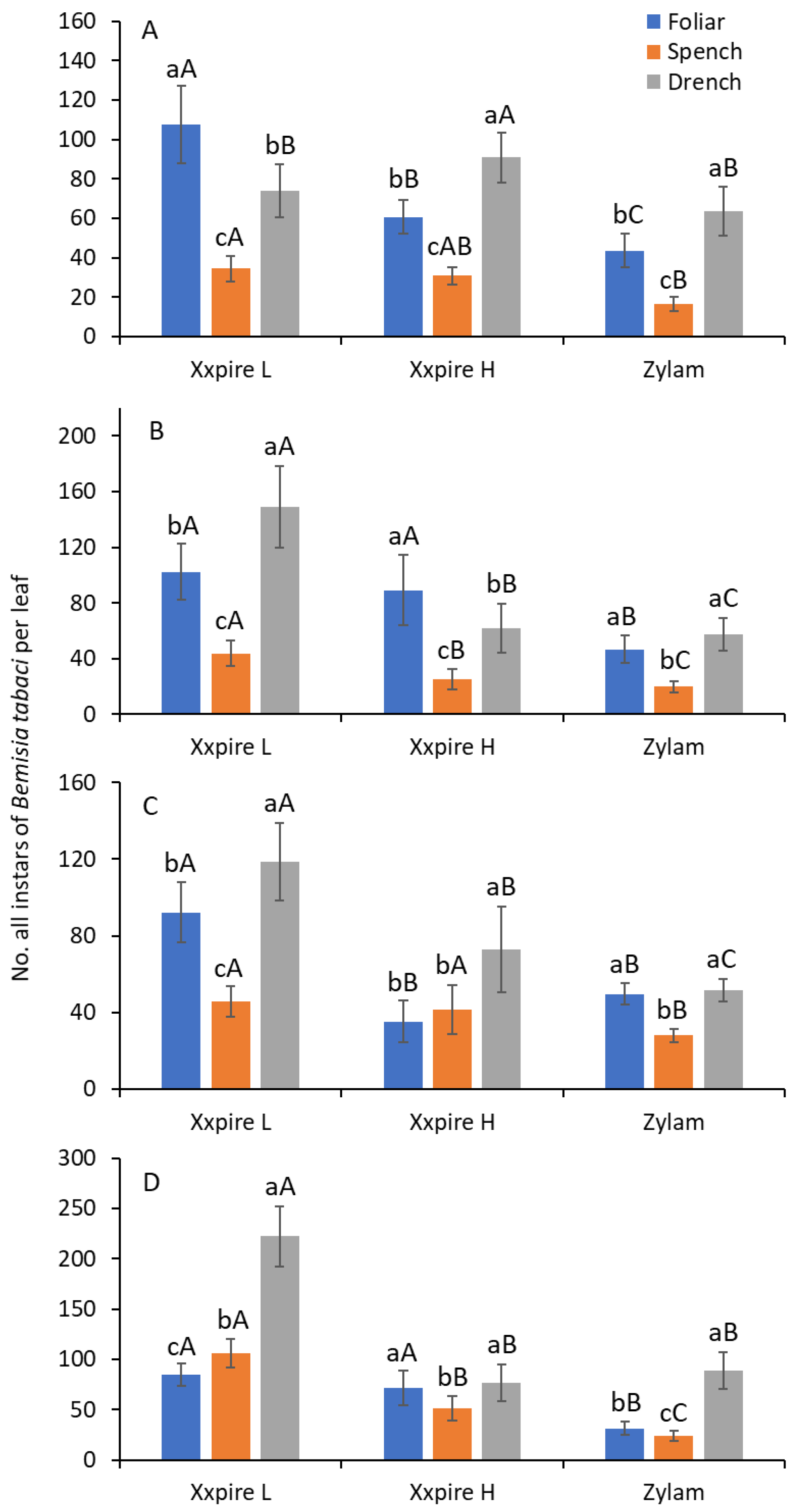

| After Application | Treatment | Young Instars * | Late Instars † | All Immature Stages | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | ||
| 7 DAA | Insecticide a | - | - | - | - | - | - | 86.7 | 2.56 | <0.001 |
| Method b | - | - | - | - | - | - | 293.0 | 2.56 | <0.001 | |
| Insecticide × Method | - | - | - | - | - | - | 35.4 | 4.56 | <0.001 | |
| 14 DAA | Insecticide | 133.7 | 2.56 | <0.001 | 95.1 | 2.56 | <0.001 | 240.4 | 2.56 | <0.001 |
| Method | 107.2 | 2.56 | <0.001 | 244.7 | 2.56 | <0.001 | 343.9 | 2.56 | <0.001 | |
| Insecticide × Method | 35.6 | 4.56 | <0.001 | 8.6 | 4.56 | <0.001 | 32.1 | 4.56 | <0.001 | |
| 21 DAA | Insecticide | 92.7 | 2.55 | <0.001 | 102.3 | 2.55 | <0.001 | 173.8 | 2.55 | <0.001 |
| Method | 39.7 | 2.55 | <0.001 | 157.5 | 2.55 | <0.001 | 164.3 | 2.55 | <0.001 | |
| Insecticide × Method | 26.1 | 4.55 | <0.001 | 3.8 | 4.55 | 0.008 | 27.2 | 4.55 | <0.001 | |
| 28 DAA | Insecticide | 172.6 | 2.55 | <0.001 | 232.0 | 2.55 | <0.001 | 416.4 | 2.55 | <0.001 |
| Method | 208.2 | 2.55 | <0.001 | 170.9 | 2.55 | <0.001 | 386.6 | 2.55 | <0.001 | |
| Insecticide × Method | 39.4 | 4.55 | <0.001 | 62.7 | 4.55 | <0.001 | 62.3 | 4.55 | <0.001 | |
| Treatment | Method | 7 DAA a | 14 DAA | 21 DAA | 28 DAA | ||||
|---|---|---|---|---|---|---|---|---|---|
| t | p | F | p | F | p | F | p | ||
| XXpire low | Foliar | −1.1 | 0.279 | −0.5 | 0.867 | 0.0 | 0.991 | 1.2 | 0.263 |
| Spench | 1.1 | 0.274 | 1.7 | 0.122 | 2.0 | 0.064 | 0.6 | 0.547 | |
| Drench | −0.5 | 0.595 | −1.2 | 0.259 | −0.9 | 0.382 | −1.5 * | 0.166 | |
| XXpire high | Foliar | 0.1 | 0.942 | −0.3 | 0.802 | 2.2 | 0.042 | 1.1 | 0.301 |
| Spench | 1.3 | 0.223 | 2.4 | 0.032 | 1.7 | 0.117 | 1.6 | 0.130 | |
| Drench | −0.8 | 0.453 | 0.8 | 0.444 | 0.2 | 0.848 | 1.1 | 0.308 | |
| Zylam | Foliar | 0.7 | 0.503 | 1.7 | 0.112 | 1.9 | 0.077 | 2.4 | 0.029 |
| Spench | 2.3 | 0.038 | 3.2 | 0.007 | 3.1 | 0.009 | 2.7 | 0.018 | |
| Drench | −0.2 | 0.879 | 1.2 | 0.239 | 1.9 | 0.073 | 0.7 | 0.521 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, S.V. The Effects of Spray Volume on the Management of Bemisia tabaci (Hemiptera: Aleyrodidae) in the Greenhouse. Appl. Sci. 2022, 12, 2178. https://doi.org/10.3390/app12042178
Joseph SV. The Effects of Spray Volume on the Management of Bemisia tabaci (Hemiptera: Aleyrodidae) in the Greenhouse. Applied Sciences. 2022; 12(4):2178. https://doi.org/10.3390/app12042178
Chicago/Turabian StyleJoseph, Shimat V. 2022. "The Effects of Spray Volume on the Management of Bemisia tabaci (Hemiptera: Aleyrodidae) in the Greenhouse" Applied Sciences 12, no. 4: 2178. https://doi.org/10.3390/app12042178
APA StyleJoseph, S. V. (2022). The Effects of Spray Volume on the Management of Bemisia tabaci (Hemiptera: Aleyrodidae) in the Greenhouse. Applied Sciences, 12(4), 2178. https://doi.org/10.3390/app12042178






