A New Era for the Early and Non-Invasive Diagnosis of Giant Cell Arteritis: The Use of Fast-Track Ultrasound in Clinical Practice
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
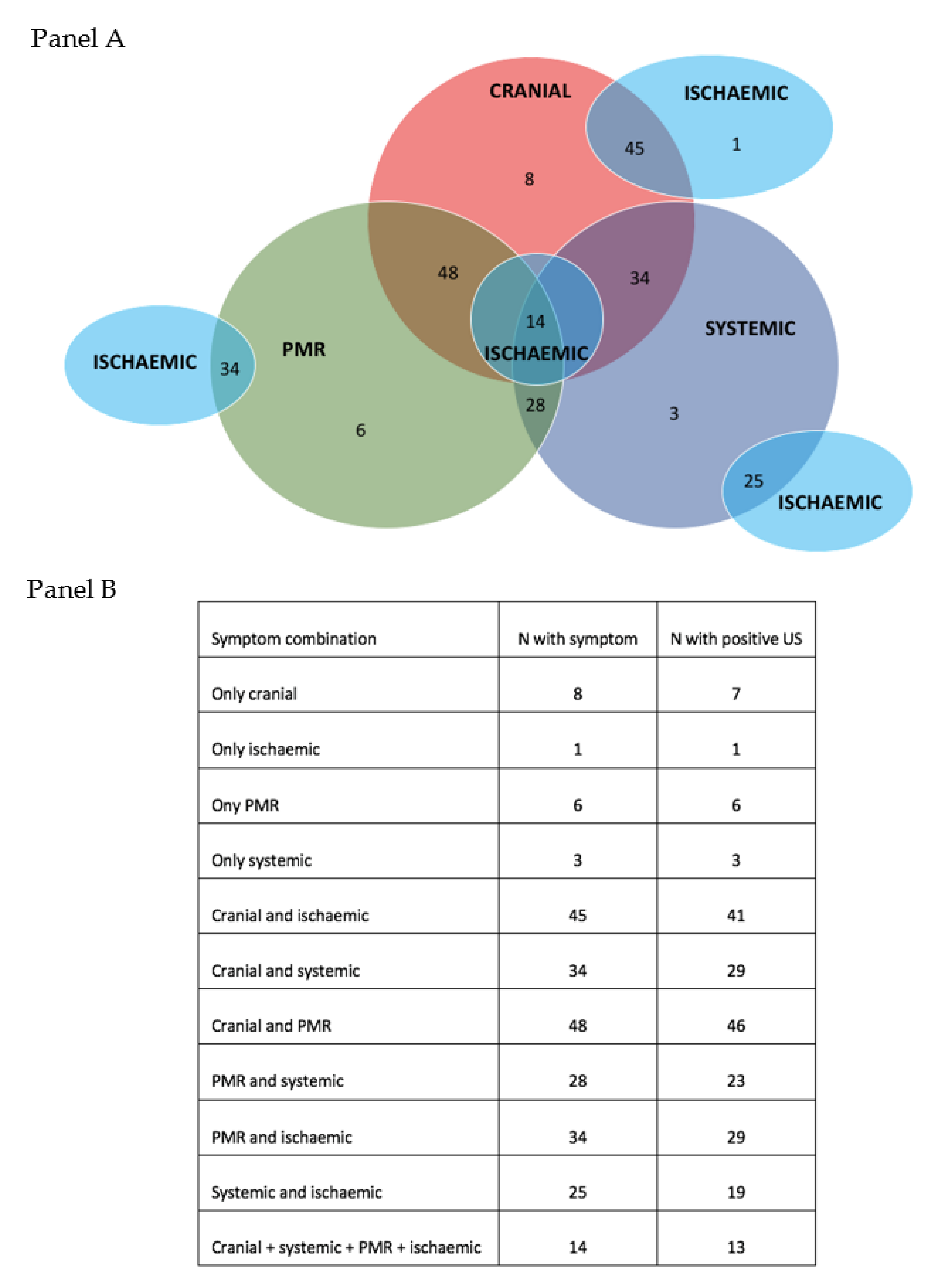
3. Results
3.1. Clinical Presentation of Patients Referred to the Fast-Track Clinic
3.2. US Findings in First Referrals
3.3. Relapsing Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvarani, C.; Cantini, F.; Boiardi, L.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. N. Engl. J Med. 2002, 347, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef]
- Chrysidis, S.; Duftner, C.; Dejaco, C.; Schäfer, V.S.; Ramiro, S.; Carrara, G.; Scirè, C.A.; Hocevar, A.; Diamantopoulos, A.P.; Iagnocco, A.; et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: A study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018, 4, e000598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luqmani, R.; Lee, E.; Singh, S.; Gillett, M.; Schmidt, W.A.; Bradburn, M.; Dasgupta, B.; Diamantopoulos, A.P.; Forrester-Barker, W.; Hamilton, W.; et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Heal. Technol. Assess. 2016, 20, 1–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Diamantopoulos, A.P.; Haugeberg, G.; Lindland, A.; Myklebust, G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: Towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016, 55, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, P.; Williams, M.; Maw, W.W.; Achilleos, K.; Elsideeg, S.; Dejaco, C.; Borg, F.; Gupta, S.; Dasgupta, B. Fast track pathway reduces sight loss in giant cell arteritis: Results of a longitudinal observational cohort study. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S89), S103–S106. [Google Scholar]
- Monti, S.; Bartoletti, A.; Bellis, E.; Delvino, P.; Montecucco, C. Fast-track ultrasound clinic for the diagnosis of giant cell arteritis changes the prognosis of the disease but not the risk of future relapse. Front. Med. 2020, 7, 589794. [Google Scholar] [CrossRef]
- Schmidt, W.A. Ultrasound in vasculitis. Clin. Exp. Rheumatol. 2014, 32 (Suppl. S80), S71–S77. [Google Scholar]
- Schmidt, W.A.; Seifert, A.; Gromnica-Ihle, E.; Krause, A.; Natusch, A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology 2008, 47, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Ponte, C.; Monti, S.; Scirè, C.A.; Delvino, P.; Khmelinskii, N.; Milanesi, A.; Teixeira, V.; Brandolino, F.; Diamantino Saraiva, F.M.; Montecucco, C.; et al. Ultrasound halo sign as a potential monitoring tool for patients with giant cell arteritis: A prospective analysis. Ann. Rheum. Dis. 2021, 80, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Floris, A.; Ponte, C.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Piper, J.; Luqmani, R. The use of ultrasound to assess giant cell arteritis: Review of the current evidence and practical guide for the rheumatologist. Rheumatology 2017, 57, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, V.S.; Jin, L.; Schmidt, W.A. Imaging for diagnosis, monitoring, and outcome prediction of large vessel vasculitides. Curr. Rheumatol. Rep. 2020, 22, 76. [Google Scholar] [CrossRef]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Mackie, S.L.; Dejaco, C.; Appenzeller, S.; Camellino, D.; Duftner, C.; Gonzalez-Chiappe, S.; Mahr, A.; Mukhtyar, C.; Reynolds, G.; de Sousa, A.W.S.; et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology 2020, 59, e1–e23. [Google Scholar] [CrossRef] [Green Version]
- Duftner, C.; Dejaco, C.; Sepriano, A.; Falzon, L.; Schmidt, W.A.; Ramiro, S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: A systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018, 4, e000612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, S.; Águeda, A.F.; Luqmani, R. The use of ultrasound in the management of large-vessel vasculitis: An evolving concept. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S114), 96–102. [Google Scholar]
- Fernández-Fernández, E.; Monjo-Henry, I.; Bonilla, G.; Plasencia, C.; Miranda-Carús, M.E.; Balsa, A.; De Miguel, E. False positives in the ultrasound diagnosis of giant cell arteritis: Some diseases can also show the halo sign. Rheumatology 2020, 59, 2443–2447. [Google Scholar] [CrossRef]
- Kanaan, M.Z.; Lorenzi, A.R.; Thampy, N.; Pandit, R.; Dayan, M. Bilateral non-arteritic anterior ischaemic optic neuropathy as the presentation of systemic amyloidosis. Neuro Ophthalmol. 2017, 41, 330–334. [Google Scholar] [CrossRef]
- Chrysidis, S.; Lewinski, M.; Schmidt, W.A. Temporal arteritis with ultrasound halo sign in eosinophilic granulomatosis with polyangiitis. Rheumatology 2019, 58, 2069–2071. [Google Scholar] [CrossRef]
- Monti, S.; Delvino, P.; Bellis, E.; Milanesi, A.; Brandolino, F.; Montecucco, C. Impact of delayed diagnoses at the time of COVID-19: Increased rate of preventable bilateral blindness in giant cell arteritis. Ann. Rheum. Dis. 2020, 79, 1658–1659. [Google Scholar] [CrossRef]
- Sebastian, A.; Tomelleri, A.; Kayani, A.; Prieto-Pena, D.; Ranasinghe, C.; Dasgupta, B. Probability-based algorithm using ultrasound and additional tests for suspected GCA in a fast-track clinic. RMD Open 2020, 6, e001297. [Google Scholar] [CrossRef]

| GCA (N= 111) | NOT GCA (N = 176) | Overall (N = 287) | p | |
|---|---|---|---|---|
| Headache | 74 (66.6%) | 65 (36.9%) | 138 (47.9%) | <0.0001 |
| Jaw claudication | 40 (36%) | 4 (2.3%) | 50 (17.3%) | <0.0001 |
| Tongue claudication | 11 (9.9%) | 0 | 11 (3.8%) | <0.0001 |
| Blurred vision | 6 (5.4%) | 4 (2.3%) | 10 (3.5%) | 0.165 |
| Visual loss | 12 (10.8%) | 8 (4.5%) | 20 (6.9%) | 0.041 |
| Bilateral visual loss | 3 (2.7%) | 0 | 5 (1.7%) | 0.029 |
| Scalp tenderness | 25 (22.5%) | 1 (0.56%) | 26 (9%) | <0.0001 |
| Polymyalgia rheumatica | 61 (54.9%) | 66 (37.5%) | 127 (44%) | 0.039 |
| Systemic symptoms | 47 (42.3%) | 32 (18.1%) | 79 (27.4%) | <0.0001 |
| ≥2 systemic symptoms | 10 (9%) | 4 (2.3%) | 14 (4.9%) | 0.011 |
| Isolated raised inflammatory markers | 1 (0.9%) | 9 (5.1%) | 10 (3.5%) | 0.058 |
| Normal inflammatory markers | 2 (1.8%) | 45 (25.6%) | 47 (16.3%) | <0.0001 |
| Fever | 29 (26.1%) | 13 (7.4%) | 42 (24.5%) | <0.0001 |
| Weight loss | 33 (29.7%) | 19 (10.7%) | 52 (18%) | <0.0001 |
| Arthritis | 5 (4.5%) | 7 (3.9%) | 12 (4.2%) | 0.804 |
| Non-productive cough | 3 (2.7%) | 2 (1.1%) | 5 (1.7%) | 0.312 |
| Mean ESR value (mm/h) | 59 ± 35 | 58 ± 35 | 58 ± 35 | 0.814 |
| Mean CRP value (mg/L) | 43 ± 50 | 42 ± 50 | 42 ± 50 | 0.869 |
| Mean prednisone-equivalent dose on the day of US scan (mg/day) | 18 ± 64 | 17 ± 64 | 17 ± 64 | 0.897 |
| Not treated with glucocorticoids on the day of US scan | 21 (19%) | 66 (38%) | 87 (30%) | 0.0007 |
| Relapse Confirmed (N = 46) | Relapse Not Confirmed (N = 42) | Overall (N = 88) | p | |
|---|---|---|---|---|
| Headache | 13 (28%) | 19 (45.2%) | 32 (36%) | 0.095 |
| Jaw claudication | 1 (2.2%) | 0 | 1 (1.1%) | 0.0336 |
| Tongue claudication | 0 | 0 | 0 | na |
| Blurred vision | 1 (2.2%) | 2 (4.7%) | 3 (3.4%) | 0.520 |
| Visual loss | 0 | 0 | 0 | na |
| Bilateral visual loss | 0 | 0 | 0 | na |
| Scalp tenderness | 0 | 1 (2.3%) | 1 (1.1%) | 0.304 |
| Polymyalgia rheumatica | 15 (32%) | 0 | 15 (17%) | 0.0001 |
| Systemic symptoms | 5 (11%) | 4 (9.5%) | 9 (10%) | 0.818 |
| ≥2 systemic symptoms | 2 (4.3%) | 1 (2.3%) | 3 (3.4%) | 0.604 |
| Isolated raised inflammatory markers | 10 (21.7%) | 13 (30.9%) | 23 (25.8%) | 0.329 |
| Normal inflammatory markers | 10 (21.7%) | 7 (16.6%) | 17 (19.1%) | 0.547 |
| Fever | 2 (4.3%) | 2 (4.7%) | 4 (2.3%) | 0.928 |
| Weight loss | 4 (8.6%) | 2 (4.7%) | 6 (6.7%) | 0.418 |
| Arthritis/arthralgia | 1 (2.2%) | 3 (7.1%) | 4 (2.3%) | 0.273 |
| Non-productive cough | 0 | 1 (2.3%) | 1 (1.1%) | 0.304 |
| Mean ESR value (mm/h) | 36 ± 24 | 37 ± 30 | 36 ± 24 | 0.863 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, S.; Delvino, P.; Montecucco, C. A New Era for the Early and Non-Invasive Diagnosis of Giant Cell Arteritis: The Use of Fast-Track Ultrasound in Clinical Practice. Appl. Sci. 2022, 12, 1621. https://doi.org/10.3390/app12031621
Monti S, Delvino P, Montecucco C. A New Era for the Early and Non-Invasive Diagnosis of Giant Cell Arteritis: The Use of Fast-Track Ultrasound in Clinical Practice. Applied Sciences. 2022; 12(3):1621. https://doi.org/10.3390/app12031621
Chicago/Turabian StyleMonti, Sara, Paolo Delvino, and Carlomaurizio Montecucco. 2022. "A New Era for the Early and Non-Invasive Diagnosis of Giant Cell Arteritis: The Use of Fast-Track Ultrasound in Clinical Practice" Applied Sciences 12, no. 3: 1621. https://doi.org/10.3390/app12031621
APA StyleMonti, S., Delvino, P., & Montecucco, C. (2022). A New Era for the Early and Non-Invasive Diagnosis of Giant Cell Arteritis: The Use of Fast-Track Ultrasound in Clinical Practice. Applied Sciences, 12(3), 1621. https://doi.org/10.3390/app12031621






