Effect of Sodium on Methanogens in a Two-Stage Anaerobic System
Abstract
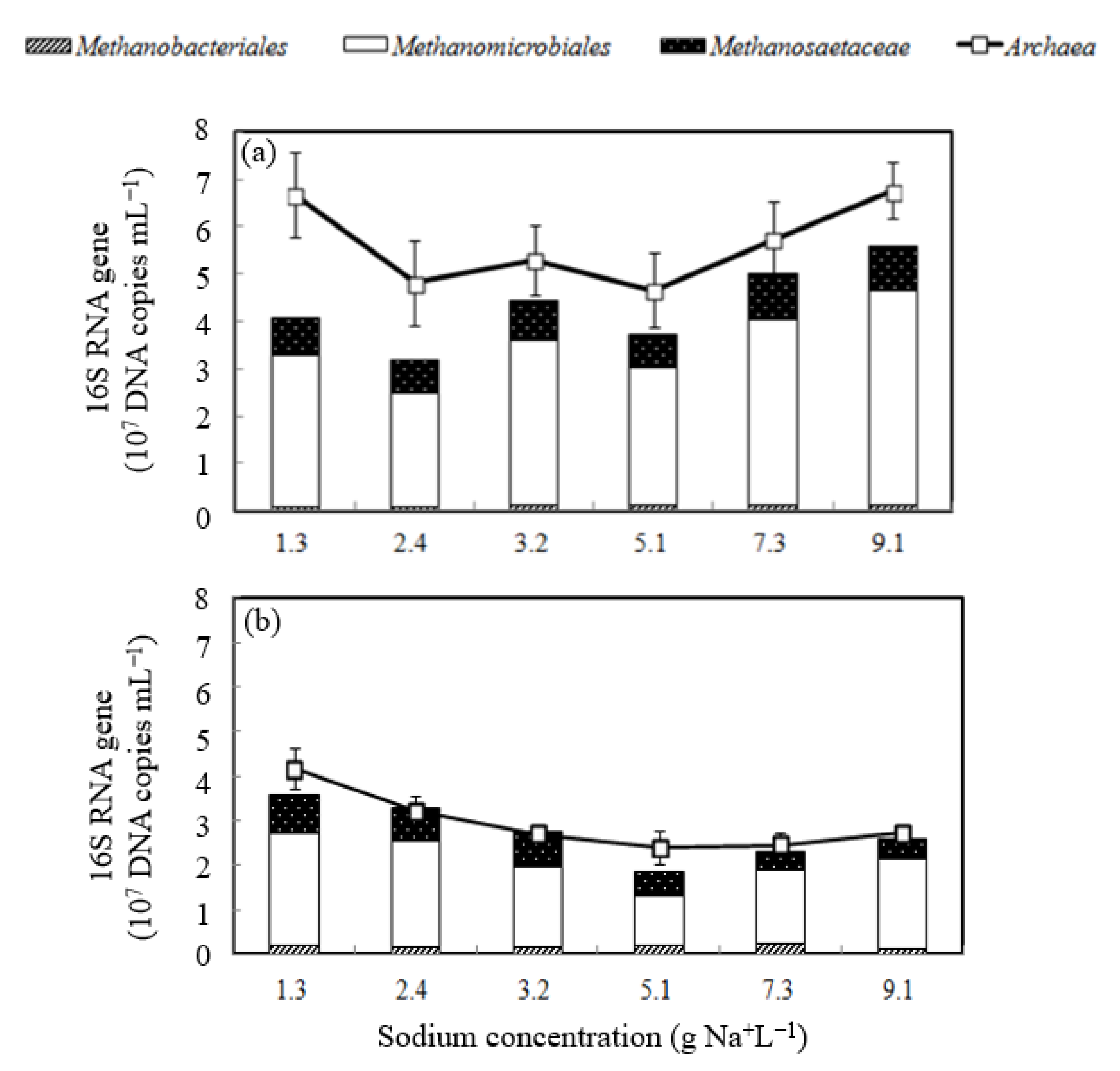
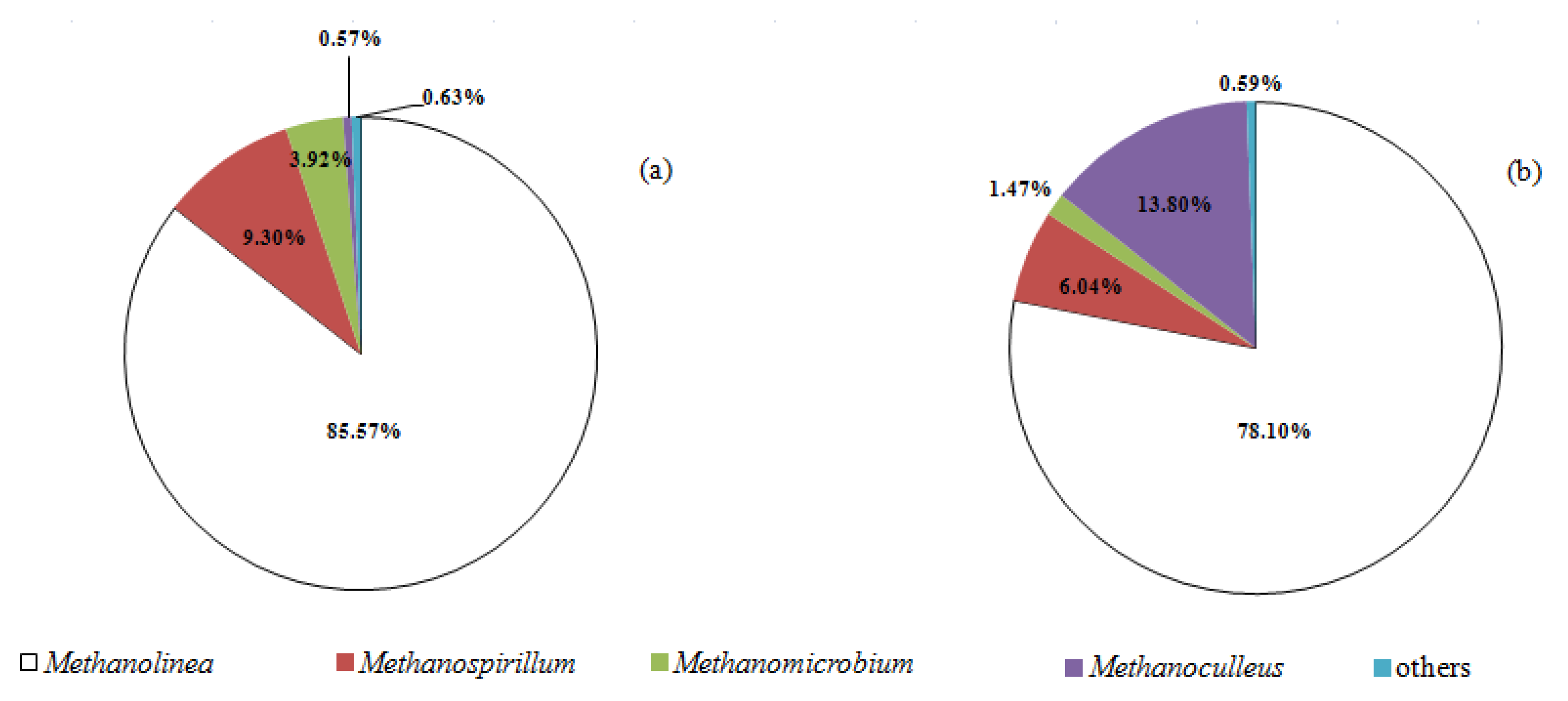
- Methanomicrobiales was the most abundant methanogen in the second-stage reactor;
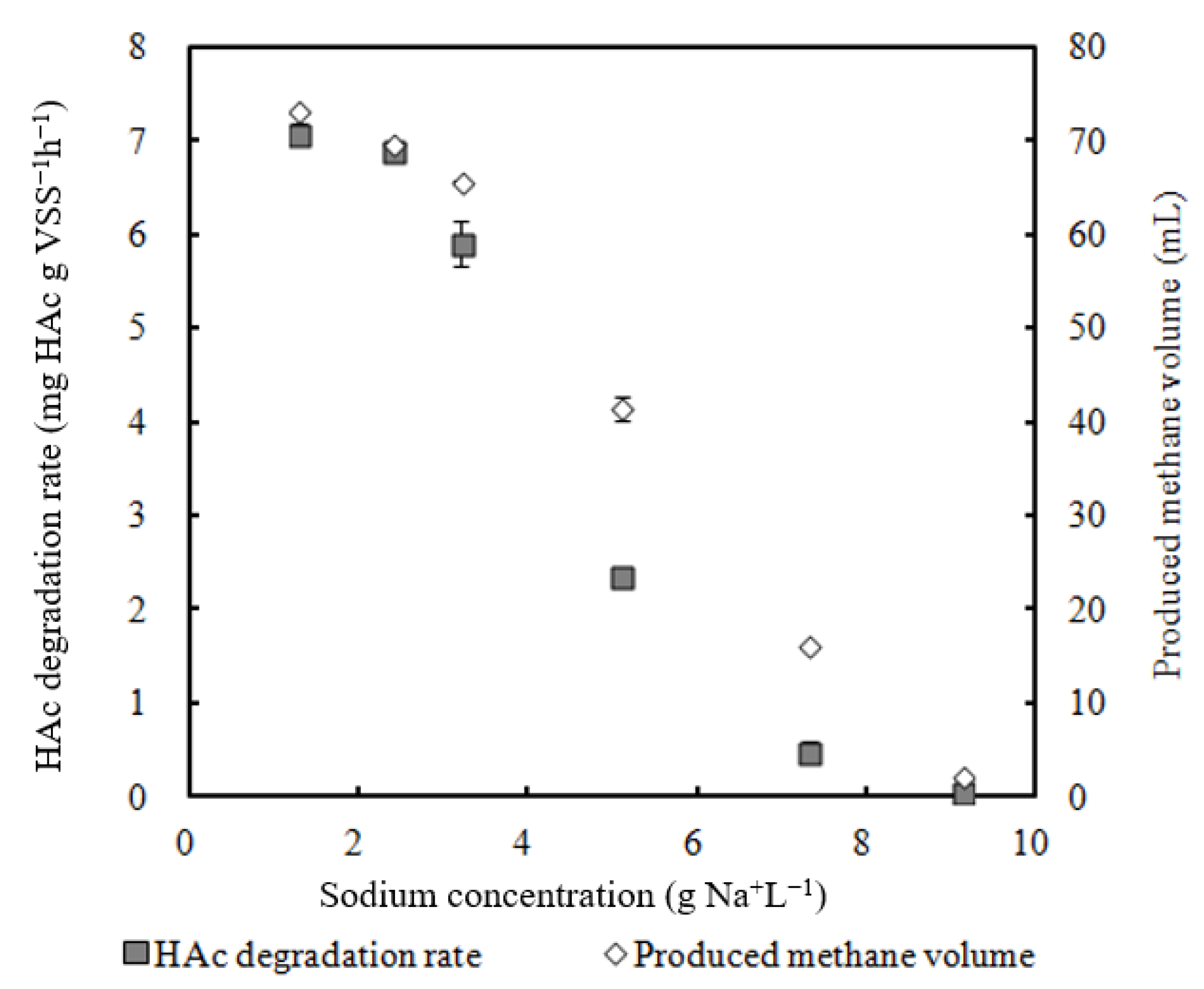
- 2.4 g Na+ L −1 sodium did not have obvious inhibition on the HAc degradation rate;
- Biomass activity decreased with Na content increased from 1.3 to 9.1 g Na+ L−1;
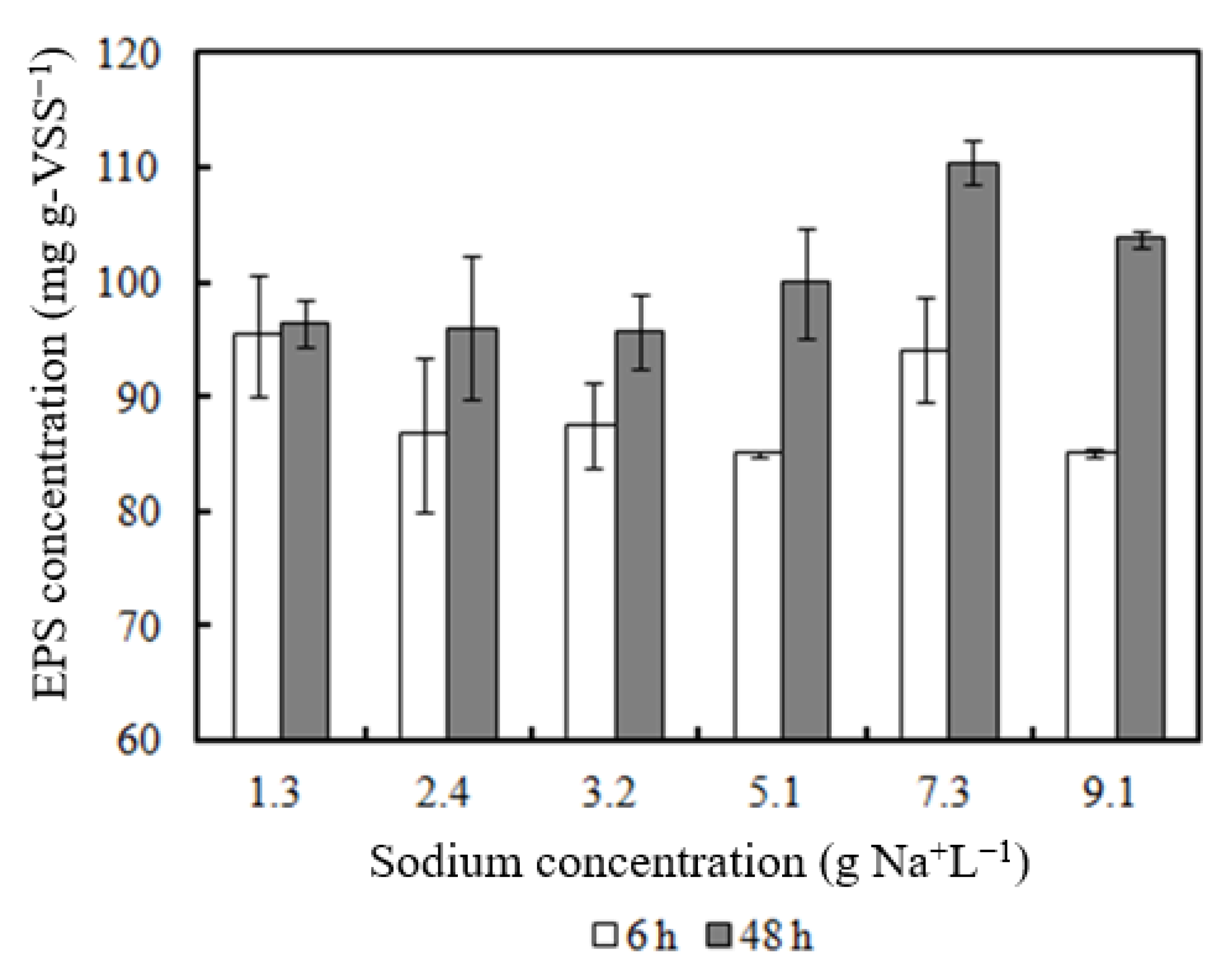
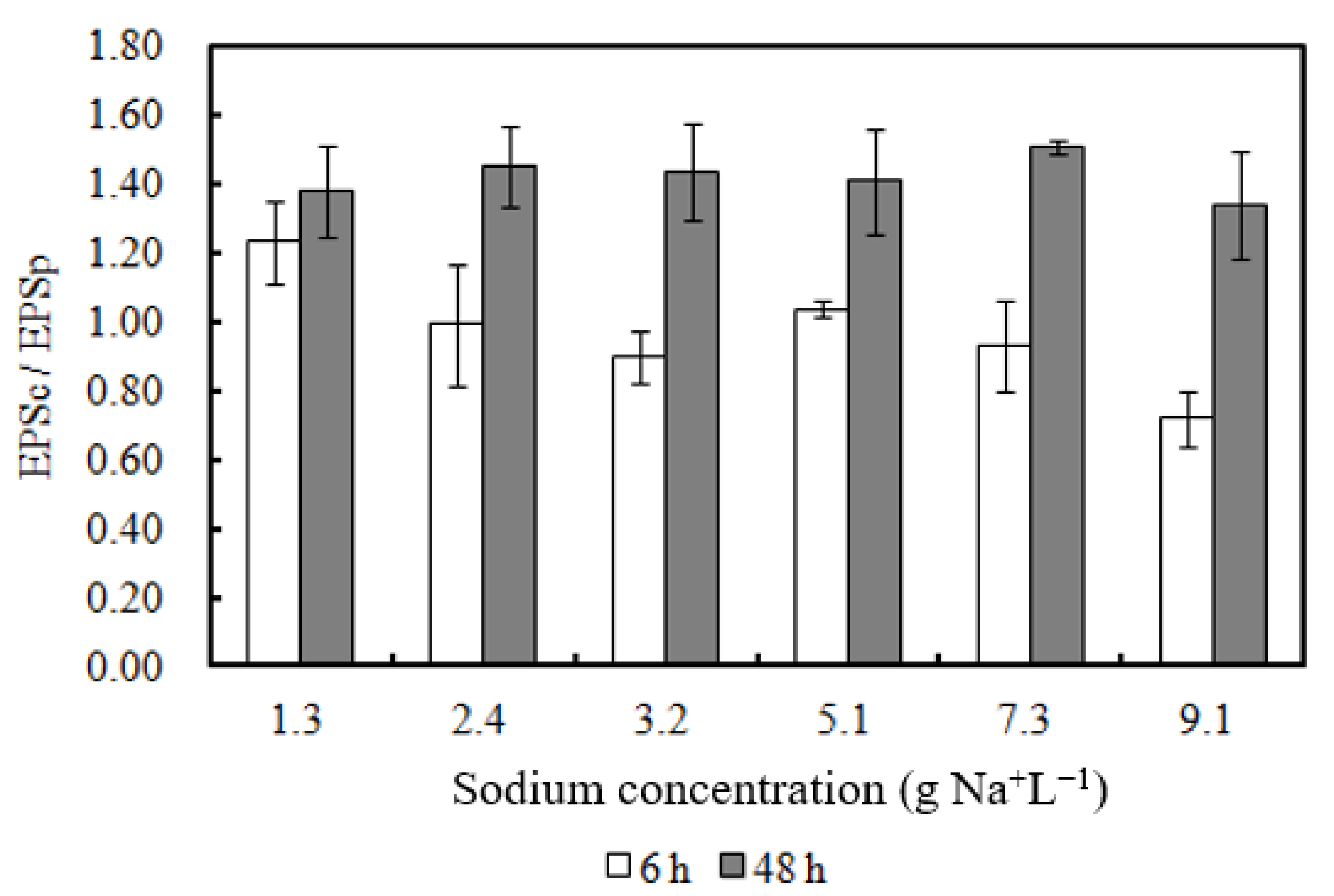
- EPS concentration increased with higher Na concentrations (7.3–9.1 g Na+ L−1).
1. Introduction
2. Materials and Methods
2.1. Bioreactor Set-Up
2.2. Inocula for Batch Tests
2.3. Experimental Set-Up
2.4. Analytical Methods
2.5. Microbial Community Analysis
2.5.1. Viability of Methanogens Incubated with Sodium
2.5.2. Microbial Shift within the Single-Stage and Second-Stage Reactor Populations
3. Results
3.1. Effect of Sodium on Methanogen Activity
3.2. Viability of Methanogens Incubated with Sodium
3.3. Microbial Shift of Biomass
3.4. Activity Evaluation of Biomass by ATP Test
3.5. EPS Content of Biomass Incubated with Sodium
4. Discussion and Conclusions
4.1. Comparison of Tolerance to Sodium
4.2. Sodium Inhibition Mechanism in the Second-Stage Reactor
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ar | Argon |
| CH4 | Methane |
| CO2 | Carbon Dioxide |
| COD | Chemical Oxygen Demand |
| CSTR | Continuous Stirred Tank Reactor |
| EPS | Extracellular Polymeric Substances |
| EPSc | The Carbohydrate Composition of Extracellular Polymeric Substances |
| EPSp | The Protein Composition of Extracellular Polymeric Substances |
| GC | Gas Chromatography |
| H2 | Hydrogen |
| HAc | Acetic Acid |
| He | Helium |
| HRT | Hydraulic Retention Time |
| N2 | Nitrogen |
| Na2CO3 | Sodium Carbonate |
| NaHCO3 | Sodium Bicarbonate |
| NaOH | Sodium Hydroxide |
| O2 | Oxygen |
| PCR | Polymerase Chain Reaction |
| PMA | Propidium Monoazide |
| qPCR | Quantitative Polymerase Chain Reaction |
| SAOB | Syntrophic Acetate Oxidizing Bacteria |
| TCOD | Total Chemical Oxygen Demand |
| TCD | Thermal Conductivity Detector |
| VFA | Volatile Fatty Acids |
| VSS | Volatile Suspended Solids |
References
- Li, H.; Li, C.; Liu, W.; Zou, S. Optimized alkaline pretreatment of sludge before anaerobic digestion. Bioresour. Technol. 2012, 123, 189–194. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef]
- Heo, N.H.; Park, S.C.; Lee, J.S.; Kang, H. Solubilization of waste activated sludge by alkaline pretreatment and biochemical methane potential (BMP) tests for anaerobic co-digestion of municipal organic waste. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2003, 48, 211–219. [Google Scholar] [CrossRef]
- Saiki, Y.; Imabayashi, S.; Iwabuchi, C.; Kitagawa, Y.; Okumura, Y.; Kawamura, H. Solubilization of excess activated sludge by self-digestion. Water Res. 1999, 33, 1864–1870. [Google Scholar] [CrossRef]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition in the anaerobic digestion process: Antagonism and adaptation phenomena. Enzym. Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Hao, X.; Zhou, M.; Yu, H.; Shen, Q.; Lei, L. Effect of Sodium Ion Concentration on Hydrogen Production from Sucrose by Anaerobic Hydrogen-producing Granular Sludge. Chin. J. Chem. Eng. 2006, 14, 511–517. [Google Scholar] [CrossRef]
- Rinzema, A.; van Lier, J.; Lettinga, G. Sodium inhibition of acetoclastic methanogens in granular sludge from a UASB reactor. Enzym. Microb. Technol. 1988, 10, 24–32. [Google Scholar] [CrossRef]
- Liu, Y.; Boone, D.R. Effects of salinity on methanogenic decomposition. Bioresour. Technol. 1991, 35, 271–273. [Google Scholar] [CrossRef]
- Chen, W.H.; Han, S.K.; Sung, S. Sodium inhibition of thermophilic methanogens. J. Environ. Eng. 2003, 129, 506–512. [Google Scholar] [CrossRef]
- Bahareh, K.; Babak, B.; Shahrzad, F. The effect of salt on the performance and characteristics of a combined anaerobic–aerobic biological process for the treatment of synthetic wastewaters containing Reactive Black 5. Chem. Eng. J. 2013, 221, 363–372. [Google Scholar]
- Kargi, F.; Dincer, A.R. Effect of salt concentration on biological treatment of saline wastewater by fed-batch operation. Enzym. Microb. Technol. 1996, 19, 529–537. [Google Scholar] [CrossRef]
- Dinçer, A.R.; Kargi, F. Performance of rotating biological disc system treating saline wastewater. Process Biochem. 2001, 36, 901–906. [Google Scholar] [CrossRef]
- Onodera, T.; Sase, S.; Choeisai, P.; Yoochatchaval, W.; Sumino, H.; Yamaguchi, T.; Ebie, Y.; Xu, K.; Tomioka, N.; Mizuochi, M.; et al. Development of a treatment system for molasses wastewater: The effects of cation inhibition on the anaerobic degradation process. Bioresour. Technol. 2013, 131, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, K.; Ding, L.; Ren, H. Effect of salinity on extracellular polymeric substances (EPS) and soluble microbial products (SMP) in anaerobic sludge systems. Fresenius Environ. Bull. 2009, 18, 1456–1461. [Google Scholar]
- Merlino, G.; Rizzi, A.; Schievano, A.; Tenca, A.; Scaglia, B.; Oberti, R.; Adani, F.; Daffonchio, D. Microbial community structure and dynamics in two-stage vs single-stage thermophilic anaerobic digestion of mixed swine slurry and market bio-waste. Water Res. 2013, 47, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef]
- Nasr, N.; Elbeshbishy, E.; Hafez, H.; Nakhla, G.; Hesham El Naggar, M. Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Bioresour. Technol. 2012, 111, 122–126. [Google Scholar] [CrossRef]
- Orozco, A.M.; Nizami, A.S.; Murphy, J.D.; Groom, E. Optimizing the thermophilic hydrolysis of grass silage in a two-phase anaerobic digestion system. Bioresour. Technol. 2013, 143, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kyazze, G.; Dinsdale, R.; Guwy, A.J.; Hawkes, F.R.; Premier, G.C.; Hawkes, D.L. Performance characteristics of a two-stage dark fermentative system producing hydrogen and methane continuously. Biotechnol. Bioeng. 2007, 97, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.K.; Guo, C.H.; Zhou, Y.; Maspolim, Y.; Wang, J.Y.; Ng, W.J. Acetic acid inhibition on methanogens in a two-phase anaerobic process. Biochem. Eng. J. 2013, 75, 1–7. [Google Scholar] [CrossRef]
- Dulbecco, R.; Vogt, M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J. Exp. Med. 1954, 99, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, V.; Smith, C.; Donovan, A. Microwave digestion of sediment, soils and urban particulate matter for trace metal analysis. Talanta 2003, 60, 715–723. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Water Work Association: Washington, DC, USA, 2005. [Google Scholar]
- Zhou, Y.; Ganda, L.; Lim, M.; Yuan, Z.; Kjelleberg, S.; Ng, W.J. Free nitrous acid (FNA) inhibition on denitrifying poly-phosphate accumulating organisms (DPAOs). Appl. Microbiol. Biotechnol. 2010, 88, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Youming, T.; Maszenan bin Abdul, M.; Santosh, P.; Antonius Yudi, S.; Dongzhe, L.; Jerry Jian Lin, L.; Yan, Z.; Wun Jern, N. Effect of a high strength chemical industry wastewater on microbial community dynamics and mesophilic methane generation. J. Environ. Sci. 2014, 26, 875–884. [Google Scholar]
- Chang, I.-S.; Lee, C.-H. Membrane filtration characteristics in membrane-coupled activated sludge system—The effect of physiological states of activated sludge on membrane fouling. Desalination 1998, 120, 221–233. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Yue, Z.B. Factors influencing the production of extracellular polymeric substances by Rhodopseudomonas acidophila. Int. Biodeterior. Biodegrad. 2006, 58, 89–93. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Shanmugam, P.; Horan, N.J. Simple and rapid methods to evaluate methane potential and biomass yield for a range of mixed solid wastes. Bioresour. Technol. 2009, 100, 471–474. [Google Scholar] [CrossRef]
- Chen, H. ATP as an indicator of biomass activity in thermophilic upflow anaerobic sludge blanket reactor. J. Environ. Sci. 2000, 12, 380–384. [Google Scholar]
- Hwang, S.; Hansen, C.L. Evaluating a correlation between volatile suspended solid and adenosine 5′-triphosphate levels in anaerobic treatment of high organic suspended solids wastewater. Bioresour. Technol. 1998, 63, 243–250. [Google Scholar] [CrossRef]
- Nocker, A.; Camper, A.K. Selective removal of DNA from dead cells of mixed bacterial communities by use of ethidium monoazide. Appl.Environ. Microbiol. 2006, 72, 1997–2004. [Google Scholar] [CrossRef]
- Nocker, A.; Sossa-Fernandez, P.; Burr, M.D.; Camper, A.K. Use of propidium monoazide for live/dead distinction in microbial ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef]
- Nocker, A.; Cheung, C.Y.; Camper, A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Wuertz, S. Discrimination of viable and dead fecal Bacteroidales bacteria by quantitative PCR with propidium monoazide. Appl. Environ. Microbiol. 2009, 75, 2940–2944. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef]
- Kugelman, I.J.; Chin, K.K. Toxicity, synergism, and antagonism in anaerobic waste treatment processes. Adv. Chem. Ser. 1971, 105, 55–90. [Google Scholar]
- Kugelman, I.J.; McCarty, P.L. Cation Toxicity and Stimulation in Anaerobic Waste Treatment; Water Pollution Control Federation, Department of Civil Engineering, New York University: New York, NY, USA, 1965. [Google Scholar]
- Ahring, B.; Alatriste-Mondragon, F.; Westermann, P.; Mah, R. Effects of cations on Methanosarcina thermophila TM-1 growing on moderate concentrations of acetate: Production of single cells. Appl. Microbiol. Biotechnol. 1991, 35, 686–689. [Google Scholar] [CrossRef]
- Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition and sulphate reduction in the anaerobic treatment of mussel processing wastewaters. J. Chem. Technol. Biotechnol. 1993, 58, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Shimada, T.; Morgenroth, E.; Tandukar, M.; Pavlostathis, S.G.; Smith, A.; Raskin, L.; Kilian, R.E. Syntrophic acetate oxidation in two-phase (acid-methane) anaerobic digesters. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2011, 64, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Knapp, B.A.; Farbmacher, T.; Ebner, C.; Insam, H.; Franke-Whittle, I.H. Searching for links in the biotic characteristics and abiotic parameters of nine different biogas plants. Microb. Biotechnol. 2012, 5, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Bialek, K.; Kim, J.; Lee, C.; Collins, G.; Mahony, T.; O’Flaherty, V. Quantitative and qualitative analyses of methanogenic community development in high-rate anaerobic bioreactors. Water Res. 2011, 45, 1298–1308. [Google Scholar] [CrossRef]
- O’Reilly, J.; Lee, C.; Collins, G.; Chinalia, F.; Mahony, T.; O’Flaherty, V. Quantitative and qualitative analysis of methanogenic communities in mesophilically and psychrophilically cultivated anaerobic granular biofilims. Water Res. 2009, 43, 3365–3374. [Google Scholar] [CrossRef]
- Jaenicke, S.; Ander, C.; Bekel, T.; Bisdorf, R.; Droge, M.; Gartemann, K.H.; Junemann, S.; Kaiser, O.; Krause, L.; Tille, F.; et al. Comparative and joint analysis of two metagenomic datasets from a biogas fermenter obtained by 454-pyrosequencing. PLoS ONE 2011, 6, e14519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Lee, C. Absolute dominance of hydrogenotrophic methanogens in full-scale anaerobic sewage sludge digesters. J. Environ. Sci. 2013, 25, 2272–2280. [Google Scholar] [CrossRef]
- Claus, H.; Akca, E.; Debaerdemaeker, T.; Evrard, C.; Declercq, J.P.; Konig, H. Primary structure of selected archaeal mesophilic and extremely thermophilic outer surface layer proteins. Syst. Appl. Microbiol. 2002, 25, 3–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hattori, S. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. JSME 2008, 23, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef]
- Lee, M.J.; Zinder, S.H. Isolation and Characterization of a Thermophilic Bacterium Which Oxidizes Acetate in Syntrophic Association with a Methanogen and Which Grows Acetogenically on H(2)-CO(2). Appl. Environ. Microbiol. 1988, 54, 124–129. [Google Scholar] [CrossRef]
- Balk, M.; Weijma, J.; Stams, A.J. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int. J. Syst. Evol. Microbiol. 2002, 52, 1361–1368. [Google Scholar]
- Schnurer, A.; Schink, B.; Svensson, B.H. Clostridium ultunense sp. nov., a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogenic bacterium. Int. J. Syst. Bacteriol. 1996, 46, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Kamagata, Y.; Hanada, S.; Shoun, H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 4, 1601–1609. [Google Scholar] [CrossRef]
- Westerholm, M.; Roos, S.; Schnurer, A. Syntrophaceticus schinkii gen. nov., sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter. FEMS Microbiol. Lett. 2010, 309, 100–104. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, P.-J.; Shao, L.-M. Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res. 2011, 45, 1711–1719. [Google Scholar] [CrossRef]
- de Baere, L.A.; Devocht, M.; Van Assche, P.; Verstraete, W. Influence of high NaCl and NH4Cl salt levels on methanogenic associations. Water Res. 1984, 18, 543–548. [Google Scholar] [CrossRef]
- Kargi, F.; Dinçer, A.R. Saline Wastewater Treatment By Halophile-Supplemented Activated Sludge Culture in an Aerated Rotating Biodisc Contactor. Enzym. Microb. Technol. 1998, 22, 427–433. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 1507–1538. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Neethling, J.B. Microbial activity measurement for anaerobic sludge digestion. Water Pollut. Control Fed. 1989, 61, 343–349. [Google Scholar]
- Chung, Y.C.; Neethling, J.B. ATP as a measure of anaerobic sludge digester activity. Water Environ. Fed. 1988, 60, 107–112. [Google Scholar]
- Myoung-Joo, L.; Tae-Hyeong, K.; Booki, M.; Sun-Jin, H. Sodium(Na+) concentration effects on metabolic pathway and estimation of ATP use in dark fermentation hydrogen production through stoichiometric analysis. J. Environ. Manag. 2012, 108, 22–26. [Google Scholar]
- Liu, H.; Buskey, E.J. Hypersalinity enhances the production of extracellular polymeric substance (eps) in the texas brown tide alga, aureoumbra lagunensis (PELAGOPHYCEAE). J. Phycol. 2000, 36, 71–77. [Google Scholar] [CrossRef]
- Zhang, X.; Bishop, P.L. Spatial distribution of extracellular polymeric substances in biofilms. J. Environ. Eng. 2001, 127, 850–856. [Google Scholar] [CrossRef]

| Test Substrate | Microorganisms | Test System | Sodium Concentration (g Na+L−1) | Sodium Concentration (g Na+L−1) Leading to 10% Inhibition | References |
|---|---|---|---|---|---|
| HAc | Mixed culture, presumably Methanosarcinaceae | CSTR, shock exposure | 1.15 | 4.8 | [39] |
| HAc | Mixed culture, presumably Methanosarcinaceae | CSTR, continuous exposure | 0.46 | 6.1 | [38] |
| HAc | Mixed culture | Anaerobic sequencing batch reactors, 55 °C | 0 | 1.3 | [37] |
| HAc | Mixed culture | Anaerobic sequencing batch reactors, 55 °C | 4.1 | 1.6 | [7] |
| HAc | Mixed culture | Anaerobic sequencing batch reactors, 55 °C | 7.1 | 1 | [7] |
| HAc | Mixed culture | Anaerobic sequencing batch reactors, 55 °C | 12 | 1.2 | [7] |
| HAc | Mixed culture | Up-flow anaerobic sludge blanket, 30 °C | 10 | 4.8 | [7] |
| HAc | Mixed culture | Anaerobic filter, 37 °C | 5–10 | 8.5 | [5] |
| HPr | Mixed culture | Anaerobic filter, 37 °C | 5–10 | 5.0 | [5] |
| HBu | Mixed culture | Anaerobic filter, 37 °C | 5–10 | 12.5 | [5] |
| HAc | Methanosarcina thermophila | Batch study, 52 °C | 1.15 | 0.7–3 | [40] |
| VFA | Mixed culture | Anaerobic filter, 37 °C | 10 | 9.0 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, K.; Maspolim, Y.; Zhou, Y.; Guo, C.; Ng, W.J. Effect of Sodium on Methanogens in a Two-Stage Anaerobic System. Appl. Sci. 2022, 12, 956. https://doi.org/10.3390/app12030956
Xiao K, Maspolim Y, Zhou Y, Guo C, Ng WJ. Effect of Sodium on Methanogens in a Two-Stage Anaerobic System. Applied Sciences. 2022; 12(3):956. https://doi.org/10.3390/app12030956
Chicago/Turabian StyleXiao, Keke, Yogananda Maspolim, Yan Zhou, Chenghong Guo, and Wun Jern Ng. 2022. "Effect of Sodium on Methanogens in a Two-Stage Anaerobic System" Applied Sciences 12, no. 3: 956. https://doi.org/10.3390/app12030956
APA StyleXiao, K., Maspolim, Y., Zhou, Y., Guo, C., & Ng, W. J. (2022). Effect of Sodium on Methanogens in a Two-Stage Anaerobic System. Applied Sciences, 12(3), 956. https://doi.org/10.3390/app12030956







