Abstract
Gamma-decalactone (GDL) is a fragrance compound obtained in the process of β-oxidation of ricinoleic acid, which is derived from the hydrolysis of castor oil. The biotechnological method of the synthesis of this lactone has been improved for over two decades, but the vast majority of research results have been based only on determining the concentration of the lactone by chromatographic methods without separating it from the biotransformation medium. In this study, we attempted to separate GDL from the medium in which the lactone was synthesized by Yarrowia lipolytica from castor oil. The effectiveness of liquid–liquid extraction, hydrodistillation, and adsorption on the porous materials (zeolite, vermiculite and resin Amberlite XAD-4) was compared. The influence of the solvent on the efficiency of GDL extraction, the influence of the acidity of the medium on the amount of GDL in the distillate, and the level of lactone adsorption on the above-mentioned adsorbents were compared by calculating the initial adsorption rate. The adsorption isotherm was determined for the most effective adsorbent. Among the five solvents tested, the most effective was diethyl ether, used at the ratio of 1:1. The extraction was characterized by higher efficiency than hydrodistillation; the difference in GDL determinations by these two methods ranged from 12.8 to 22%. The purity of the distillates was much higher than that of the extracts at 88.0 ± 3.4% compared to 53.0 ± 1.8%. The acidification of the biotransformation medium increased the concentration of the lactone in both the reaction mixture and the distillate. GDL was most efficiently adsorbed on Amberlite XAD-4 resin, for which the lactone isotherm adsorption was linear. The amount of lactone adsorbed on Amberlite XAD-4 within 1 h was approx. 80% (2.45 g), of which 1.96 g was then desorbed with ethanol. In the context of industrial applications, adsorption of GDL on Amberlite XAD-4 seems to be the most appropriate method due to material costs, the ease of the process, and low environmental burden.
1. Introduction
Lactones are one of the most important groups of substances that shape the sensory properties of food and cosmetic products. They exhibit a pleasant, fruity-floral scent and are a group of compounds that are often isolated from the natural environment. However, the possibilities of using industrial extraction from natural sources are limited, as the sensory-relevant plant components are often present in low concentration, and therefore, isolating and obtaining them in sufficiently large amounts is usually expensive. The technically troublesome and economically unprofitable isolation of cyclic esters from the natural environment, with the simultaneous willingness to meet consumer trends for a healthy lifestyle and interest in what is natural, contributed to the dissemination of the research on the biotechnological method of synthesizing these compounds [1,2].
In the last two decades, a particularly great interest has been noted in the biotechnological synthesis of gamma-decalactone (GDL), a compound with a characteristic peach fragrance used in the production of confectionery, cakes, wafers, chewing gums, and granulated and powder beverages [3]. The research conducted by the scientists from France (Waché, Dufossé, Blin-Perrin) [4,5,6], Belgium (Alchihab, Destain) [7,8], Portugal (Aguedo, Gomes, Braga) [9,10], and Brazil (Soares, Pereira de Andrade) [11,12] has provided extensive knowledge on the biotransformation of fatty acids to gamma-decalactone, based on β-oxidation, which takes place in yeast cell peroxisomes. Particular attention was paid to the β-oxidation process of ricinoleic acid [(R)-12-hydroxy-9-octadecanoic acid], which is the main component (80–90%) of castor oil obtained from castor bean seeds (Ricinus communis). The conversion of this acid into GDL involves cleavage from the carboxy terminus of the eight-carbon fragment as a result of four β-oxidation cycles. From the 18-carbon chain, the final 10-carbon compound (4-hydroxydecanoic acid) is obtained, which is then cyclized in an acidic environment to gamma-decalactone [13].
Many microorganisms show the capability of the biosynthesis of GDL (Candida, Pichia, Sporobolus, Rhodotorula, Yarrowia) and are highly active in the process of β-oxidation and reduction of the unsaturated bond in ricinoleic acid (gamma-decalactone precursor) [6,7,13,14,15,16]. Among them, Yarrowia lipolytica yeast is characterized by the highest production efficiency, especially when castor oil is used as a substrate in the reactions. This is due to the high extracellular lipolytic activity of this yeast induced by hydrophobic substrates [17,18].
The numerous studies conducted so far on the biotechnological synthesis of GDL have mainly focused on the improvement of reaction efficiency through the selection and optimization of individual process parameters, as well as the selection of the appropriate strain, which is often subject to additional genetic modifications [12,19,20,21,22]. However, these studies have been mostly carried out on a small scale, and the reaction efficiency, at the level of a few grams of lactone per liter of the culture medium, has been estimated on the basis of chromatographic or spectrophotometric determinations without separating a product from the mixture. The most frequently used analytical methods include GC [19,22,23,24], GC-MS [7,9,25,26], solid-phase microextraction in combination with GC-MS (SPEME-GC-MS) [27], and less often, a spectrophotometric method based on the ferric hydroxamate reaction [28].
So far, only a few research papers on the possibility of separating GDL from the culture medium have been published. Generally, the recovery of products in the bioprocesses that involve microorganisms is a difficult stage, especially for fragrances, due to their volatility and low solubility as well as the complexity of the biotransformation medium itself. Liquid–liquid extraction (LLE), adsorption, or pervaporation are most often used to extract fragrances [29,30,31]. So far, attempts to recover GDL from biotransformation media have been carried out with the use of activated carbon Acticarbone NC35 [32,33] and porous resins, e.g., Porapak Q, Chromosorb 105, SM4 Tenax-TA, Amberlite XAD-2 and XAD-4, and Macronet (MN-202, MN-102 and MN-100) [30,32,33,34].
In this study, we conducted research to verify the possibility of secreting GDL separation from biotransformation media, in which a lactone was synthesized from castor oil via Yarrowia lipolytica KKP379 yeast. The Y. lipolytica KKP379 yeast strain was selected for the study due to our experience in working with this strain and knowledge of some optimal conditions for its efficient biosynthesis of gamma-decalactone [35]. The level of GDL separation efficiency was compared with the use of traditional liquid–liquid extraction, hydrodistillation, and adsorption.
2. Materials and Methods
2.1. Microorganisms and Reagents
Yarrowia lipolytica KKP379, used for biosynthesis of GDL, was obtained from the Collection of Industrial Microorganisms, Prof. Wacław Dąbrowski Institute of Agricultural and Food Biotechnology, State Research Institute (Warsaw, Poland). The culture media components, yeast extract, peptone, glucose, and agar, were obtained from BTL Sp. Z o.o. (Łódź, Poland). The castor oil was from Carl Roth (Karlsruhe, Germany). The Tween 80 came from Acros Organics (Geel, Belgium). Diethyl ether, ethyl alcohol 96%, hexane, heptane, dichloromethane, chloroform, magnesium sulfate, and hydrochloric acid were provided by Avantor Performance Materials (Gliwice, Poland). Gamma-decalactone >98% and gamma-undecalactone 98% were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Amberlite XAD-4, zeolite, and vermiculite were from Merck (Darmstadt, Germany). The general characteristics of the adsorbents, in accordance with the manufacturer’s declarations, are presented in Table 1.

Table 1.
Characteristics of the adsorbents.
2.2. Cultivation of Microorganisms
Yarrowia lipolytica KKP379 was multiplied for 48 h on petri dishes with YPGA medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, 20 g/L agar) at 27 °C and inoculated to a 250 mL Erlenmeyer flask containing 50 mL of YPG medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose). Flasks were shaken at 140 rpm and 27 °C for 24 h, until the cultures reached the late-logarithmic growth phase. This preculture was used to inoculate the biotransformation medium.
2.3. Biotransformation of Castor Oil into Gamma-Decalactone
One milliliter of the preculture was transferred into 500 mL Erlenmeyer flasks with biotransformation medium (20 g/L peptone, 100 g/L castor oil, 5 g/L Tween 80). The final biotransformation medium volume was 100 mL and the initial concentration of yeast cells was about OD600 ≈ 0.25. The biotransformation was conducted at 27 °C and 140 rpm for 7 days. After finishing the reaction, to stop metabolism of yeast and to achieve the complete lactonization of 4-hydroxydecanoic acid, 1 M HCl was added to the samples. Before the separation of GDL, the pH of the mixture was adjusted to approximately 3.
2.4. Liquid–Liquid Extraction
Extraction in the liquid–liquid system was carried out using model water solutions and biotransformation mediums. The 100 mL of model solutions contained 0.3 g of GDL and 10 g of castor oil (the composition was similar to the biotransformation mediums). Solutions were emulsified before extraction using an Ultra Turrax T25 homogenizer (IKA, Staufen im Breisgau, Germany), and homogenized for 5 min at 15,000 rpm. The liquid–liquid extraction method was adapted from the methodology of Groguenin et al. [24]. For extraction, 1.5 mL or 3 mL of model solutions or biotransformation mediums were taken. They were then added to 20 µL of internal standard (gamma-undecalactone) and extracted with 1.5 mL of the either diethyl ether, hexane, heptane, chloroform, or dichloromethane as the solvent. The organic layer was separated and dehydrated by anhydrous MgSO4. Finally, 1 µL of aliquots was injected into GC system.
2.5. Hydrodistillation
GDL was separated by hydrodistillation from a 100 mL sample of the biotransformation mediums. Yeast cells were centrifuged (speed of 8000 rpm for 5 min, Centrifuge MPW-223) from the medium prior to distillation. To improve the distillation process, 20 mL of distilled water was added to the samples. One hundred milliliters of distillate was collected, which was then subjected to liquid–liquid extraction (1:1, v:v) using diethyl ether and determined by gas chromatography.
2.6. Adsorption of Gamma-Decalactone
Absorption of GDL from biotransformation mediums was carried out with the aid of three different adsorbents: zeolite, Amberlite XAD-4, and vermiculite. Prior to use, the adsorbents were cleaned for 24 h with ethyl alcohol. Sequentially, the washed adsorbents were dried for 8 h at 80 °C to eliminate alcohol residues.
The adsorption process was carried out at a temperature of 25 °C. The adsorbents were added to the biotransformation mediums at a concentration of 30 g/L. The mediums with adsorbents were stirred at 140 rpm for 6 h, and every hour, the samples were taken for chromatographic analysis.
2.7. Recovery of Gamma-Decalactone from the Adsorbents
After the specified adsorption time, the adsorbents were separated from the culture broth by filtration on the Büchner funnel. Porous materials were washed three times with distilled water and dried at room temperature for 2 h. GDL was extracted from the adsorbents with ethyl alcohol at a ratio of 3 mL of ethanol par 1 g of adsorbent, using 5 extraction cycles. For quantification, the ethanolic fractions were collected and dried by anhydrous magnesium sulfate, and gamma-undecalactone was added as an internal standard. The ethanolic extract was analyzed by gas chromatography.
2.8. Quantification of Gamma-Decalactone
The content of GDL was analyzed using a gas chromatography instrument (YL 6100 Young Lin Instrument) equipped with a capillary column BPX (30 m × 0.25 mm) and a flame ionization detector. The N2 was the carrier gas, and flow rate was 1.1 mL/min. The analysis conditions were as follows: a 1 µL sample was injected at 250 °C, the detector temperature was 280 °C, and the oven temperature was maintained at 165 °C for 1 min, increased to 180 °C at a rate of 3 °C/min, raised to 220 °C at a rate of 5 °C/min and held constant for 2 min.
The identification of other lactones in the broth and the purity of the samples were determined using a GC-MS system (Varian 3400 GC with a CPWAX-52-CB column, coupled to Varian Saturn II MS). The conditions of mass spectroscopy were as follows: electron ionization source was set to 80 eV, the emission current was 35.5 μA, mass spectrometry (MS) Quad was 160 °C, and the MS source was 220 °C.
2.9. Statistical Analysis
Statistical analysis was performed using Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA). The results were considered statistically different at α = 0.05. The data were expressed as mean ± relative standard deviation (±RSD).
3. Results
In the studies on the effectiveness of GDL secretion, model media were used which were similar in composition to the biotransformation or microbiological media with the addition of 10 g/dm3 of castor oil, from which the lactone was synthesized by Yarrowia lipolytica yeast by β-oxidation. The effectiveness of liquid–liquid extraction (using a few selected solvents), hydrodistillation, and adsorption on natural materials and polymer resins was analyzed.
3.1. Recovery of Gamma-Decalactone by Liquid–Liquid Extraction
Solvent extraction is a very simple and rapid method of isolating fragrance compounds. Research on the separation of GDL began with analyzing the influence of the type of solvent used at the level of its recovery. For this purpose, model solutions were used with a composition that reflected the microbiological media for lactone synthesis. The model solutions contained GDL at the concentration of 3 g/L and castor oil. The concentration of the ingredients was selected based on the previous research on GDL synthesis, in which, an average of about 3 g/L of lactone was obtained from 10 g/L of castor oil by biotransformation reactions with the participation of Yarrowia lipolytica yeast [35].
In this study, five different organic solvents with different polarities were compared in order to obtain the best recovery of GDL. Table 2 presents a comparison of the results of the recovery test with two different solvent-to-sample ratios.

Table 2.
Gamma-decalactone recovery degree from model solutions by different solvents.
The data indicate that diethyl ether is one of the best solvents for GDL extraction. The recovery with its participation reached almost 100% (99.9 ± 1.8%). Slightly lower or less accurate (burdened with a bigger error) extraction efficiency was obtained with the use of chloroform or dichloromethane (with a recovery of 98.8 ± 1.3% and 101.1 ± 2.5%, respectively). The use of a larger volume of organic solvent for lactone extraction clearly improved the level of its recovery, regardless of the solvent used. With a 1:1 (v:v) solvent-to-sample ratio, 0.1% (for diethyl ether) to 13% (for heptane) of the GDL was not extracted. For a 1:2 (v:v) solvent-to-sample extraction ratio, 10.6% (for dichloromethane) to 27.9% (for heptane) of the GDL remained in the solution. The RSD values were less than 5% in all the experiments, with higher deviations for the 1:2 (v:v) extraction, regardless of the type of solvent. The extraction method that used equal amounts of solvent and sample for diethyl ether, chloroform, and dichloromethane showed good recoveries and high repeatability.
However, the problem with using this technique is the purity of the extracted compound. In the extracts, the GDL purity was only about 51.5 ± 1.2%–53.0 ± 1.8% (the results obtained using GC-MS analyses). The samples were contaminated mainly with ricinoleic acid derived from the hydrolysis of castor oil as well as with triglycerides of the unreacted substrate.
3.2. Recovery of Gamma-Decalactone from Biotransformation Media by Hydrodistillation
Attempts to separate GDL from biotransformation media were also carried out via hydrodistillation (HD). Castor oil biotransformation reactions (10 g/L) were performed with Yarrowia yeast for eight days. Each day the reaction mixture from the flask was distilled, and the GDL concentration in the distillate was compared with the values obtained from the direct liquid–liquid extraction with diethyl ether. The data presented in Figure 1 show that the use of hydrodistillation allowed the separation of GDL from the media, regardless of the level of the substrate conversion, and the use of this method is associated with a partial loss of the lactone. The concentration of the compound in the distillates on each sampling day was lower than in extracts, and the difference in determinations with the lactone content above 0.5 g/L ranged from 12.8% to 22% (from 0.25 g/L to 0.63 g/L). In the range of lower lactone concentrations, the differences in the distillates compared to the extracts were as high as 73%. However, this may be due to an error of sample determination as it is difficult to separate the lactone effectively at its low concentrations. However, taking into account the purity of the product in the distillate (about 88% ± 3.4%), this process seems to be more advantageous than liquid–liquid extraction.
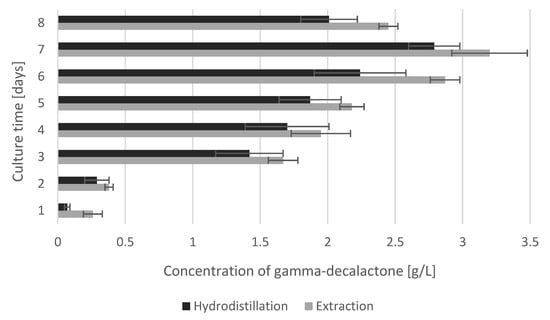
Figure 1.
Comparison of gamma-decalactone concentration in biotransformation medium by extraction and hydrodistillation methods.
While conducting research on GDL hydrodistillation, the influence of the pH of the reaction mixture on the level of GDL concentration in the distillate was analyzed. On the seventh day of cultivation, when the lactone concentration was at its maximum (Figure 1) or close to it, the medium was acidified or alkalized to the pH at the levels of 1, 3, 5, or 7, and then the GDL was separated with the usage of hydrodistillation and determined chromatographically. The results of GDL concentration in the media before and after pH modifications as well as in the distillate and distillation residue are summarized in Table 3.

Table 3.
Influence of acidity on gamma-decalactone levels in biotransformation medium and distillate.
The presented data clearly show that the pH of the medium has a significant influence on the concentration of GDL in the biotransformation medium, and thus, finally, in the distillate. With the decrease in the pH (acidification of the medium), the concentration of the lactone in the post-reaction mixture increased. The highest content of the lactone was recorded at the pH of 3 or 1. Acidification contributed to an increase in the concentration of the GDL in the extracted samples by approximately 33.3% and 8.8% (from 2.76 ± 0.09 to 3.68 ± 0.26 for pH 3, and from 2.97 ± 0.21 to 3.25 ± 0.15 for pH = 1). This is due to the spontaneous cyclization of 4-hydroxydecanoic acid into the neutral lactone form. However, this reaction is not total and some of the acid is not transformed into GDL.
Alkalization of the medium to pH 7, in relation to the natural acidity at the pH level of approximately 5.7, gave the opposite result. The concentration of the lactone in the medium, and thus, in the distillate, decreased by about 36% on average. With an almost identical concentration of the fragrance compound in the microbiological media after 7 days of the reaction, the differences in the concentration of the lactone between the media with pH 3 and 7 were close to 2 g/L. Therefore, the acidification of the post-reaction mixture makes sense in the context of the higher GDL concentration in the distillate. The efficiency of the distillation process, regardless of the acidity of the solutions, was at the level of 70–80%. After distillation, part of the GDL remained in the flask, which is a result of good solubility of this compound in the hydrophobic fraction (unreacted castor oil fraction).
3.3. Adsorption of Gamma-Decalactone on Porous Materials
The possibility of using adsorption was also analyzed in the research on GDL secretion. For this purpose, one of the selected adsorbents, Amberlite XAD-4, zeolite, or vermiculite, at the concentration of 30 g/L, was introduced into the biotransformation media after the finished reaction and separated from the yeast cells.
The GDL adsorption kinetics were analyzed within 6 h of the introduction of the adsorbent by monitoring the decrease in GDL concentration in the solutions (Figure 2). The quantity of the GDL adsorbed corresponded to the difference between the initial concentration (measured immediately after the finished biotransformation reaction) and the final concentration (after the adsorption).
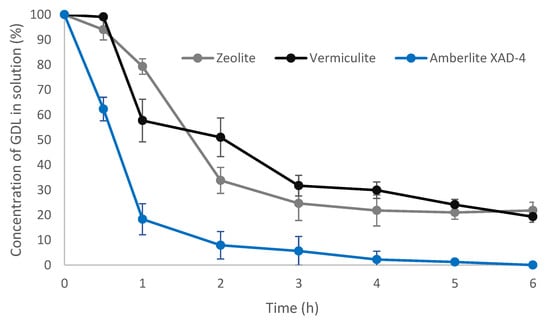
Figure 2.
Kinetics of gamma-decalactone adsorption from biotransformation medium on three selected adsorbents: zeolite, vermiculite, and Amberlite XAD-4.
The data presented in Figure 2 show that at a concentration of GDL in the biotransformation media of about 3 g/L, the GDL was most effectively adsorbed on Amberlite XAD-4. After 1 h, the amount of the compound adsorbed was over 80%, which was approximately 2.45 g. In the case of vermiculite, after the same time, approximately 43% of the compound was absorbed, i.e., nearly 2 times less. The level of adsorption was the lowest with zeolite; after 1 h, there was still about 80% of the unadsorbed peach lactone in the medium. However, regardless of the type of the adsorbent used, the equilibrium state was reached after approximately 3 h of contact. At equilibrium, the adsorption capacities of zeolite and vermiculite were similar at about 70–80%, while for Amberlite XAD-4 it was over 95%.
In order to obtain a more complete picture of the adsorption kinetics from biotransformation media, the initial adsorption rate (based on the first hour of the process) was determined for each of the adsorbents used (Table 4). The data confirm that in the first hour of the process, the highest adsorption rate, at the level of 1.47 × 10−3 g GDL/(adsorbent (g) × minute), is shown by Amberlite XAD-4. This value is twice as high as compared to vermiculite and more than four times as high as compared to zeolite.

Table 4.
Initial adsorption rate of gamma-decalactone on the zeolite, vermiculite, and Amberlite XAD-4 after 1 h.
As the Amberlite XAD-4 resin showed the highest GDL adsorption efficiency, an adsorption isotherm was determined for it, through which the aroma compound separation was fully characterized (Figure 3). The equilibrium concentration of GDL was measured in the range of 0.1—5 g GDL/L (concentration range so far achieved in a microbial culture, depending on the initial concentration of castor oil).
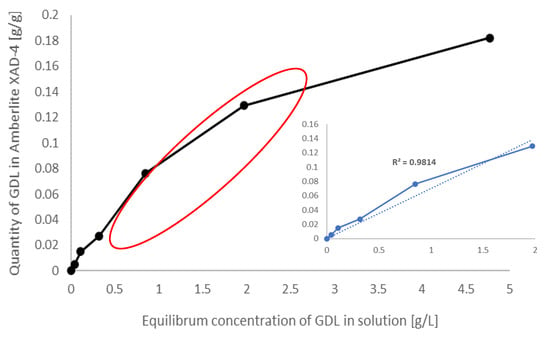
Figure 3.
Adsorption isotherm of gamma-decalactone on the Amberlite XAD-4.
The curve shown in Figure 3 is a type 1 isotherm with a visible and achievable level of saturation, characteristic for sorbents in which sorption is limited by the steric effect to one or two molecular layers. Lower lactone concentrations in the medium are up to 4 g/L on average (preadsorption concentration). The adsorption isotherm has a linear relationship R2 = 0.9814 (points marked with a red line, highlighted in the additional image).
Considering the possibility of using adsorption in the separation of GDL from biotransformation media, the effectiveness of removing the adsorbed lactone was also analyzed. The desorption was carried out in 5 cycles with ethyl alcohol, using the ratio of 3 mL of ethanol/g of adsorbent. Each of the adsorbents showed good desorption abilities. Figure 4 shows the amount of the desorbed GDL in 5 extraction cycles, taking into account the efficiency of each stage. The most effective desorption was with the Amberlite XAD-4 resin; about 81% of the lactone was desorbed. In the case of zeolite and vermiculite, the level of desorption was comparable and amounted to 75–76%. It is noteworthy that the ricinoleic acid was extracted from the adsorbents together with the desorbed lactone. Its concentration in the extracts after desorption ranged from 2.03 ± 0.44 g/L (in the first desorption cycle) to about 0.22 ± 0.12 g/L (in the fourth or fifth desorption cycles). With each subsequent cycle, the concentration of the desorbed lactone and ricinoleic acid decreased.
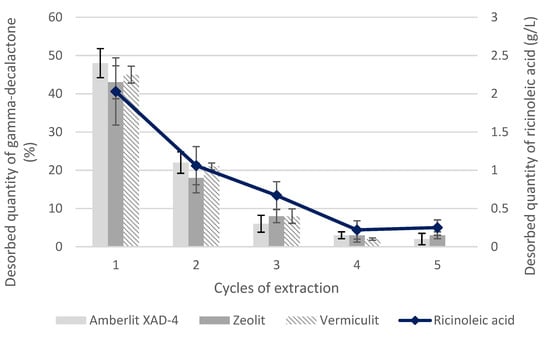
Figure 4.
Desorption of the gamma-decalactone from adsorbents (zeolite, vermiculite, and Amberlite XAD-4) using five cycles of extraction with ethyl alcohol.
4. Discussion
Despite the large amount of literature in the field of the optimization of GDL biosynthesis, there are no data on the separation and purification of this compound from the post-reaction mixtures. The published results are based only on the chromatographic determinations of the samples taken directly from individual culture stages. Considering the fact that the efficiency of the extraction, purification, and isolation of this compound is a key stage which generates significant costs in relation to the entire biotechnological cycle and is decisive for the application of the methods of biotechnological synthesis of GDL in industry, research was undertaken on how to effectively isolate this compound from reaction mixtures. Our focus was on extraction, hydrodistillation, and lactone adsorption.
Liquid–liquid extraction is the most mature and commonly used method of separating fragrances compounds. Solvents commonly used in the extraction are dichloromethane, chloroform, ethyl acetate, diethyl ether, and n-hexane [36,37]. They were also used (except for ethyl acetate) in GDL extraction. The best efficiency obtained in this study with the use of diethyl ether (99.9%) justifies its widespread use in the extraction of lactones from microbiological media [19,24,25,26]. The similar efficiency and reproducibility of GDL extraction for dichloromethane and chloroform allow the conclusion that due to difficulties in extraction on a larger scale with diethyl ether (due to its high volatility and tendency to form explosive peroxides), it is possible to replace it with these solvents. The effectiveness of dichloromethane and chloroform is also confirmed in the literature. In the research on quantitative determination of N-acylhomoserine lactones, conducted by the team of Morin et al. [38], the best extraction results with almost the same yields were obtained for the above-mentioned solvents. Canbay [37], conducting liquid–liquid extraction of the volatile compounds from rose aromatic water, obtained complete extraction for chloroform, dichloromethane, and ethyl acetate. n-Hexane showed the worst extraction parameters, similar to our GDL extraction study. The effectiveness of dichloromethane in the extraction of terpenoids from aqueous solutions was also confirmed by the team of Agarwal et al. [39].
The role of the volume of the solvent used in the extraction is highlighted by Obi et al. [40], who conducted research on determination of lactones in milk fat. The authors conducted their study with the use of 0.5 to 1.5 mL of the solvent per 0.5 g of the extracted sample. The results showed that the use of a large amount of an organic solvent for the extraction of lactone clearly improves the recovery rate. This conclusion is consistent with the data obtained in this study, where solvent-to-sample ratios of 1:2 or 1:1 (v:v) were used for GDL extraction. The difference in the extraction efficiency for the solvents used in these two volume variants was from 9 to 17%. Obi et al. [40] showed that with a small amount of an organic solvent relative to the sample, long-chain lactones (above C6) are captured by triacylglycerols that are present in the samples due to their hydrophobic nature. When a larger volume of solvent is used for extraction, the lactones are not affected by the triacylglycerol matrix effect. Since the biotechnological synthesis of GDL uses an oil-in-water emulsion base (addition of 10 g/L castor oil as a precursor to GDL), it is highly probable that the lactone partially dissolves in the triglycerides of the unreacted oil. This may also explain the poorer reproducibility of the extraction results for the samples extracted with a 1:2 solvent-to-sample ratio (higher standard deviations).
Although the efficiency of the performed GDL extraction is high, it is necessary to point out a disadvantage of using organic solvents in the media with the addition of lipids. In the target biotransformation media, the precursor of GDL synthesized by microorganisms is castor oil or ricinoleic acid. These are introduced into the media in excess, as they are also sources of carbon for the growth and maintenance of the vital functions of microorganisms. Not fully hydrolyzed castor oil and the ricinoleic acid not used in the β-oxidation cycle are extracted together with GDL, contaminating it significantly (unpublished data). Hence, the extraction technique itself is not sufficient and should be supported, e.g., with chromatography or fractional distillation. However, this technique is not always effective due to the small differences in the boiling points of individual compounds.
Considering the significant contamination of GDL with the lipid fraction, which is extracted regardless of the solvent used, attempts were made in further studies to separate the lactone by hydrodistillation. In the extensive literature on the subject of GDL biosynthesis, only one publication was found in which Alchihab et al. [7] used the hydrodistillation method to isolate GDL. Hydrodistillation is a traditional technique to recover essential oils and bioactive compounds from plants [41]. Despite its disadvantages, including long process time and low efficiency as well as partial degradation of unsaturated compounds due to thermal effects, it is widely used [41,42,43]. The use of hydrodistillation in this research to separate GDL from biotransformation media allowed for its recovery, and the efficiency of this technique was approximately 12–22% lower than in liquid–liquid extraction. It is assumed that the lower concentration of lactone in distillation may be partly due to its loss as a result of long-term heating of samples and possible hydrolysis of this compound [44]. Based on the data from Figure 1, it can be concluded that at the concentration of GDL in the biotransformation medium below 0.5 g/L, distillation exhibits low effectiveness. However, it should be emphasized that the purity of the distillates was higher than that of the extracts. In the distillates, apart from GDL, 3-hydroxy-γ-decalactone, dec-2-en-olide, and dec-3-en-olide were identified. These compounds had been previously detected during the fermentation process that was conducted by the yeast Yarrowia lipolytica W29 on solid media, as mentioned by Try et al. [25].
Since the microbial synthesis of GDL is based on the transformation in the β-oxidation cycle of ricinoleic acid to 4-hydroxydecanoic acid, which subsequently cyclizes to GDL, it was justified to analyze the influence of the reaction environment on the level of acid cyclization, and hence, the final concentration of GDL in the distillate. Acidity turned out to be an important factor that partially determined the concentration of GDL in the distillate. Increasing the acidity of the reaction mixture from the natural pH of about 5.68 to the pH of 3 or 1 resulted in an increase in the concentration of the lactone in the biotechnological medium, ranging from 0.25 g to 0.75 g/L (percentage range 8–33%; Table 3). The results of Feron et al. [45] confirm this research. The team carried out the bioconversion of methyl ricinoleate to gamma-decalactone using the yeast Sporidiobolus ruinenii and observed that acidification of the reaction medium before the extraction process increases the concentration of lactone in the aqueous phase. By changing the acidity of the post-reaction mixture from pH 6 to pH 2, an over 2.5-fold increase in the GDL concentration was observed. Romero-Guido et al. [46] also emphasizes that lactonization of 4-hydroxydecanoic acid occurred particularly easily in an acidic environment.
In the research on effective separation of GDL from biotransformation media, adsorption on porous adsorbents (Amberlite XAD-4 resin, zeolite, and vermiculite) was also tested. Porous materials are widely and willingly used due to their properties, mainly their large specific surface, high porosity, absorptive capacity, low chemical activity, high mechanical strength, and possibility of regeneration [47]. The use of porous sorbents has often been successful in increasing microorganism production and recovery of volatile compounds from fermentation broth [32,33,34,48]. However, it should be remembered that adsorption from biotransformation media causes certain difficulties which result from the fact that many components are present in cell culture supernatants. These ingredients come from both the growth medium and the extracellular products that are produced by the cells of microorganisms. Additionally, GDL adsorption takes place in the presence of unreacted lipid precursor (ricinoleic acid) (Figure 4) and lipid substrate (castor oil).
Among the three tested adsorbents, the highest adsorption efficiency was obtained for Amberlite XAD-4. After 6 h of adsorption on this resin, less than 1% GDL in the solution was determined. Probably, the ability of Amberlite XAD-4 resin to adsorb GDL is higher than that of zeolite or vermiculite due to the higher specific surface area (750 m2/g) compared to other materials (700 m2/g and 394 m2/g, respectively). Nongonierma et al. [47] confirms that the adsorbents with the largest surface area seem to be the most effective in the separation of organic compounds. According to Alchihab et al. [34], hydrophobic interactions, chemical structure, and high specific surface area are the main driving forces behind the adsorption of GDL to Amberlite XAD-4 resin in aqueous solutions.
In order to better understand the mechanism of GDL adsorption from biotransformation media, which is influenced by the interactions between the components of the culture medium (especially lipids) and lactone, the sorption isotherm was drawn for the most effective Amberlite XAD-4 adsorbent. The obtained linear dependence over a certain range of GDL concentrations in the biotransformation medium (up to 4 g/L) can be attributed to the low solubility of this lactone in water. This is mentioned in the research by Alchihab et al. [34], who obtained a linear relationship for each of the sorbents by carrying out the adsorption of GDL on the MN-100, MN-102, and MN-202 resins. The peach lactone adsorption method was closely related to the solubility of this compound in water, which is about 0.6 g/L. The same conclusions were made by Souchon et al. [33] while testing activated carbon and three porous polystyrene-type polymers (Porapak Q, Chromosorb 105 and Resin SM4).
Desorption of GDL from individual adsorbents, carried out with ethyl alcohol, presented relatively good efficiency; 75–81% of adsorbed lactone. The chromatographic analysis of the extracts after desorption from Amberlite XAD-4 revealed the content of unreacted ricinoleic acid (Figure 4). Its amount decreased during individual cycles of the process, and the purity of the GDL after desorption was approximately 77 ± 2%. The phenomenon of ricinoleic acid adsorption on the surface of adsorbents has been reported by Guan et al. [49].
5. Conclusions
The development of an effective method of GDL separation from biotransformation media seems to be important in the context of the attempts to apply biotechnological synthesis of peach lactone in industry. Due to the existing gap in the research on GDL separation, an attempt was made to compare the effectiveness of three techniques: liquid–liquid extraction, hydrodistillation, and adsorption, in fragrance recovery. The relatively low concentration of GDL in the fermentation broth (approx. 3 g/L) and the presence of an unreacted lipid substrate (castor oil or ricinoleic acid), in which the lactone is partially dissolved, make its recovery difficult.
Bearing in mind the selectivity of the process, its efficiency, and the speed of execution, among the three techniques used, adsorption on Amberlite XAD-4 is recommended. The six-hour adsorption on this resin resulted in less than 1% of lactone remaining in the biotransformation medium. GDL adsorption on resin is based, inter alia, on the use of Van der Waals forces, which enable quick adsorption and easy desorption of the fragrance. Moreover, in the context of industrial GDL bioproduction and its separation from the medium, Amberlite XAD-4 has several advantages: it is inexpensive, does not chemically react with the fragrance, is easily regenerated, and can be easily adapted on an industrial scale. In addition, considering the aspect of environmental protection, it must be remembered that the desorption of GDL from Amberlite XAD-4 resin requires only small amounts of ethanol and takes place with an efficiency of approximately 80%.
In the context of the purity of the released GDL, the hydrodistillation process seemed to be more advantageous, as the purity of the distillate was about 10% higher than that of the ethanol extract after lactone desorption from the resin (88 ± 3.4% and 77 ± 2%, respectively). However, by choosing a separation method, one should consider the resultant of many factors.
This article only outlines the direction of further research that is necessary to optimize the GDL adsorption process, both in economic terms (studies on higher adsorption efficiency by selecting, e.g., the amount of adsorbent in relation to the adsorbed compound) and ecological terms (minimizing the amounts of reagents used).
Author Contributions
Conceptualization, J.M.; methodology, J.M. and J.B.; formal analysis, J.M., M.W.-W. and E.O.-L.; investigation, J.M. and J.B.; writing—original draft preparation, J.M.; writing—review and editing, J.M. and A.G.; visualization, J.M., E.O.-L. and M.W.-W.; supervision, J.M. and A.G.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by the Ministry of Education and Science within funds of the Institute of Food Sciences of Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Longo, A.M.; Sanromán, M.A. Production of Food Aroma Compounds: Microbial and Enzymatic Methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Braga, A.; Guerreiro, C.; Belo, I. Generation of flavors and fragrances through biotransformation and de novo synthesis. Food Bioproc. Tech. 2018, 11, 2217–2228. [Google Scholar] [CrossRef]
- Gopinath, M.; Vijayakumar, L.; Dhanasekar, R.; Viruthagiri, T. Microbial biosynthesis of γ-decalactone and its applications—A review. Glob. J. Biotechnol. Biochem. 2008, 3, 60–68. [Google Scholar]
- Blin-Perrin, C.; Molle, D.; Dufossé, L.; Le-Quere, J.L.; Viel, C.; Mauvais, G.; Feron, G. Metabolism of ricinoleic acid into gamma-decalactone: Beta-oxidation and long chain acyl intermediates of ricinoleic acid in the genus Sporidiobolus sp. FEMS Microbiol. Lett. 2000, 188, 69–74. [Google Scholar] [CrossRef][Green Version]
- Dufossé, L.; Feron, G.; Mauvais, G.; Bonnarme, P.; Durand, A.; Spinnler, H.E. Production of γ-decalactone and 4-hydroxy-decanoic acid in the genus Sporidiobolus. J. Ferment. Bioeng. 1998, 86, 169–173. [Google Scholar] [CrossRef]
- Waché, Y.; Aguedo, M.; Choquet, A.; Gatfield, I.L.; Nicaud, J.M.; Belin, J.M. Role of β-oxidation enzymes in γ-decalactone production by the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2001, 67, 5700–5704. [Google Scholar] [CrossRef] [PubMed]
- Alchihab, M.; Destain, J.; Aguedo, M.; Majad, L.; Ghalfi, H.; Wathelet, J.P.; Thonart, P. Production of gamma-decalactone by a psychrophilic and a mesophilic strain of the yeast Rhodotorula aurantiaca. Appl. Biochem. Biotechnol. 2009, 158, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Alchihab, M.; Destain, J.; Aguedo, M.; Wathelet, J.P.; Thonart, P. The utilization of gum tragacanth to improve the growth of Rhodotorula aurantiaca and the production of gamma-decalactone in large scale. Appl. Biochem. Biotechnol. 2010, 162, 233–241. [Google Scholar] [CrossRef]
- Aguedo, M.; Gomes, N.; Escamilla Garcia, E.; Waché, Y.; Mota, M.; Teixeira, J.A.; Belo, I. Decalactone production by Yarrowia lipolytica under increased O2 transfer rates. Biotechnol. Lett. 2005, 27, 1617–1621. [Google Scholar] [CrossRef][Green Version]
- Gomes, N.; Aguedo, M.; Teixeira, J.; Belo, I. Oxygen mass transfer in a biphasic medium: Influence on the biotransformation of methyl ricinoleate into γ-decalactone by the yeast Yarrowia lipolytica. Biochem. Eng. J. 2007, 35, 380–386. [Google Scholar] [CrossRef]
- Soares, G.P.A.; Souza, K.S.T.; Vilela, L.F.; Schwan, R.F.; Dias, D.R. γ-decalactone production by Yarrowia lipolytica and Lindnera saturnus in crude glycerol. Prep. Biochem. Biotechnol. 2017, 47, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Andrade, D.; Carvalho, B.F.; Schwan, R.F.; Dias, D.R. Production of γ-decalactone by yeast strains under different conditions. Food Technol. Biotechnol. 2017, 55, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Waché, Y.; Aguedo, M.; LeDall, M.T.; Nicaud, J.M.; Belin, J.M. Optimization of Yarrowia lipolytica’s β-oxidation pathway for γ-decalactone production. J. Mol. Catal. B Enzym. 2002, 19–20, 347–351. [Google Scholar] [CrossRef]
- Okui, S.; Uchiyama, M.; Mizugaki, M. Metabolism of hydroxy fatty acids. II. intermediates of the oxidative breakdown of ricinoleic acid by genus Candida. J Biochem. 1963, 54, 536–540. [Google Scholar] [CrossRef]
- Endrizzi, A.; Awadé, A.C.; Belin, J.M. Presumptive involvement of methyl ricinoleate β-oxidation in the production of γ-decalactone by the yeast Pichia guilliermondii. FEMS Microbiol Lett. 1993, 114, 153–159. [Google Scholar] [CrossRef]
- Lee, S.L.; Cheng, H.Y.; Chen, W.C.; Chou, C.C. Effect of physical factors on the production of γ-decalactone by immobilized cells of Sporidiobolus salmonicolor. Process Biochem. 1999, 34, 845–850. [Google Scholar] [CrossRef]
- Najjar, A.; Robert, S.; Guérin, C.; Violet-Asther, M.; Carrière, F. Quantitative study of lipase secretion, extracellular lipolysis, and lipid storage in the yeast Yarrowia lipolytica grown in the presence of olive oil: Analogies with lipolysis in humans. Appl. Microbiol. Biotechnol. 2011, 89, 1947–1962. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Hussain, M.S.; Gambill, L.; Blenner, M. Alternative substrate metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 1077. [Google Scholar] [CrossRef]
- Braga, A.; Belo, I. Production of γ-decalactone by Yarrowia lipolytica: Insights into experimental conditions and operating mode optimization. J. Chem. Technol. Biotechnol. 2015, 90, 559–565. [Google Scholar] [CrossRef]
- Małajowicz, J.; Nowak, D.; Fabiszewska, A.; Iuliano, A. Comparison of gamma-decalactone biosynthesis by yeast Yarrowia lipolytica MTLY40-2p and W29 in batch-cultures. Biotechnol. Biotechnol. Equip. 2020, 34, 330–340. [Google Scholar] [CrossRef]
- Guo, Y.; Song, H.; Wang, Z.; Ding, Y. Expression of POX2 gene and disruption of POX3 genes in the industrial Yarrowia lipolytica on the γ-decalactone production. Microbiol. Res. 2012, 167, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, C.; Song, H.; Wang, Z.; Ren, Q.; Wang, R. Effect of POX3 gene disruption using self-cloning CRF1 cassette in Yarrowia lipolytica on the γ-decalactone production. World J. Microbiol. Biotechnol. 2011, 27, 2807–2812. [Google Scholar] [CrossRef]
- Gomes, N.; Braga, A.; Teixeira, J.A.; Belo, I. Impact of lipase-mediated hydrolysis of castor oil on γ-decalactone production by Yarrowia lipolytica. J. Am. Oil Chem. Soc. 2013, 90, 1131–1137. [Google Scholar] [CrossRef]
- Groguenin, A.; Waché, Y.; Escamilla Garciaa, E.; Aguedo, M.; Husson, F.; LeDall, M.T.; Nicaud, J.M.; Belin, J.M. Genetic engineering of the β-oxidation pathway in the yeast Yarrowia lipolytica to increase the production of aroma compounds. J. Mol. Catal. B Enzym. 2004, 28, 75–79. [Google Scholar] [CrossRef]
- Try, S.; De-Coninck, J.; Voilley, A.; Chunhieng, T.; Waché, Y. Solid state fermentation for the production of γ-decalactones by Yarrowia lipolytica. Process Biochem. 2018, 64, 9–15. [Google Scholar] [CrossRef]
- Moradi, H.; Asadollahi, M.A.; Nahvi, I. Improved γ-decalactone production from castor oil by fed-batch cultivation of Yarrowia lipolytica. Biocatal. Agric. Biotechnol. 2013, 2, 64–68. [Google Scholar] [CrossRef]
- Pérez-Olivero, S.J.; Pérez-Pont, M.L.; Conde, J.E.; Pérez-Trujillo, J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2014, 863019. [Google Scholar] [CrossRef]
- Zhao, Y.; Mu, X.; Nie, Y.; Xu, Y. A new rapid spectrophotometric quantitative determination method for γ-decalactone and application in high-throughput screening for γ-decalactone producing strains. Food Sci. Biotechnol. 2014, 23, 1935–1940. [Google Scholar] [CrossRef]
- Rong, S.; Guan, X.; Li, Q.; Guan, S.; Cai, B.; Zhang, S. Biotransformation of 12-hydroxystearic acid to gamma-decalactone: Comparison of two separation systems. J. Microbiol. Methods 2020, 178, 106041. [Google Scholar] [CrossRef]
- Medeiros, A.B.P.; Pandey, A.; Vandenberghe, L.P.S.; Pastore, G.M.; Soccol, C.R. Production and recovery of aroma compounds produced by solid-state fermentation using different adsorbents. Food Technol. Biotechnol. 2006, 44, 47–51. [Google Scholar]
- Bluemke, W.; Schrader, J. Integrated bioprocess for enhanced production of natural flavors and fragrances by Ceratocystis moniliformis. Biomol. Eng. 2001, 17, 137–142. [Google Scholar] [CrossRef]
- Souchon, I.; Spinnler, H.E.; Dufosse, L.; Voilley, A. Trapping of γ-decalactone by adsorption on hydrophobic sorbents: Application to the bioconversion of methyl ricinoleate by the yeast Sporidiobolus salmonicolor. Biotechnol. Technol. 1998, 12, 109–113. [Google Scholar] [CrossRef]
- Souchon, I.; Rojas, J.A.; Voilley, A.; Grevillot, G. Trapping of aromatic compounds by adsorption on hydrophobic sorbents. Sep. Sci. Technol. 1996, 31, 2473–2491. [Google Scholar] [CrossRef]
- Alchihab, M.; Aldric, J.M.; Aguedo, M.; Destain, J.; Wathelet, J.P.; Thonart, P. The use of Macronet resins to recover γ-decalactone produced by Rhodotorula aurantiaca from the culture broth. J. Ind. Microbiol. Biotechnol. 2010, 37, 167–172. [Google Scholar] [CrossRef]
- Małajowicz, J.; Kozłowska, M. Factors affecting the yield in formation of fat-derived fragrance compounds by Yarrowia lipolytica yeast. Appl. Sci. 2021, 11, 9843. [Google Scholar] [CrossRef]
- Wang, J.; Quan, C.; Wang, X.; Zhao, P.; Fan, S. Extraction, purification and identification of bacterial signal molecules based on N-acyl homoserine lactones. Microb. Biotechnol. 2011, 4, 479–490. [Google Scholar] [CrossRef]
- Canbay, H.S. Effectiveness of liquid-liquid extraction, solid phase extraction, and headspace technique for determination of some volatile water-soluble compounds of rose aromatic water. Int. J. Anal. Chem. 2017, 4870671. [Google Scholar] [CrossRef]
- Morin, D.; Grasland, B.; Vallee-Rehel, K.; Dufau, C.; Haras, D. On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acylhomoserine lactones, quorum sensing signal molecules, in the presence of biological matrices. J. Chromatogr. A 2003, 1002, 79–92. [Google Scholar] [CrossRef]
- Agarwal, S.G.; Gupta, A.; Kapahi, B.K.; Baleshwar, A.; Thappa, R.K.; Suri, O.P. Chemical composition of rose water volatiles. J. Essent. Oil Res. 2005, 17, 265–267. [Google Scholar] [CrossRef]
- Obi, J.; Yoshinaga, K.; Tago, A.; Nagai, T.; Yoshida, A.; Beppu, F.; Gotoh, N. Simple quantification of lactones in milk fat by solvent extraction using gas chromatography–mass spectrometry. J. Oleo Sci. 2018, 67, 941–948. [Google Scholar] [CrossRef]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Bernardo-Gil, M.G.; Romano, A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Khan, I.A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. J. Agric. Food Chem. 2002, 50, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Yamini, Y.; Khajeh, M.; Ghasemi, E.; Mirza, M.; Javidnia, K. Comparison of essential oil compositions of Salvia mirzayanii obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 2008, 108, 341–346. [Google Scholar] [CrossRef]
- Memarzadeh, S.M.; Ghasemi Pirbalouti, A.; AdibNejad, M. Chemical composition and yield of essential oils from Bakhtiari savory (Satureja bachtiarica Bunge. ) under different extraction methods. Ind. Crops Prod. 2015, 76, 809–816. [Google Scholar] [CrossRef]
- Feron, G.; Dufosse, L.; Pierard, E.; Bonnarme, P.; Quere, J.L.; Spinnler, H. Production, identification, and toxicity of (gamma)-decalactone and 4-hydroxydecanoic acid from Sporidiobolus spp. Appl. Environ. Microbiol. 1996, 62, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Romero-Guido, C.; Belo, I.; Ngoc Ta, T.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Thonart, P.; Teixeira, J.A.; Destain, J.; Waché, Y. Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef]
- Nongonierma, A.; Voilley, A.; Cayot, P.; Le Quéré, J.L.; Springett, M. Mechanisms of extraction of aroma compounds from foods, using adsorbents. Effect of various Parameters. Food Rev. Inter. 2006, 22, 51–94. [Google Scholar] [CrossRef]
- Holst, O.; Mattiasson, B. Solid sorbent used in extractive bioconversion. In Extractive Bioconversion; Mattiasson, B., Holst, O., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 189–205. [Google Scholar]
- Guan, S.; Rong, S.; Wang, M.; Cai, B.; Li, Q.; Zhang, S. Enhanced biotransformation productivity of gamma-decalactone from ricinoleic acid based on the expanded vermiculite delivery system. J. Microbiol. Biotechnol. 2019, 29, 1071–1077. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).