Effect of Elastomeric Expandable Additive on Compressive Strength and Linear Expansion of Fly-Ash-Based Strength-Enhanced Geopolymer Cement for Shrinkage-Resistant Oil-Well Cementing
Abstract
:1. Introduction
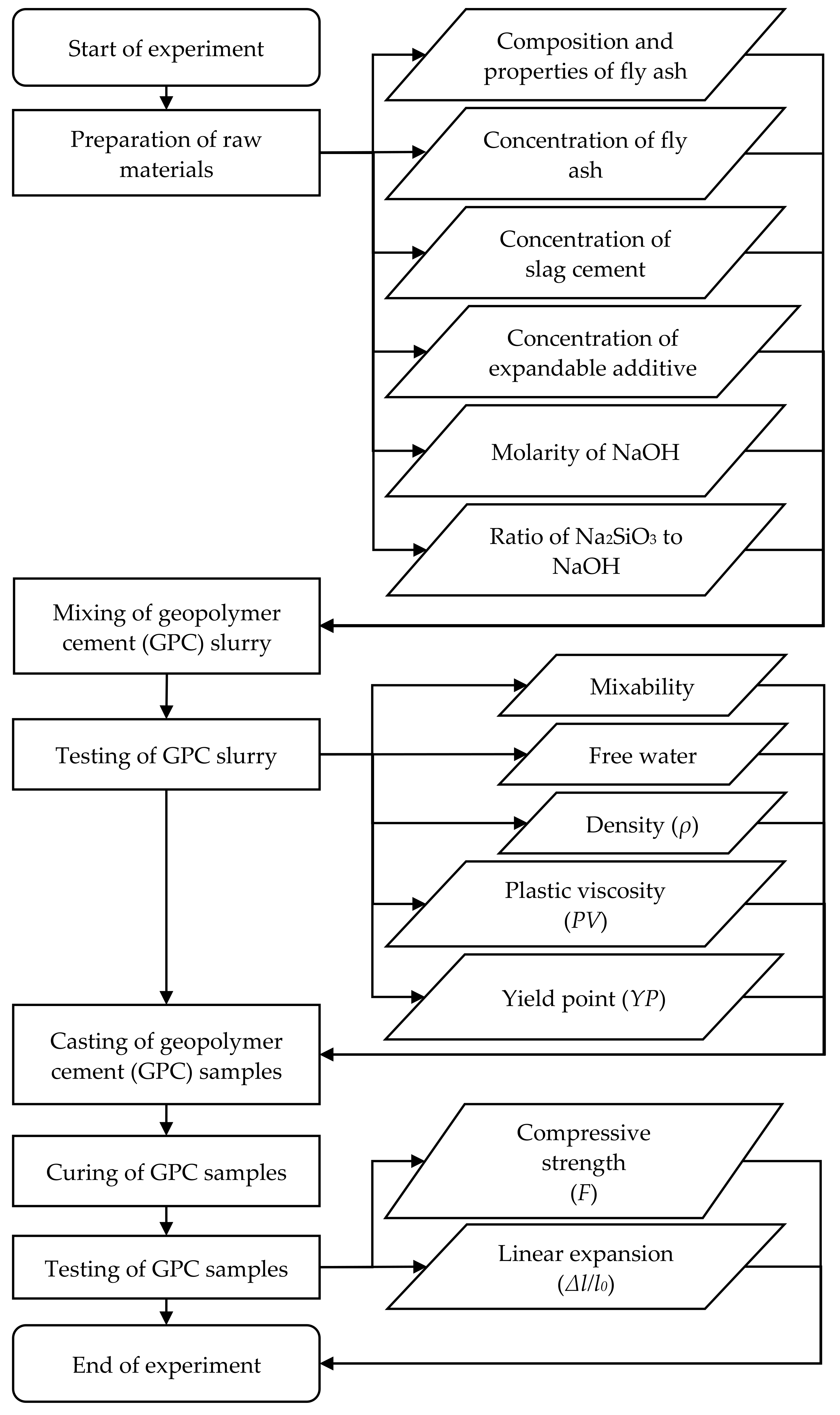
2. Materials and Methods
2.1. Preparation of Geopolymer Cement (GPC) Samples
2.2. Measurement of Compressive Strength and Linear Expansion of GPC Samples
3. Results and Discussion
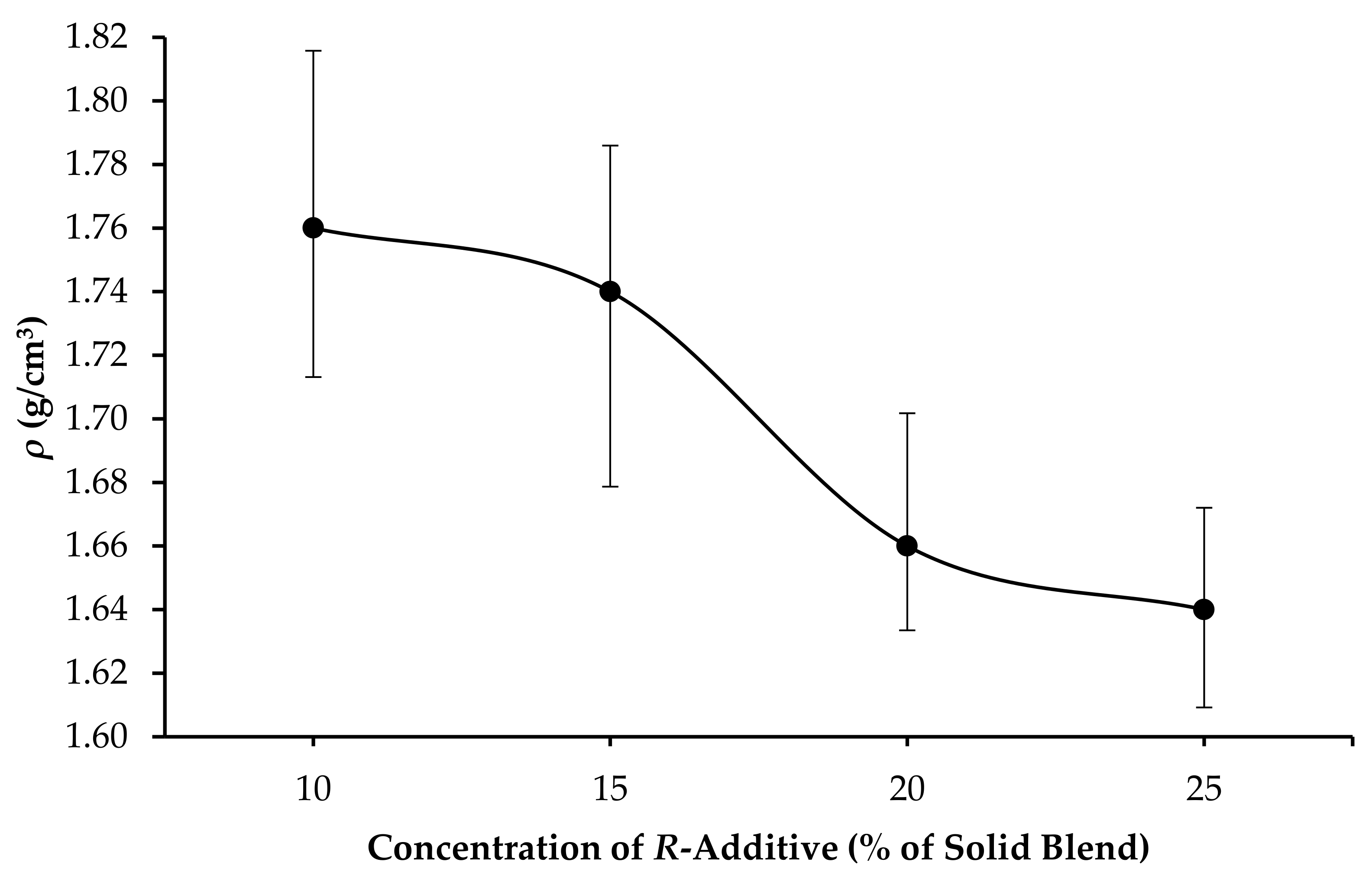
3.1. Mixability, Amount of Free Water and Density of GPC Slurry (ρ)
3.2. Rheological Properties of GPC Slurry
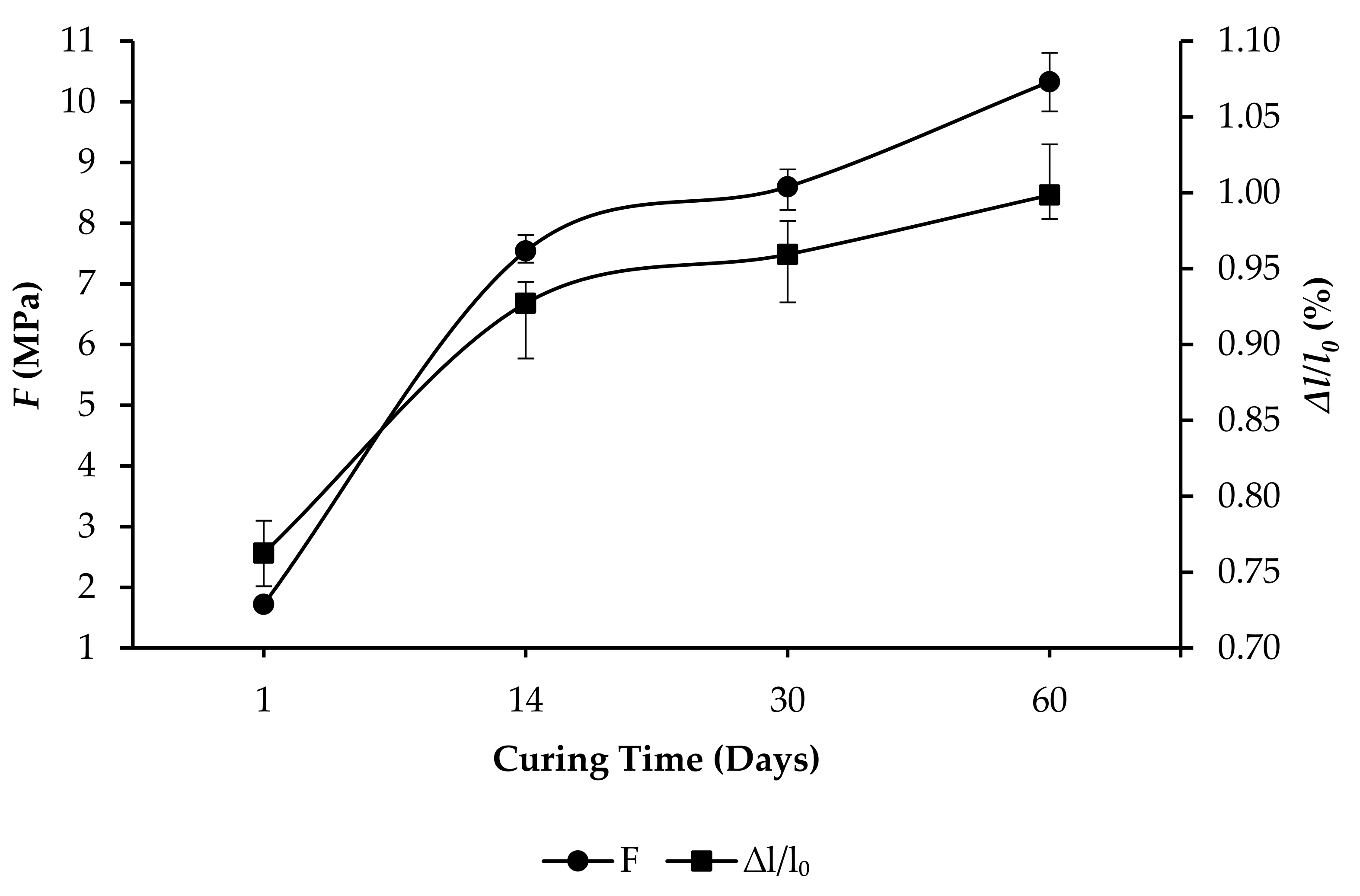
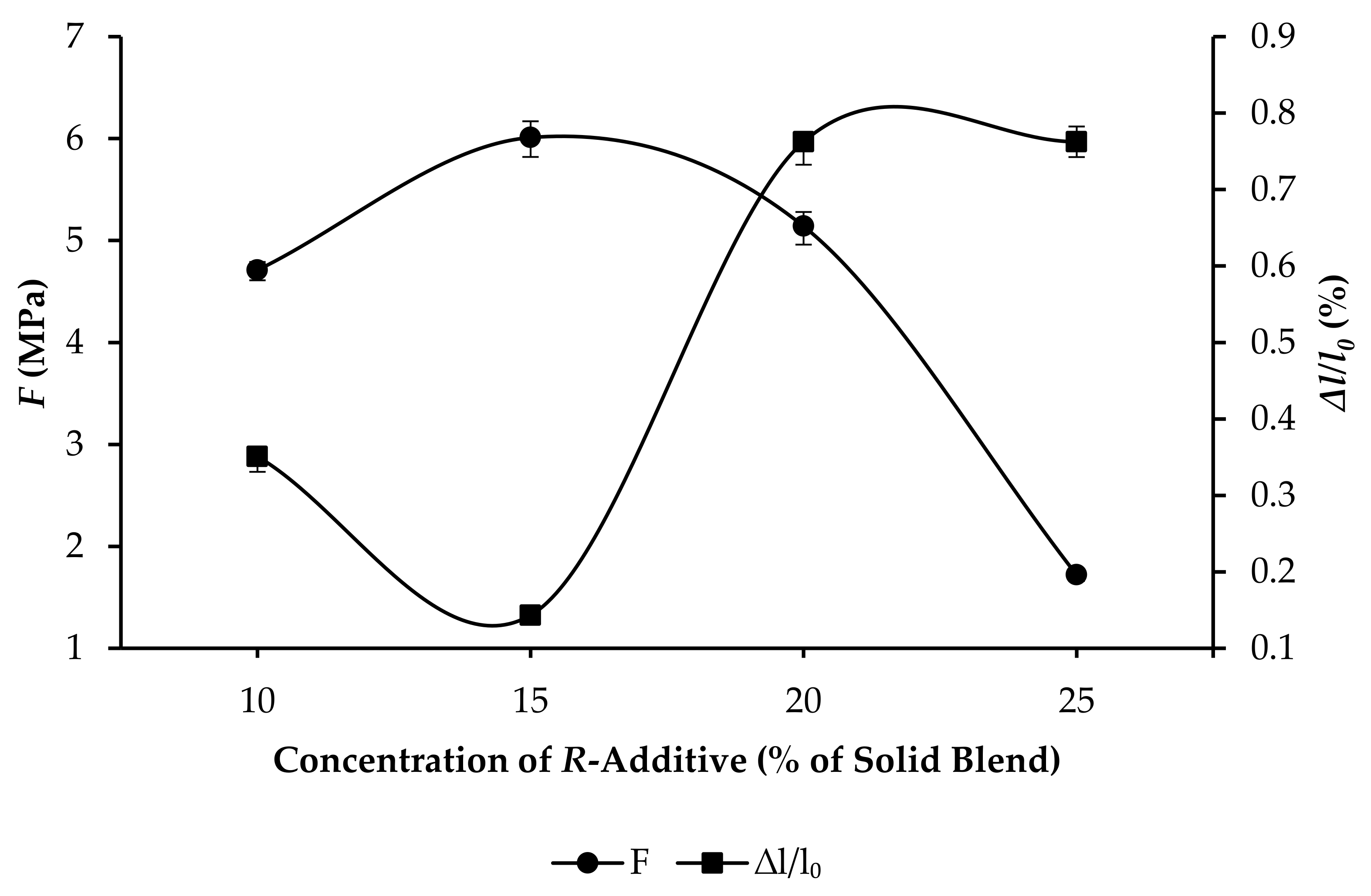
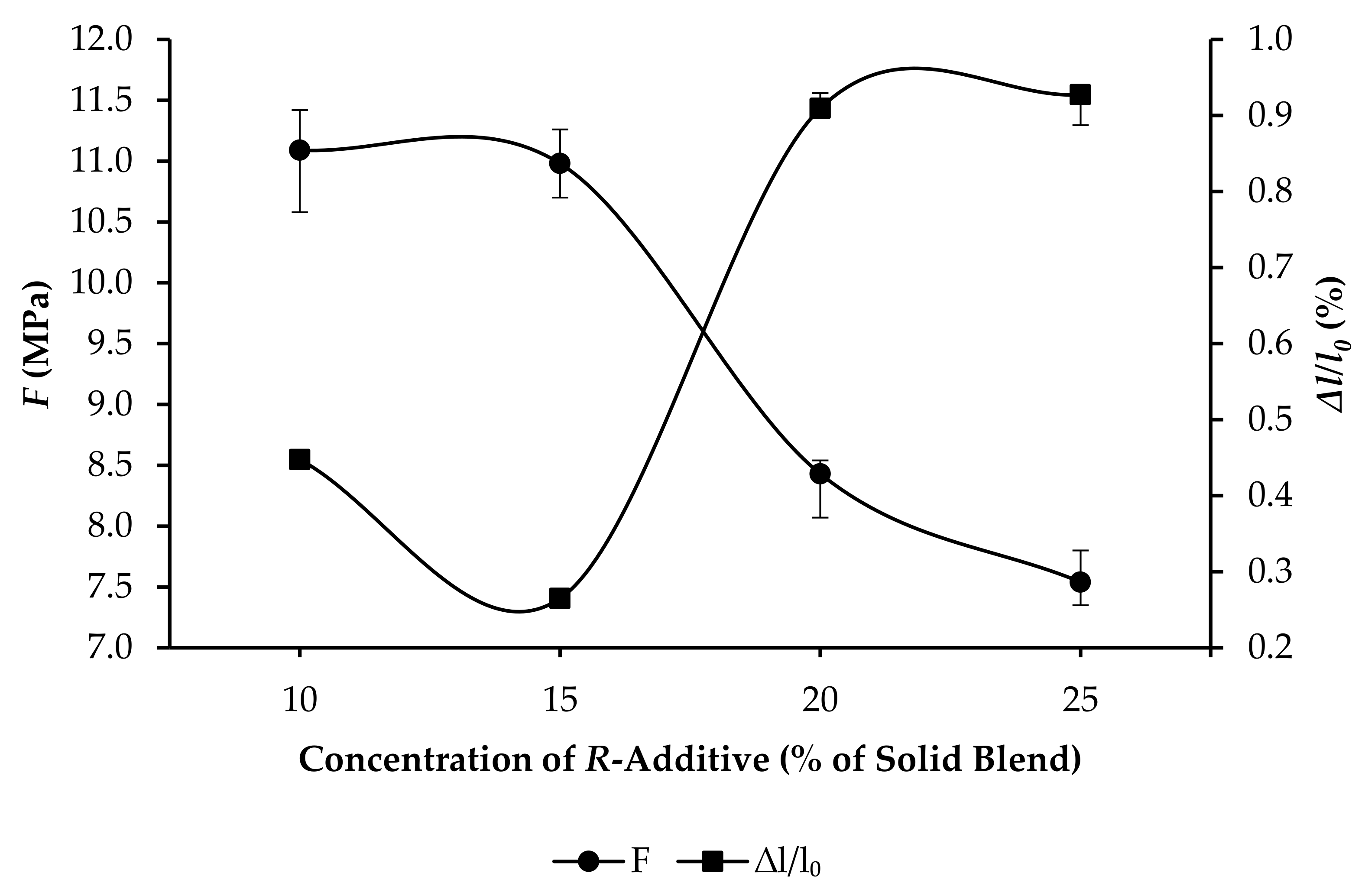
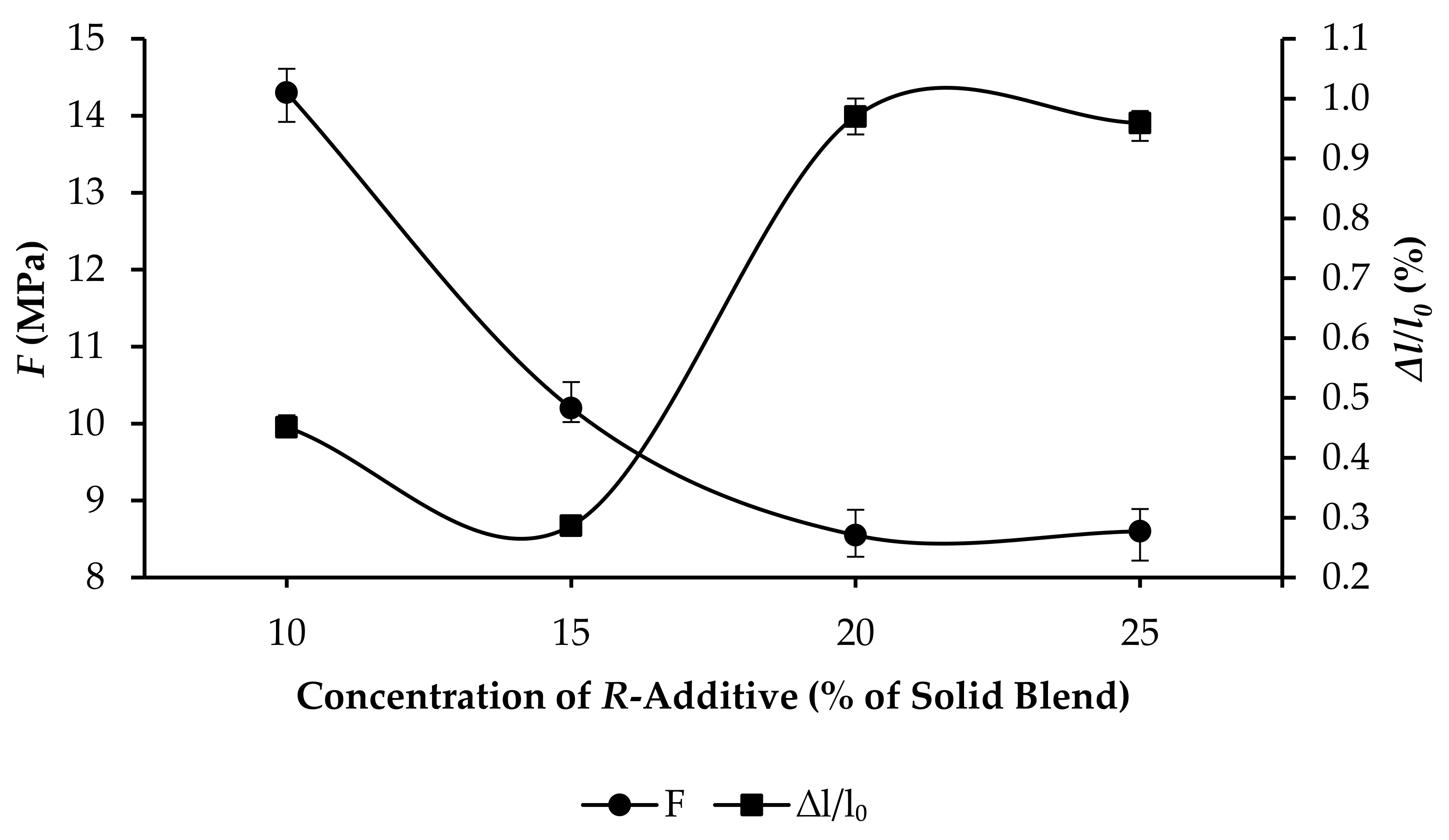
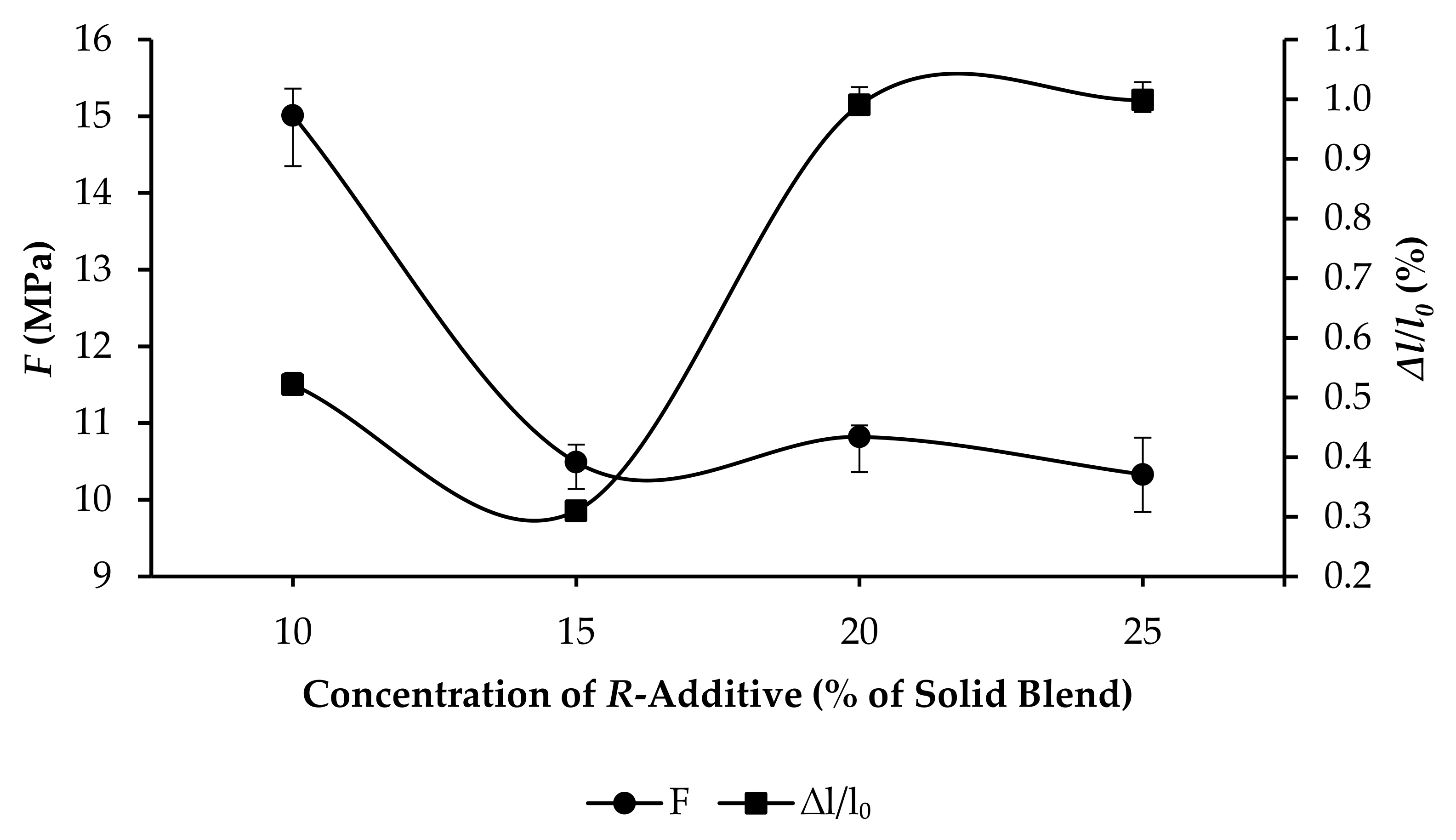
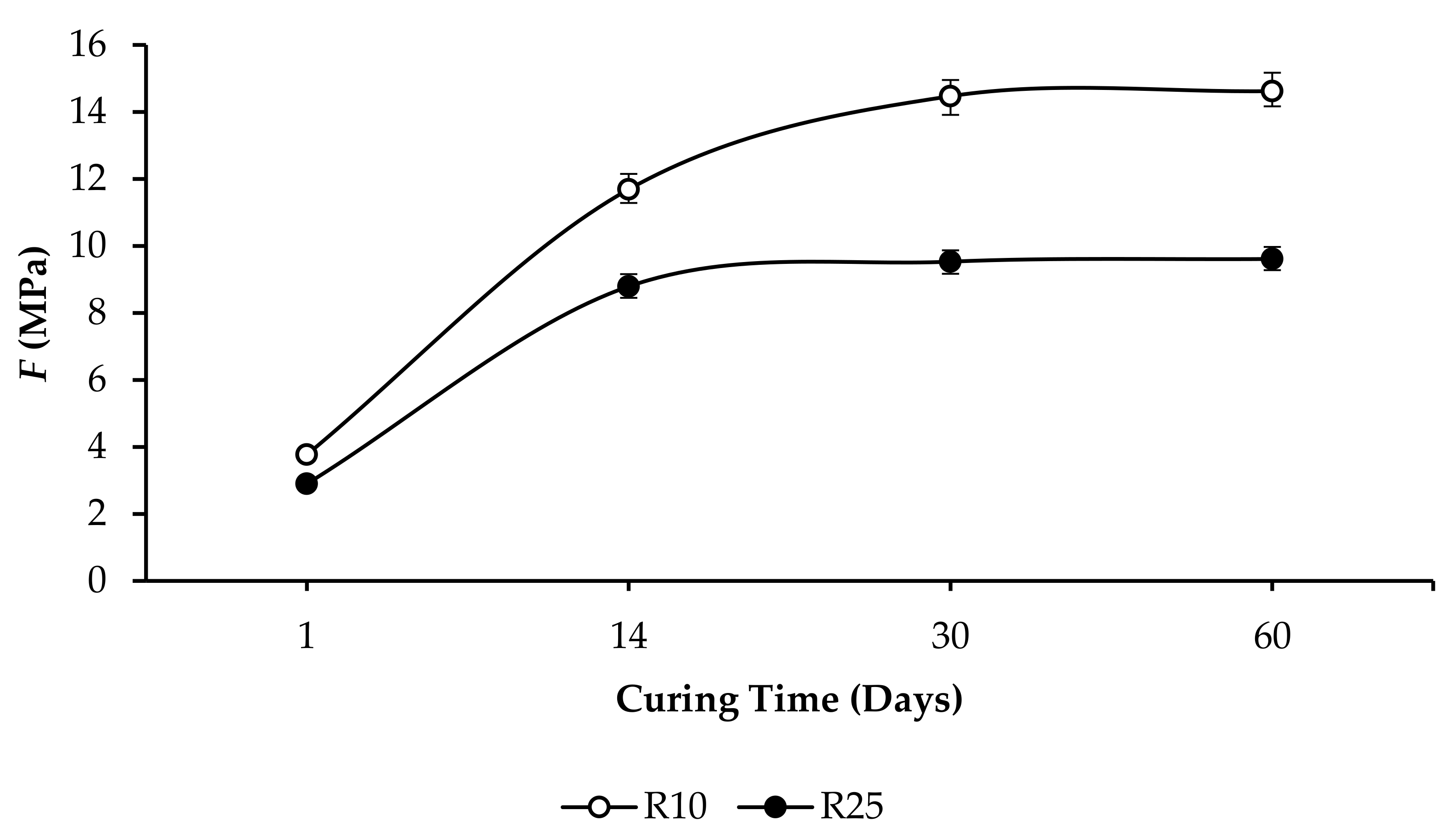
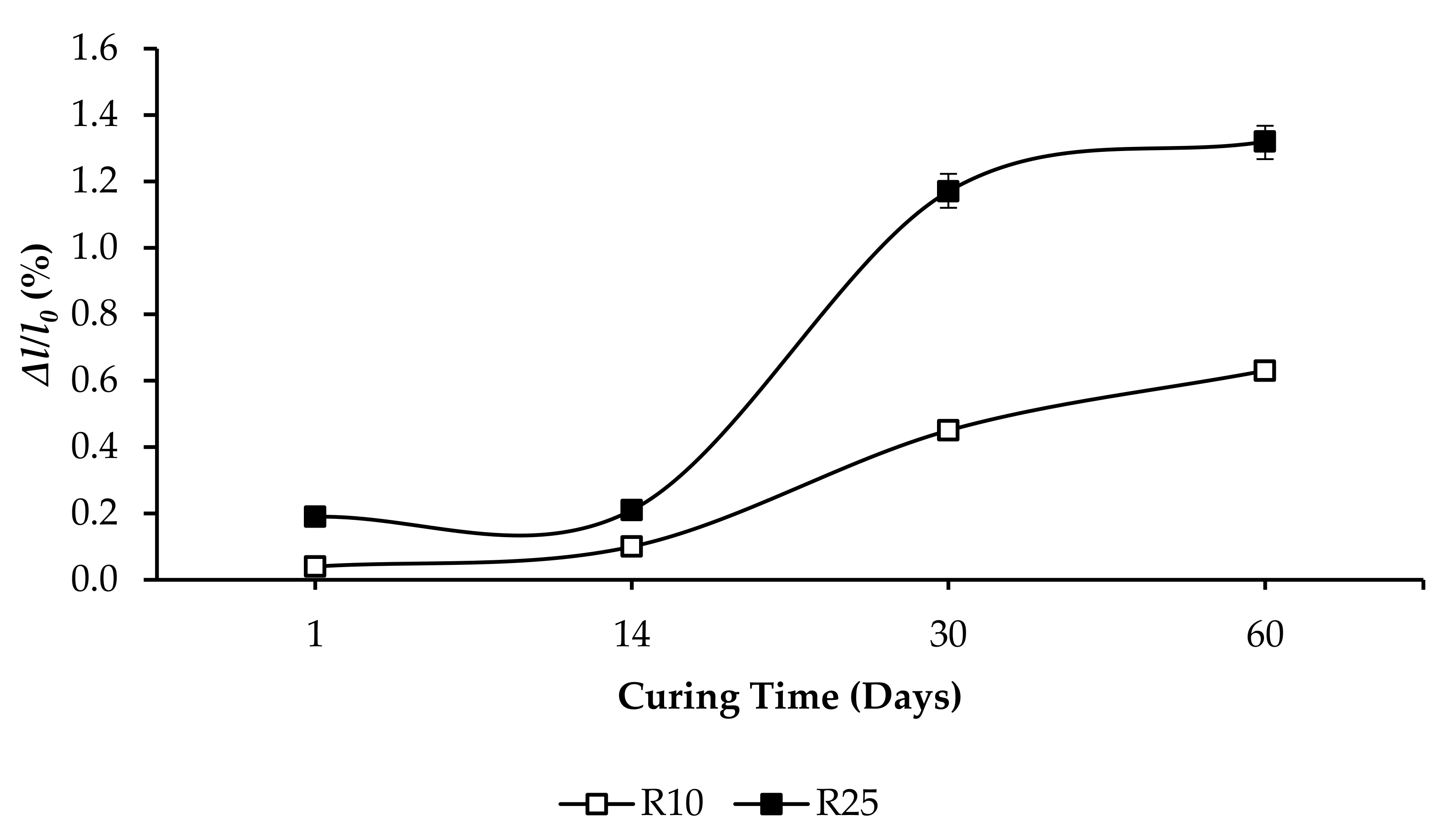
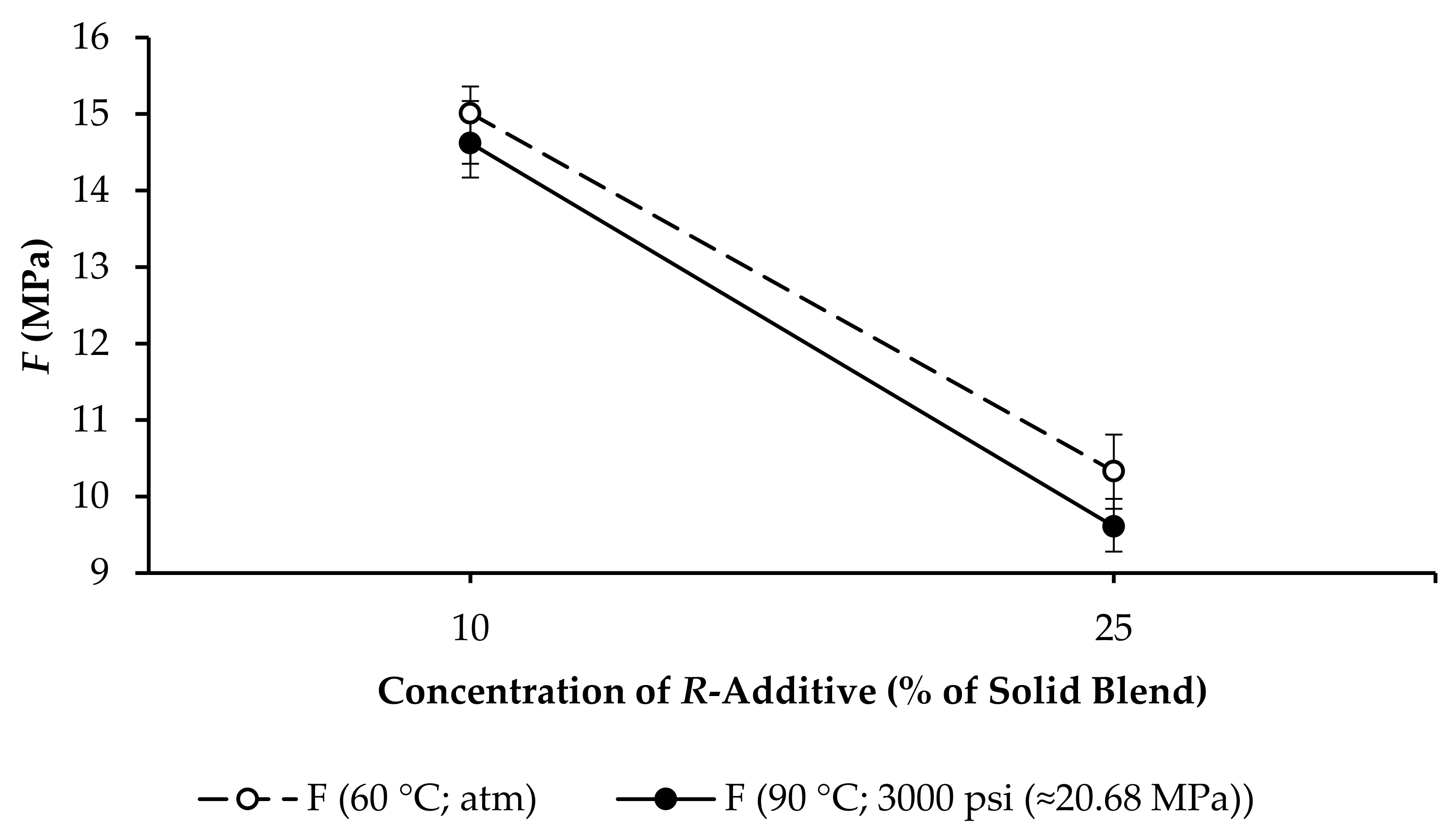
3.3. Compressive Strength and Linear Expansion of GPC Samples
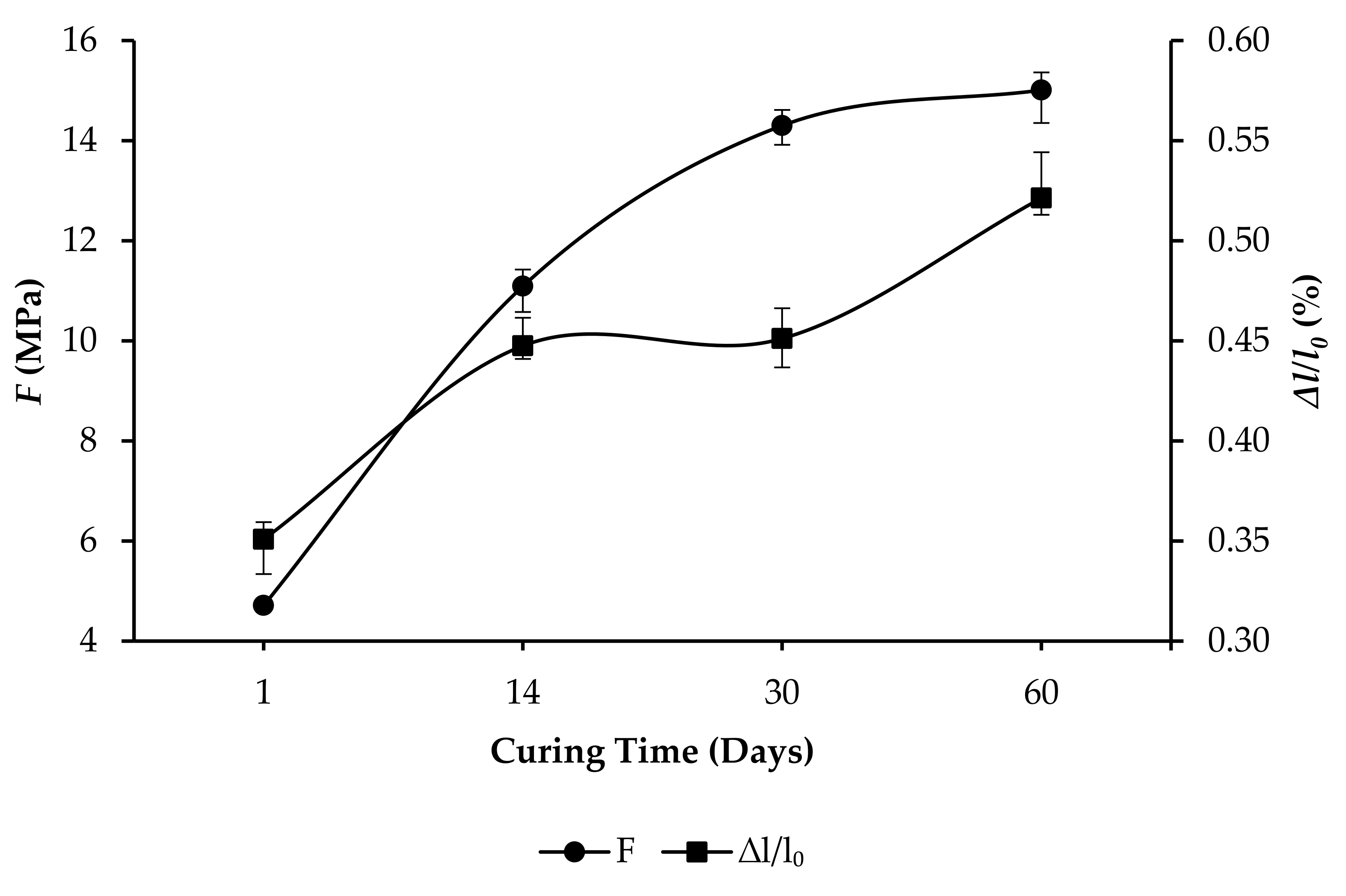
- R10: F increased from 4.71 to 15.01 MPa, and ∆l/l0 increased from 0.3508% to 0.5213%, as per Figure 7.
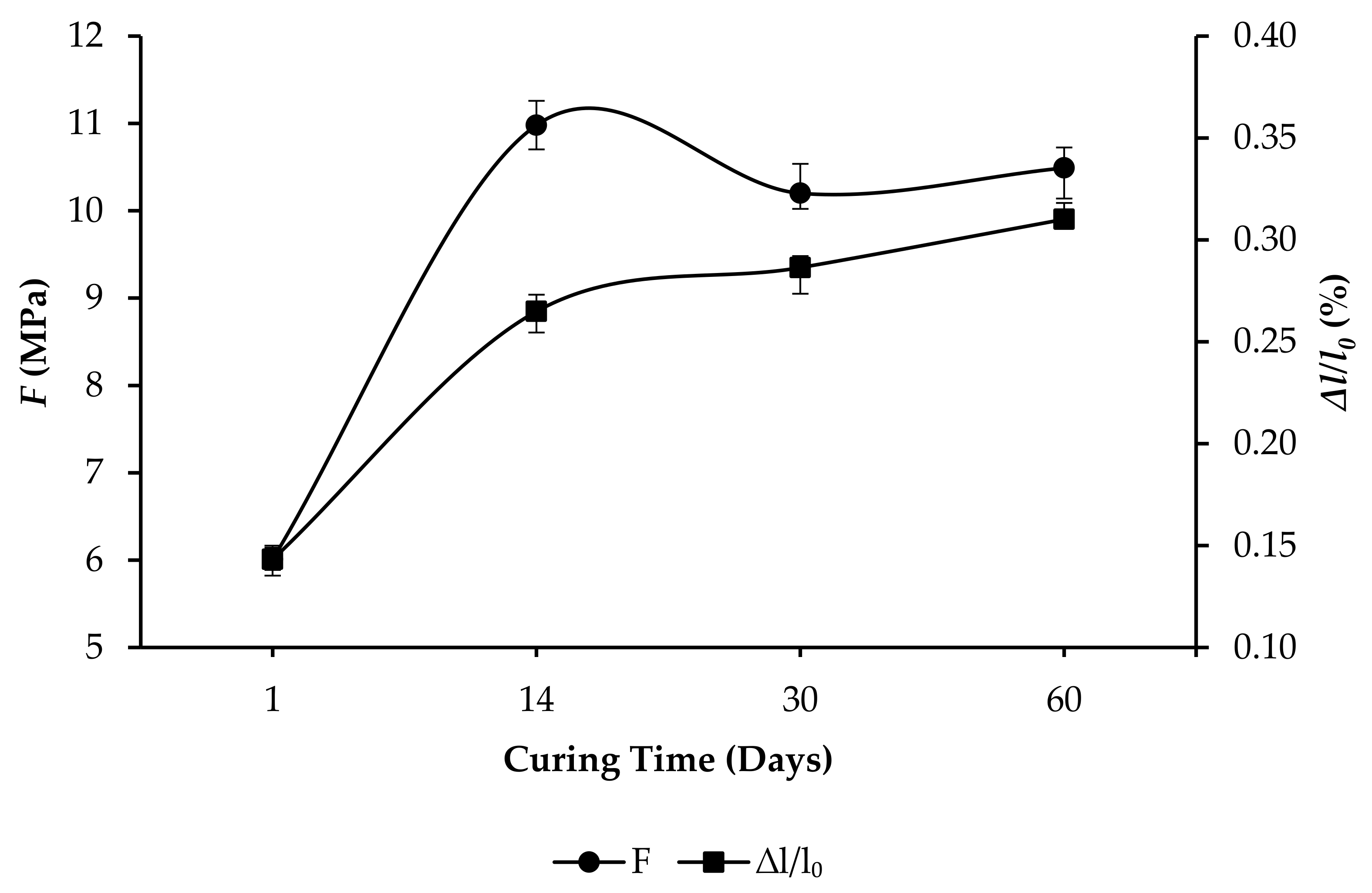
- R15: F increased from 6.01 to 10.49 MPa, and ∆l/l0 increased from 0.1432% to 0.3101%, as per Figure 8.
- R20: F increased from 5.14 to 10.82 MPa, and ∆l/l0 increased from 0.7625% to 0.9903%, as per Figure 9.
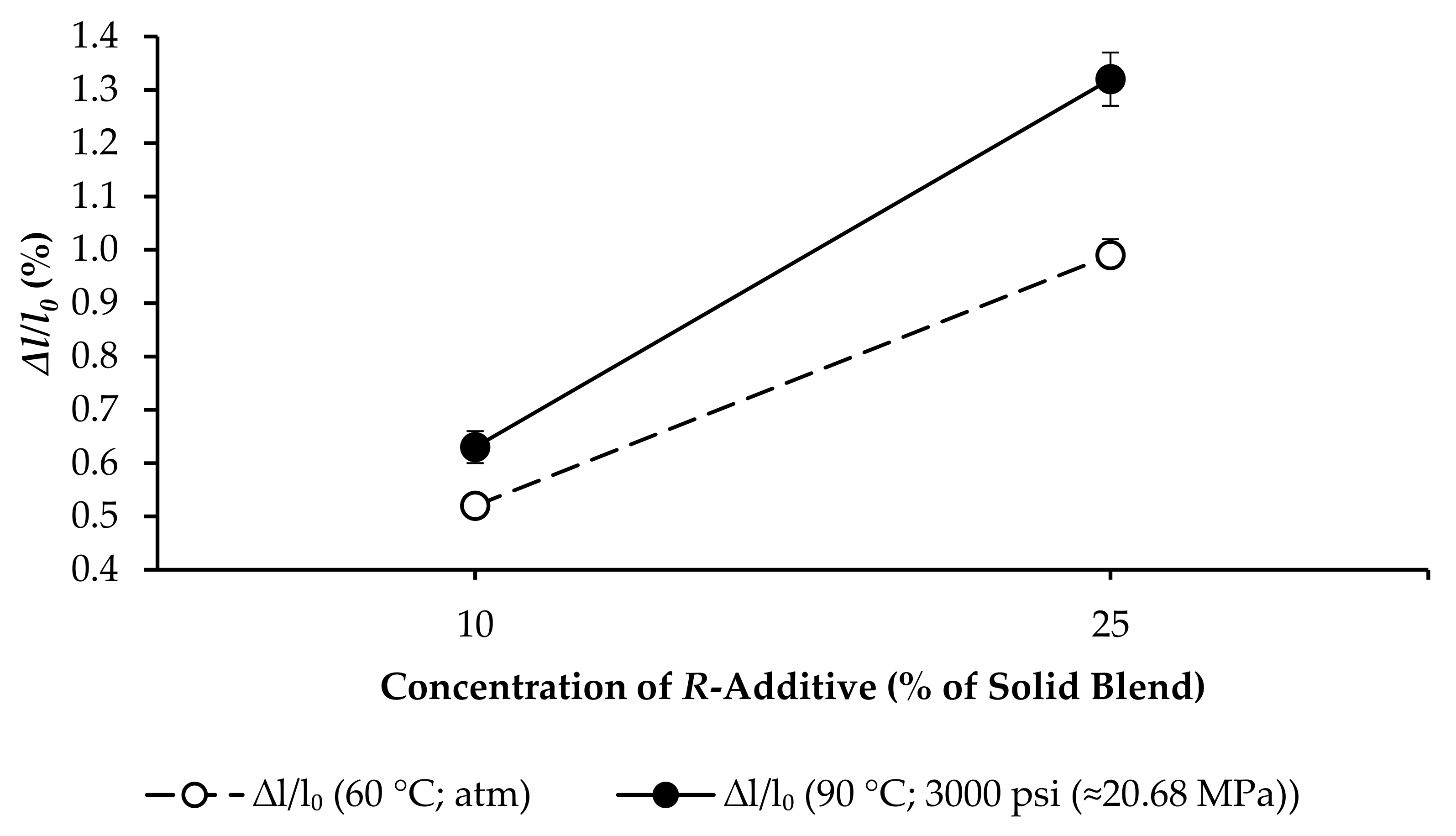
- R25: F increased from 1.72 to 10.33 MPa, and ∆l/l0 increased from 0.7625% to 0.9985%, as per Figure 10.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| API | American Petroleum Institute |
| ASTM | American Society for Testing Materials |
| atm | atmospheric pressure |
| DPAM | dual-coated polyacrylamide |
| GPC | geopolymer cement |
| OPC | ordinary Portland cement |
| R-additive | elastomeric expandable additive used in the present study |
| SCP | sustained casing pressure |
| Chemical Formulae | |
| Al2Ca6H12O24S3 | ettringite |
| Al2O3 | aluminum oxide |
| Al–O–Si | polysialate |
| Al–O–Si–Si | polysialate siloxo |
| Al–O–Si–Si–Si | polysialate disiloxo |
| CaAl2O4 | calcium aluminate |
| CaCO3 | calcium carbonate |
| CaO | calcium oxide |
| CaSO4 | calcium sulfate |
| Ca2+ | calcium cation |
| Cl | chlorine |
| CO2 | carbon dioxide |
| C-S-H | calcium-silicate-hydrate |
| Fe2O3 | iron (III) oxide |
| H2CO3 | carbonic acid |
| K2O | potassium oxide |
| K+ | potassium cation |
| MgO | magnesium oxide |
| NaOH | sodium hydroxide |
| Na2O | sodium oxide |
| Na2SiO3 | sodium silicate |
| Na+ | sodium cation |
| SiO2 | silicon dioxide |
| SO3 | sulfur trioxide |
| TiO2 | titanium (IV) Oxide |
| Notations | |
| A | cross-sectional surface area of the sample |
| ECD | equivalent circulating density |
| F | compressive strength |
| Lf | final length as measured using the expansion cell |
| Li | initial length as measured using the expansion cell |
| M | alkali cation |
| n | degree of polycondensation |
| P | compressive load at the point of failure |
| Pannular | annular pressure loss |
| PV | plastic viscosity |
| TVD | true vertical depth |
| kinematic viscosity | |
| kinematic viscosity at 100 rpm | |
| kinematic viscosity at 300 rpm | |
| YP | yield point |
| ∆l/l0 | linear expansion |
| ρ | slurry density |
References
- Abd Rahman, S.H.; Zulkarnain, N.N.; Shafiq, N. Experimental study and design of experiment using statistical analysis for the development of geopolymer matrix for oil-well cementing for enhancing the integrity. Crystals 2021, 11, 139. [Google Scholar] [CrossRef]
- Zulkarnain, N.N.; Farhan, S.A.; Sazali, Y.A.; Shafiq, N.; Abd Rahman, S.H.; Abd Hamid, A.I.; Habarudin, M.F. Reducing the waiting-on-cement time of geopolymer well cement using calcium chloride (CaCl2) as the accelerator: Analysis of the compressive strength and acoustic impedance for well logging. Sustainability 2021, 13, 6128. [Google Scholar] [CrossRef]
- Bu, Y.; Du, J.; Guo, S.; Liu, H.; Huang, C. Properties of oil well cement with high dosage of metakaolin. Constr. Build. Mater. 2016, 112, 39–48. [Google Scholar] [CrossRef]
- Khalifeh, M.; Saasen, A.; Hodne, H.; Godøy, R.; Vrålstad, T. Geopolymers as an Alternative for Oil Well Cementing Applications: A Review of Advantages and Concerns. In Proceedings of the 36th International Conference on Ocean, Offshore and Arctic Engineering, Trondheim, Norway, 25–30 June 2017; The American Society of Mechanical Engineers: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- API SPEC 10A; Cements and Materials for Well Cementing. American Petroleum Institute (API): Washington, DC, USA, 2019. Available online: https://standards.globalspec.com/std/14208303/api-spec-10a (accessed on 30 September 2021).
- Nasvi, M.C.M.; Ranjith, P.G.; Sanjayan, J. Comparison of Mechanical Behaviors of Geopolymer and Class G Cement as Well Cement at Different Curing Temperatures for Geological Sequestration of Carbon Dioxide. In Proceedings of the 46th U.S. Rock Mechanics/Geomechanics Symposium, Chicago, IL, USA, 24–27 June 2012; American Rock Mechanics Association: Alexandria, VA, USA, 2012. ARMA-2012-232. [Google Scholar]
- Barlet-Gouédard, V.; Rimmelé, G.; Goffé, B.; Porcherie, O. Well technologies for CO2 geological storage: CO2-resistant cement. Oil Gas Sci. Technol.-Rev. IFP 2007, 62, 325–334. [Google Scholar] [CrossRef]
- Liteanu, E.; Spiers, C.J.; Peach, C.J. Failure behaviour wellbore cement in the presence of water and supercritical CO2. Energy Procedia 2009, 1, 3553–3560. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Khattak, M.J.; Ali, N.; Ezeakacha, C.; Saleh, F.K. Study and use of geopolymer mixtures for oil and gas well cementing applications. J. Energy Resour. Technol. 2018, 140, 012908. [Google Scholar] [CrossRef]
- Salehi, S.; Ezeakacha, C.P.; Khattak, M.J. Geopolymer Cements: How Can You Plug and Abandon a Well with New Class of Cheap Efficient Sealing Materials. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium, Oklahoma City, OK, USA, 27–31 March 2017; Society of Petroleum Engineers: Richardson, TX, USA, 2017. [Google Scholar] [CrossRef]
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J.D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J. Pet. Sci. Eng. 2018, 173, 478–491. [Google Scholar] [CrossRef]
- Bois, A.-P.; Garnier, A.; Galdiolo, G.; Laudet, J.-B. Use of a mechanistic model to forecast cement-sheath integrity. SPE Drill. Complet. 2012, 27, 303–314. [Google Scholar] [CrossRef]
- Santra, A.K.; Reddy, B.R.; Liang, F.; Fitzgerald, R. Reaction of CO2 with Portland Cement at Downhole Conditions and the Role of Pozzolanic Supplements. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2009. [Google Scholar] [CrossRef]
- Reddy, B.R.; Xu, Y.; Ravi, K.; Gray, D.W.; Pattillo, P. Cement shrinkage measurement in oilwell cementing—a comparative study of laboratory methods and procedures. SPE Drill. Complet. 2009, 24, 104–114. [Google Scholar] [CrossRef]
- Kosmatka, S.H.; Wilson, M.L. Design and Control of Concrete Mixtures: The Guide to Applications, Methods, and Materials, 15th ed.; Portland Cement Association: Skokie, IL, USA, 2011. [Google Scholar]
- Nasvi, M.C.M.; Ranjith, P.G.; Sanjayan, J.; Bui, H. Effect of temperature on permeability of geopolymer: A primary well sealant for carbon capture and storage wells. Fuel 2014, 117, 354–363. [Google Scholar] [CrossRef]
- Abd Rahman, S.H.; Irawan, S.; Shafiq, N.; Suppiah, R.R. Investigating the expansion characteristics of geopolymer cement samples in a water bath and compared with the expansion of ASTM Class-G cement. Heliyon 2020, 6, e03478. [Google Scholar] [CrossRef] [Green Version]
- Nagral, M.R.; Ostwal, T.; Chitawadagi, M.V. Effect of curing temperature and curing hours on the properties of geo-polymer concrete. Int. J. Comput. Eng. Res. 2014, 4, 1–11. [Google Scholar]
- Eric, B.; Joel, F.; Grace, O. Oil well cement additives: A review of the common types. Oil Gas Res. 2016, 2, 112. [Google Scholar] [CrossRef]
- Ridha, S.; Abd Hamid, A.I.; Abdul Halim, A.H.; Zamzuri, N.A. Elasticity and expansion test performance of geopolymer as oil well cement. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012147. [Google Scholar] [CrossRef] [Green Version]
- Richhariya, G.; Dora, D.T.K.; Parmar, K.R.; Pant, K.K.; Singhal, N.; Lal, K.; Kundu, P.P. Development of self-healing cement slurry through the incorporation of dual-encapsulated polyacrylamide for the prevention of water ingress in oil well. Materials 2020, 13, 2921. [Google Scholar] [CrossRef]
- Duguid, A.; Scherer, G.W. Degradation of oilwell cement due to exposure to carbonated brine. Int. J. Greenh. Gas Control 2010, 4, 546–560. [Google Scholar] [CrossRef]
- Bourgoyne, A.T.; Scott, S.L.; Regg, J.B. Sustained Casing Pressure in Offshore Producing Wells. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 3–6 May 1999. [Google Scholar] [CrossRef]
- Farkas, R.F.; England, K.W.; Roy, M.L.; Dickinson, M.; Samuel, M.; Hart, R.E. New Cementing Technology Cures 40-Year-Old Squeeze Problems. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999; Society of Petroleum Engineers: Richardson, TX, USA, 1999. [Google Scholar] [CrossRef]
- Merlini, M.; Artioli, G.; Cerulli, T.; Cella, F.; Bravo, A. Tricalcium aluminate hydration in additivated systems. a crystallographic study by SR-XRPD. Cem. Concr. Res. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Chenevert, M.E.; Shrestha, B.K. Chemical shrinkage properties of oilfield cements. SPE Drill. Eng. 1991, 6, 37–43. [Google Scholar] [CrossRef]
- Kiran, R.; Teodoriu, C.; Dadmohammadi, Y.; Nygaard, R.; Wood, D.; Mokhtari, M.; Salehi, S. Identification and evaluation of well integrity and causes of failure of well integrity barriers (a review). J. Nat. Gas Sci. Eng. 2017, 45, 511–526. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry & Applications, 3rd ed.; Institut Géopolymère: Saint-Quentin, France, 2011. [Google Scholar]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Resistance of fly ash based geopolymer mortars in sulfuric acid. ARPN J. Eng. Appl. Sci. 2009, 4, 65–70. [Google Scholar]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Dimas, D.; Giannopoulou, I.; Panias, D. Polymerization in sodium silicate solutions: A fundamental process in geopolymerization technology. J. Mater. Sci. 2009, 44, 3719–3730. [Google Scholar] [CrossRef]
- Khalifeh, M.; Saasen, A.; Vrålstad, T. Potential Utilization of Geopolymers in Plug and Abandonment Operations. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 2 April 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar] [CrossRef]
- Nasvi, M.M.C.; Gamage, R.P.; Jay, S. Geopolymer as well cement and the variation of its mechanical behavior with curing temperature. Greenh. Gas Sci. Technol. 2012, 2, 46–58. [Google Scholar] [CrossRef]
- Uehara, M. New concrete with low environmental load using the geopolymer method. Q. Rep. RTRI 2010, 51, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Aughenbaugh, K.; Nair, S.; Shuck, M.; van Oort, E. Solidification of Synthetic-Based Drilling Mud using Geopolymers. In Proceedings of the SPE Deepwater Drilling and Completions Conference, Galveston, TX, USA, 14–15 September 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar] [CrossRef]
- Khalifeh, M.; Hodne, H.; Saasen, A.; Integrity, O.; Eduok, E.I. Usability of Geopolymers for Oil Well Cementing Applications: Reaction Mechanisms, Pumpability, and Properties. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Perth, Australia, 25–27 October 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar] [CrossRef]
- Khalifeh, M.; Todorovic, J.; Vrålstad, T.; Saasen, A.; Hodne, H. Long-term durability of rock-based geopolymers aged at downhole conditions for oil well cementing operations. J. Sustain. Cem.-Based Mater. 2017, 6, 217–230. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Diaz, E.I.; Allouche, E.N. Recycling of Fly Ash into Geopolymer Concrete: Creation of a Database. In Proceedings of the 2010 IEEE Green Technologies Conference, Grapevine, TX, USA, 15–16 April 2010; Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2010; pp. 1–7. [Google Scholar] [CrossRef]
- Yang, Z.X.; Ha, N.R.; Jang, M.S.; Hwang, K.H. Geopolymer concrete fabricated by waste concrete sludge with silica fume. Mater. Sci. Forum. 2009, 620–622, 791–794. [Google Scholar] [CrossRef]
- Hewayde, E.; Nehdi, M.; Allouche, E.; Nakhla, G. Effect of geopolymer cement on microstructure, compressive strength and sulphuric acid resistance of concrete. Mag. Concr. Res. 2006, 58, 321–331. [Google Scholar] [CrossRef]
- Lloyd, N.; Rangan, B. Geopolymer Concrete with Fly Ash. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Università Politecnica delle Marche, Ancona, Italy, 28–30 June 2010; University of Wisconsin-Milwaukee: Milwaukee, WI, USA, 2010; pp. 1493–1504. [Google Scholar]
- Majidi, B. Geopolymer technology, from fundamentals to advanced applications: A review. Mater. Technol. Adv. Perform. Mater. 2009, 24, 79–87. [Google Scholar] [CrossRef]
- Davidovits, J. Environmentally driven geopolymer applications. In Proceedings of the Geopolymer Conference, Melbourne, Australia, 28–29 October 2002; Institut Géopolymère: Saint-Quentin, France, 2002. [Google Scholar]
- Lee, N.K.; Kim, E.M.; Lee, H.K. Mechanical properties and setting characteristics of geopolymer mortar using styrene-butadiene (SB) latex. Constr. Build. Mater. 2016, 113, 264–272. [Google Scholar] [CrossRef]
- Ekinci, E.; Türkmen, I.; Kantarci, F.; Karakoç, M.B. The improvement of mechanical, physical and durability characteristics of volcanic tuff based geopolymer concrete by using nano silica, micro silica and styrene-butadiene latex additives at different ratios. Constr. Build. Mater. 2019, 201, 257–267. [Google Scholar] [CrossRef]
- Our Business: Power Plant and Water Desalination Plant Locations. Available online: https://www.malakoff.com.my/Our-Business/Power-Plant-and-Water-Desalination-Plant-Locations/ (accessed on 5 November 2021).
- ASTM C618–19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- GB 175-2007/XG3-2018; Common Portland Cement, Including Amendment 3. Code of China: Beijing, China, 2019.
- Yuhuan, B.; Rui, M.; Jiapei, D.; Shenglai, G.; Huajie, L.; Letian, Z. Utilization of metakaolin-based geopolymer as a mud-cake solidification agent to enhance the bonding strength of oil well cement–formation interface. R. Soc. Open Sci. 2020, 7, 191230. [Google Scholar] [CrossRef] [Green Version]
- Kallesten, B.; Kakay, S.; Gebremariam, K. Synthesis and characterization of fly ash and slag based geopolymer concrete. IOP Conf. Ser. Mater. Sci. Eng. 2019, 700, 012032. [Google Scholar] [CrossRef]
- Kanesan, D.; Ridha, S.; Rao, P. Formulation of geopolymer cement using mixture of slag and Class F fly ash for oil well cementing. IOP Conf. Ser. Mater. Sci. Eng. 2017, 201, 012014. [Google Scholar] [CrossRef] [Green Version]
- API RP 10B-2; Recommended Practice for Testing Well Cements. 2nd ed. American Petroleum Institute (API): Washington, DC, USA, 2019.
- API RP10B-5; Recommended Practice on Determination of Shrinkage and Expansion of Well Cement Formulations at Atmospheric Pressure. American Petroleum Institute (API): Washington, DC, USA, 2005.
- Igbani, S.; Appah, D.; Ogoni, H.A. The application of response surface methodology in Minitab 16, to identify the optimal, comfort, and adverse zones of compressive strength responses in ferrous oilwell cement sheath systems. Int. J. Eng. Mod. Technol. 2020, 6, 20–39. [Google Scholar]
- Zahid, M.; Shafiq, N.; Isa, M.H.; Gil, L. Statistical modeling and mix design optimization of fly ash based engineered geopolymer composite using response surface methodology. J. Clean. Prod. 2018, 194, 483–498. [Google Scholar] [CrossRef]
- Baumgarte, C.; Thiercelin, M.; Klaus, D. Case studies of expanding cement to prevent microannular formation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999; Society of Petroleum Engineers: Richardson, TX, USA, 1999. [Google Scholar] [CrossRef]
- Sofi, A. Effect of waste tyre rubber on mechanical and durability properties of concrete—A review. Ain Shams Eng. J. 2018, 9, 2691–2700. [Google Scholar] [CrossRef]
- Barlet-Gouédard, V.; Rimmelé, G.; Porcherie, O.; Quisel, N.; Desroches, J. A solution against well cement degradation under CO2 geological storage environment. Int. J. Greenh. Gas Control 2009, 3, 206–216. [Google Scholar] [CrossRef]
- Powers, P.O.; Billmeyer, B.R. Swelling of synthetic rubbers in mineral oils. Effect of temperature and aniline point. Rubber Chem. Technol. 1945, 18, 452–459. [Google Scholar] [CrossRef]
- Shan, G.-R.; Xu, P.-Y.; Weng, Z.-X.; Huang, Z.-M. Oil-absorption function of physical crosslinking in the high-oil-absorption resins. J. Appl. Polym. Sci. 2003, 90, 3945–3950. [Google Scholar] [CrossRef]
- Abbas, G.; Irawan, S.; Kumar, S.; Elrayah, A.A.I. Improving oil well cement slurry performance using hydroxypropylmethylcellulose polymer. Adv. Mater. Res. 2013, 787, 222–227. [Google Scholar] [CrossRef]
- Mao, W.; Litina, C.; Al-Tabbaa, A. Development and application of novel sodium silicate microcapsule-based self-healing oil well cement. Materials 2020, 13, 456. [Google Scholar] [CrossRef] [Green Version]






| Element/Parameter | Weight (%) |
|---|---|
| Silicon Dioxide (SiO2) | 46.47 |
| Aluminum Oxide (Al2O3) | 25.95 |
| Iron (III) Oxide (Fe2O3) | 8.31 |
| Calcium Oxide (CaO) | 6.88 |
| Magnesium Oxide (MgO) | 4.95 |
| Potassium Oxide (K2O) | 2.11 |
| Sodium Oxide (Na2O) | 1.72 |
| Titanium (IV) Oxide (TiO2) | 1.16 |
| Sulfur Trioxide (SO3) | 0.63 |
| Chlorine (Cl) | <0.1 |
| Moisture | 0.11 |
| Loss of Ignition | 1.61 |
| Mix Formulation | Concentration (% of Solid Blend) | ||
|---|---|---|---|
| R-Additive | Fly Ash | Slag Cement | |
| R10 | 10 | 81.0 | 9.0 |
| R15 | 15 | 76.5 | 8.5 |
| R20 | 20 | 72.0 | 8.0 |
| R25 | 25 | 67.5 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Rahman, S.H.; Farhan, S.A.; Sazali, Y.A.; Shafiee, L.H.; Husna, N.; Abd Hamid, A.I.; Shafiq, N.; Zulkarnain, N.N.; Habarudin, M.F. Effect of Elastomeric Expandable Additive on Compressive Strength and Linear Expansion of Fly-Ash-Based Strength-Enhanced Geopolymer Cement for Shrinkage-Resistant Oil-Well Cementing. Appl. Sci. 2022, 12, 1897. https://doi.org/10.3390/app12041897
Abd Rahman SH, Farhan SA, Sazali YA, Shafiee LH, Husna N, Abd Hamid AI, Shafiq N, Zulkarnain NN, Habarudin MF. Effect of Elastomeric Expandable Additive on Compressive Strength and Linear Expansion of Fly-Ash-Based Strength-Enhanced Geopolymer Cement for Shrinkage-Resistant Oil-Well Cementing. Applied Sciences. 2022; 12(4):1897. https://doi.org/10.3390/app12041897
Chicago/Turabian StyleAbd Rahman, Siti Humairah, Syed Ahmad Farhan, Yon Azwa Sazali, Luqmanul Hakim Shafiee, Nadzhratul Husna, Afif Izwan Abd Hamid, Nasir Shafiq, Nurul Nazmin Zulkarnain, and Mohd Firdaus Habarudin. 2022. "Effect of Elastomeric Expandable Additive on Compressive Strength and Linear Expansion of Fly-Ash-Based Strength-Enhanced Geopolymer Cement for Shrinkage-Resistant Oil-Well Cementing" Applied Sciences 12, no. 4: 1897. https://doi.org/10.3390/app12041897
APA StyleAbd Rahman, S. H., Farhan, S. A., Sazali, Y. A., Shafiee, L. H., Husna, N., Abd Hamid, A. I., Shafiq, N., Zulkarnain, N. N., & Habarudin, M. F. (2022). Effect of Elastomeric Expandable Additive on Compressive Strength and Linear Expansion of Fly-Ash-Based Strength-Enhanced Geopolymer Cement for Shrinkage-Resistant Oil-Well Cementing. Applied Sciences, 12(4), 1897. https://doi.org/10.3390/app12041897









