SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
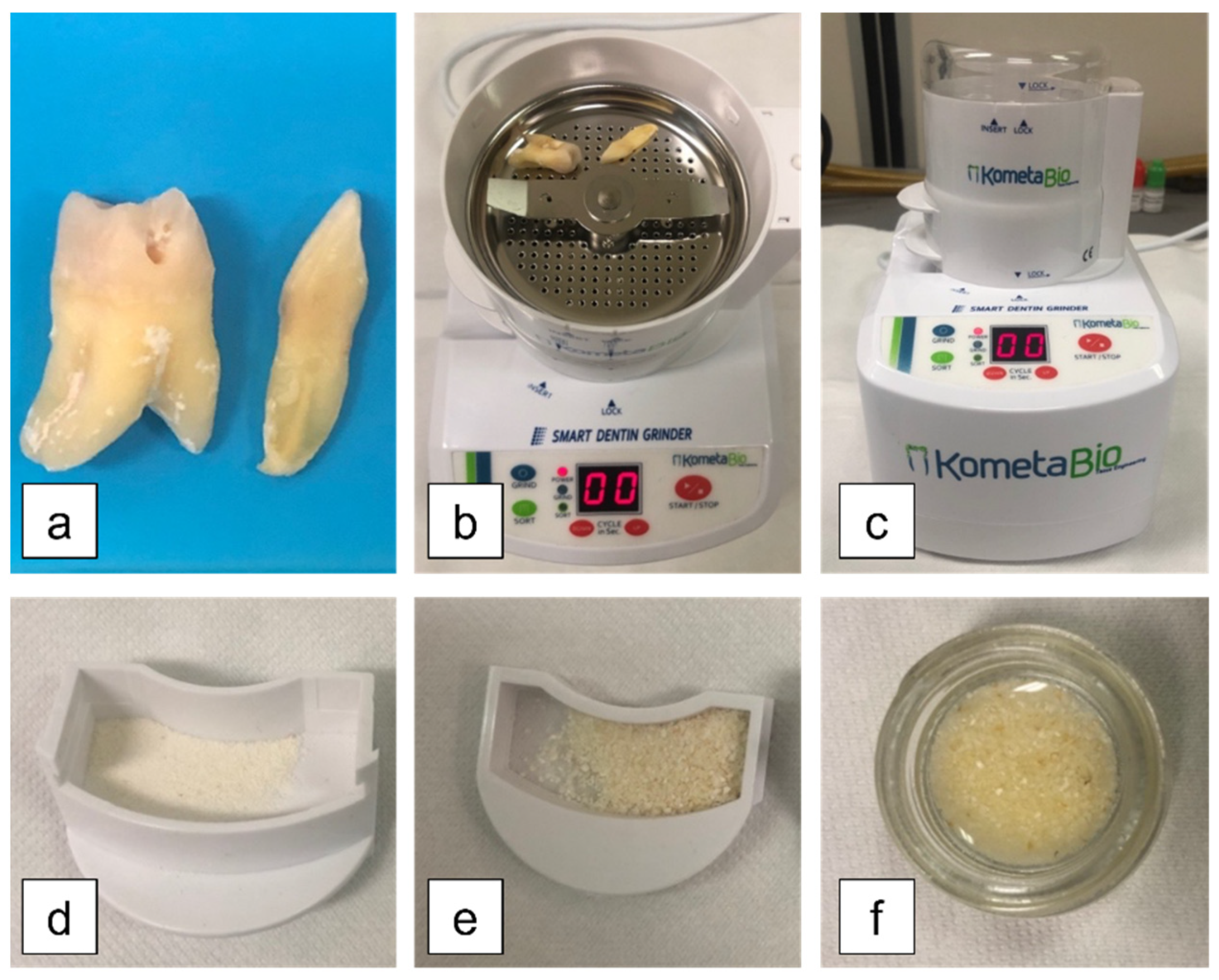
2.1. Experimental Design and Specimen Preparation
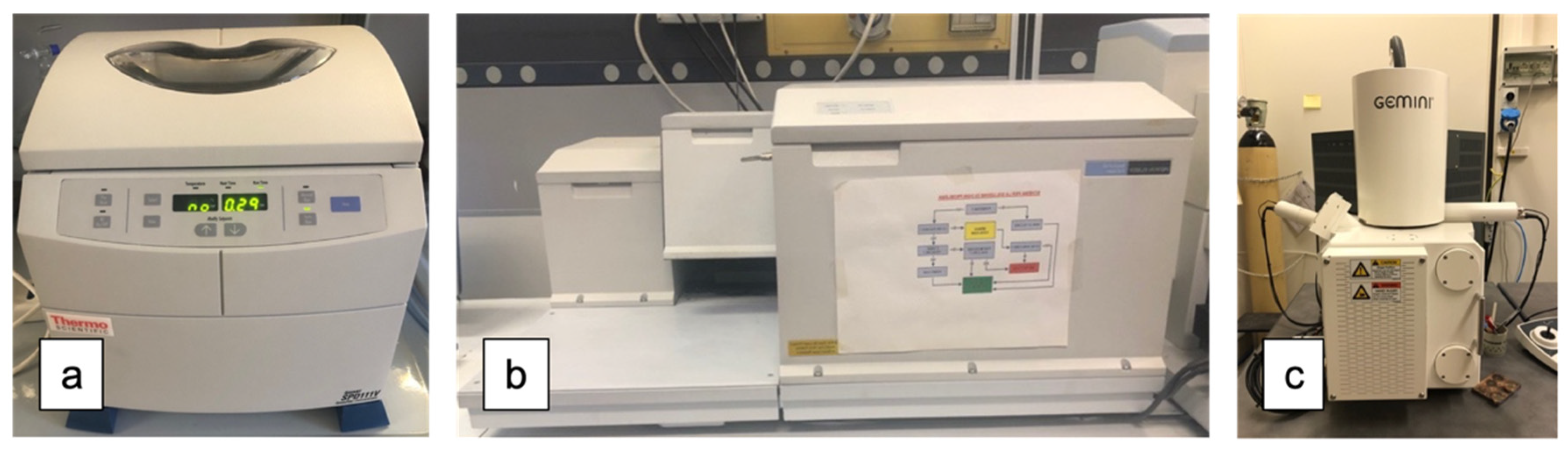
2.2. FT-MIR Analysis
2.3. SEM Analysis
2.4. Statistical Analysis
3. Results
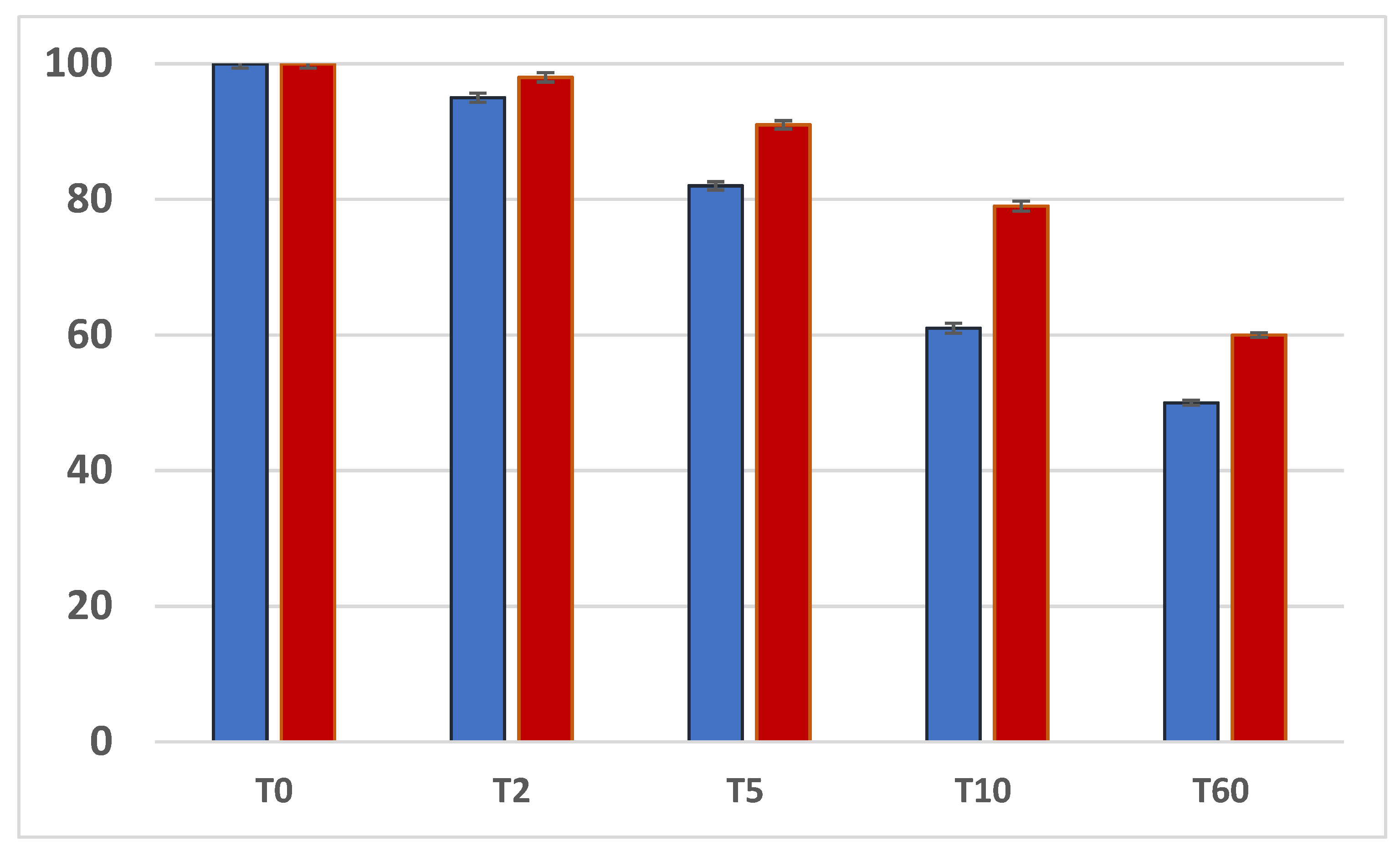
3.1. Weight Analysis of the Specimens
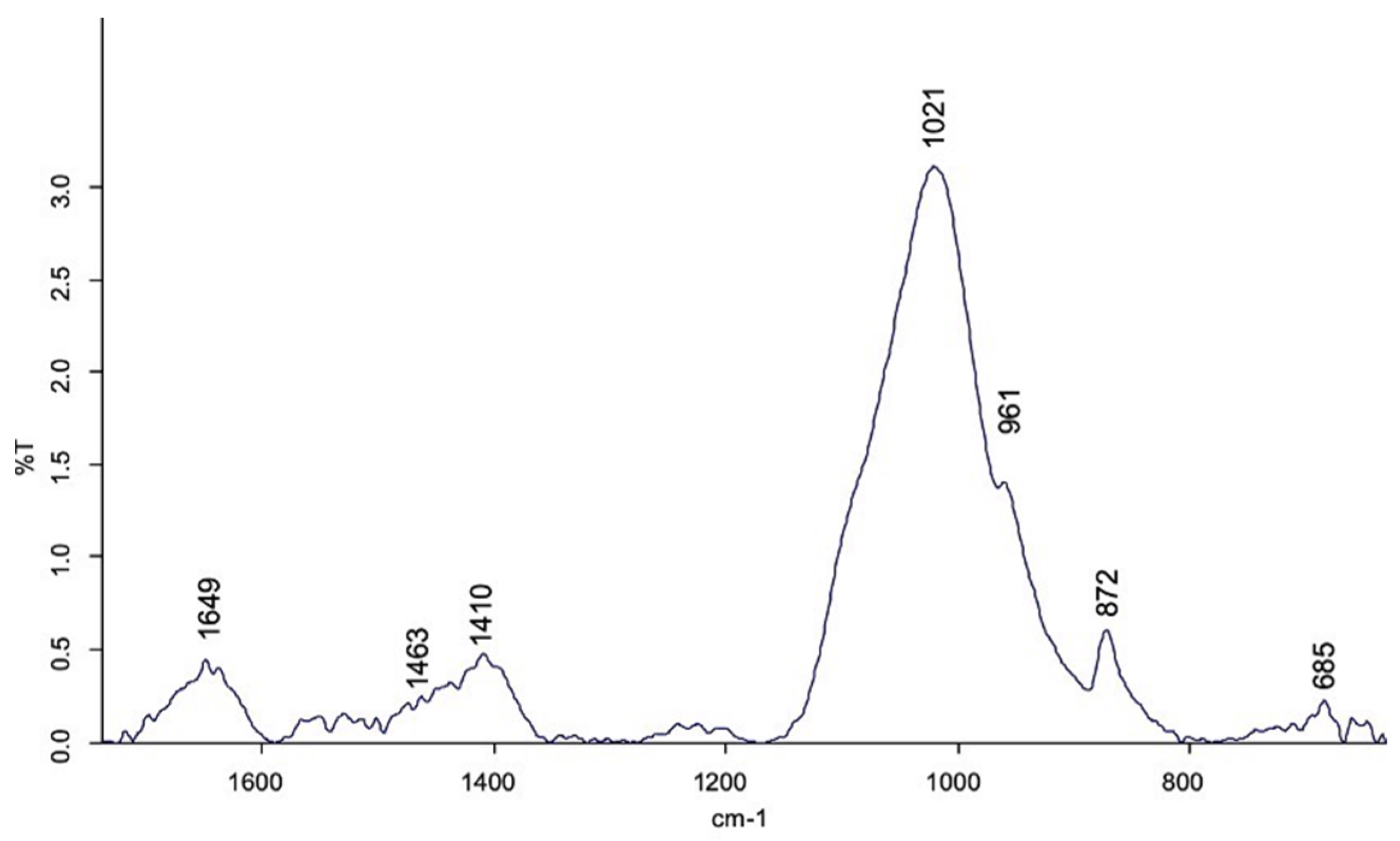
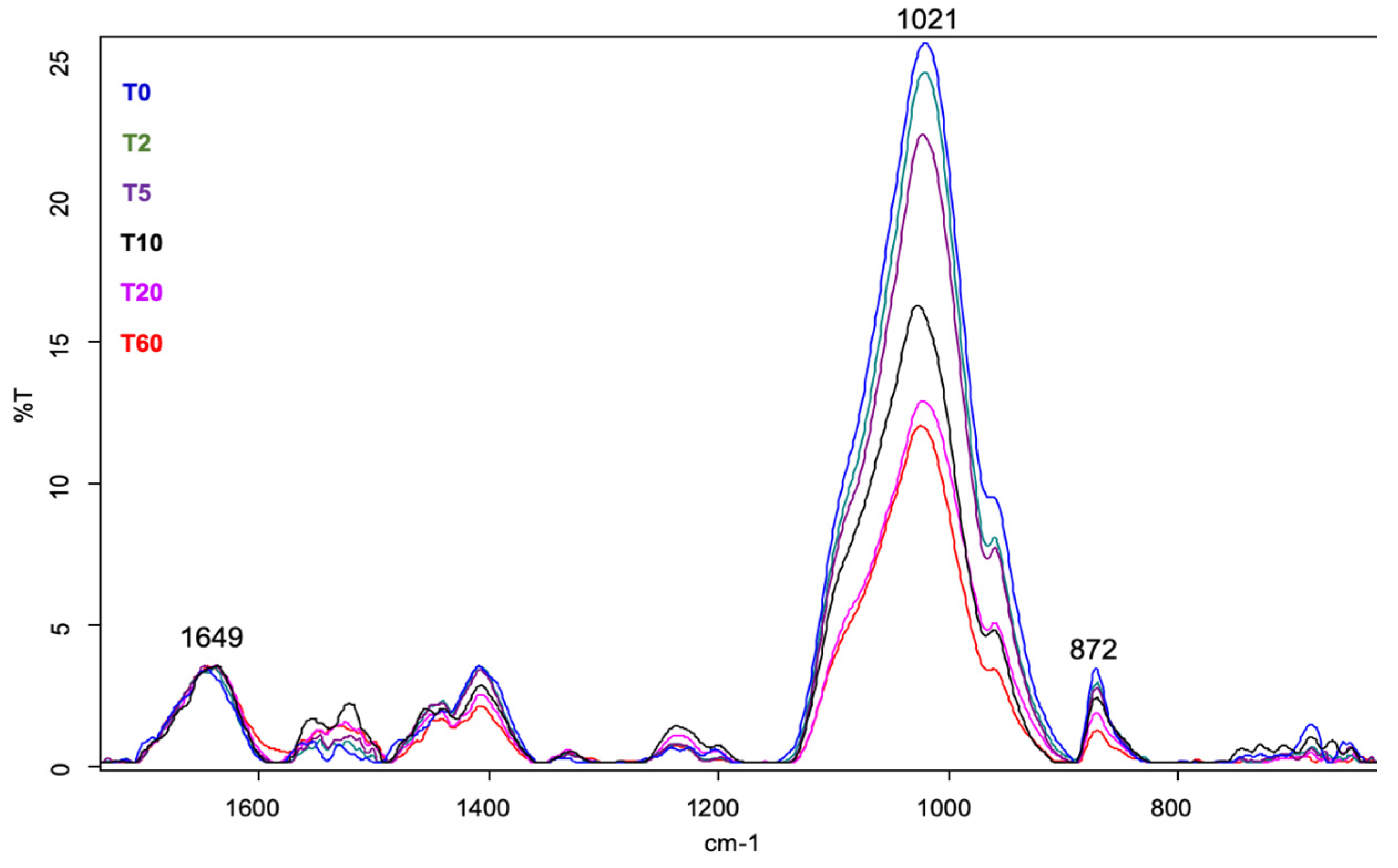
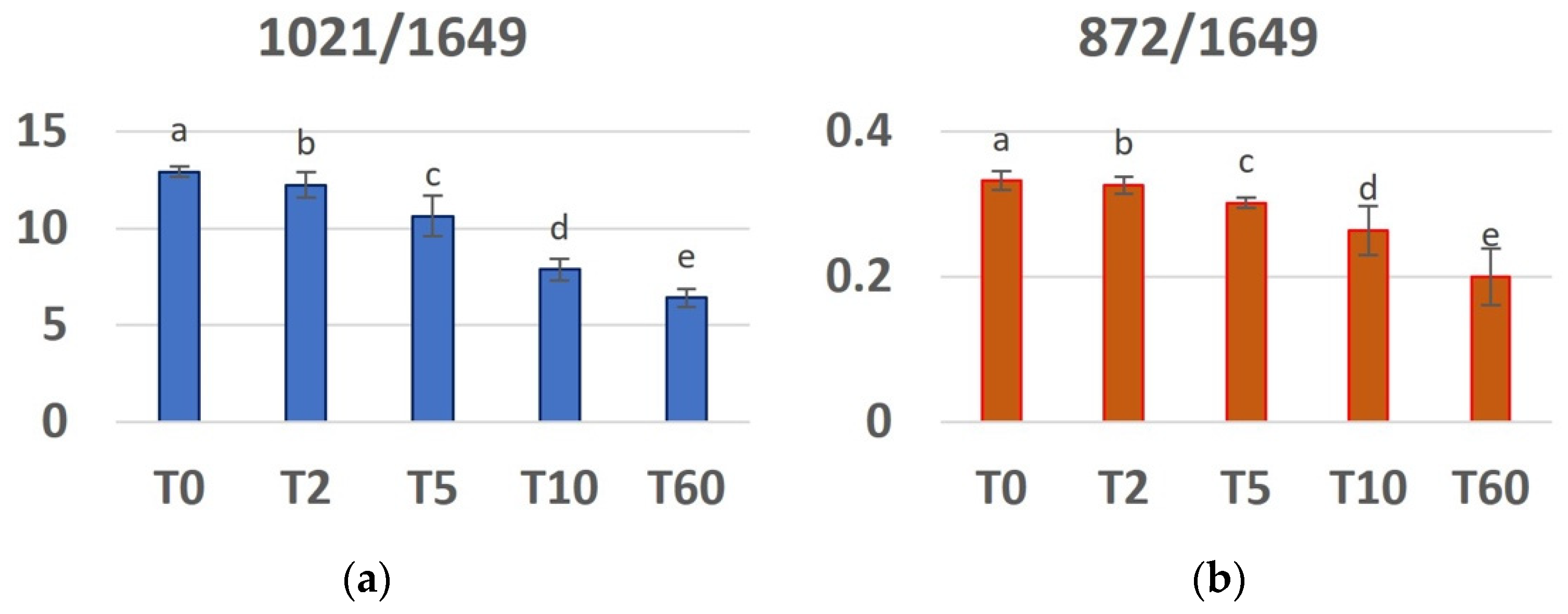
3.2. FT-MIR
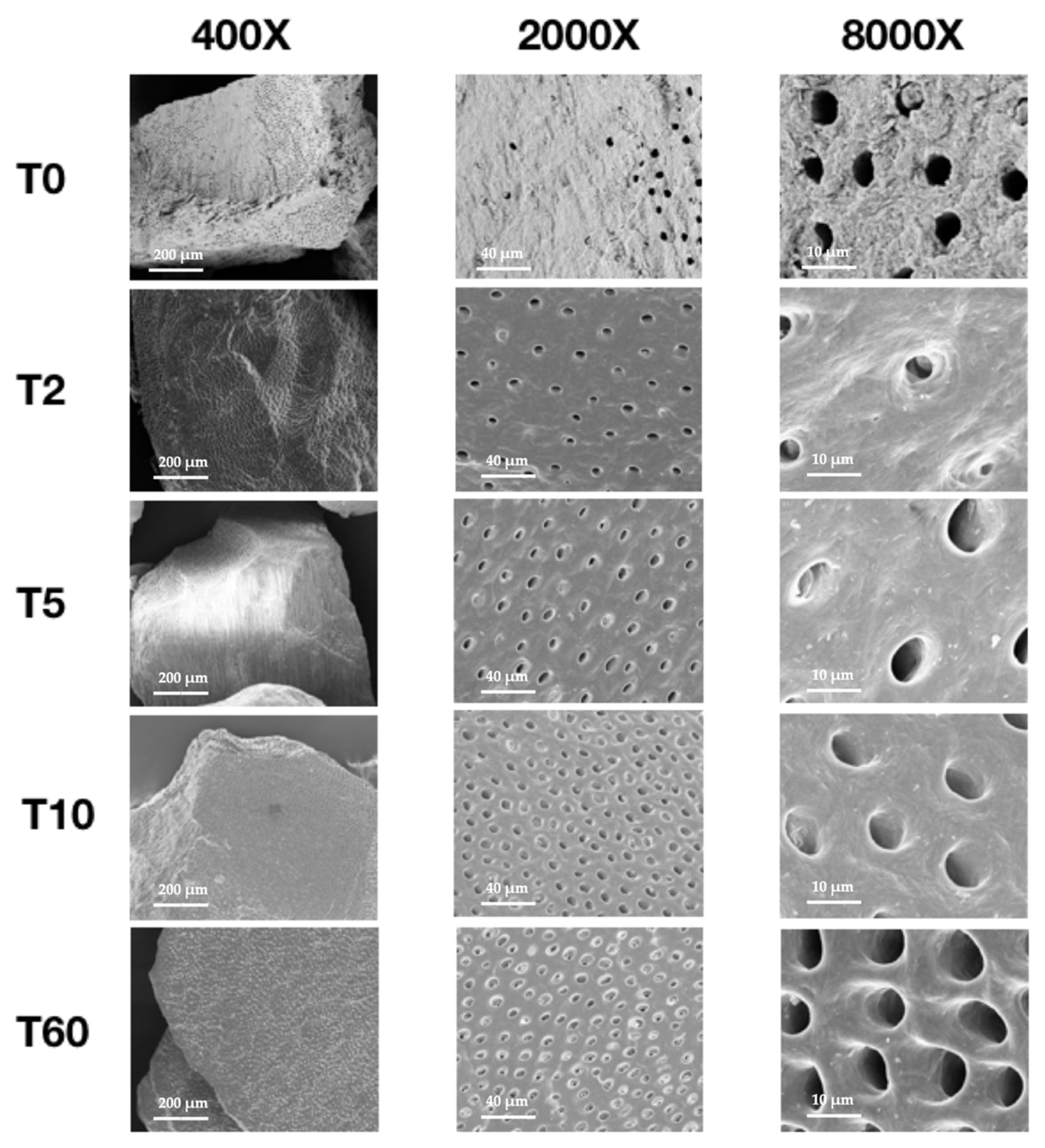
3.3. SEM
4. Discussion
5. Conclusions
- -
- there is a progressive reduction in the concentration of both PO43− and CO32− with increasing demineralization time;
- -
- the organic (protein) component does not change during the demineralization process;
- -
- increasing the duration of demineralization results in dentin particles with smoother surfaces and higher numbers of dentinal tubules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A Systematic Review of Post-Extractional Alveolar Hard and Soft Tissue Dimensional Changes in Humans. Clin. Oral Implants Res. 2012, 23 (Suppl. S5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hansson, S.; Halldin, A. Alveolar Ridge Resorption after Tooth Extraction: A Consequence of a Fundamental Principle of Bone Physiology. J. Dent. Biomech. 2012, 3, 1758736012456543. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone Healing and Soft Tissue Contour Changes Following Single-Tooth Extraction: A Clinical and Radiographic 12-Month Prospective Study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar] [PubMed]
- Jung, R.E.; Ioannidis, A.; Hämmerle, C.H.F.; Thoma, D.S. Alveolar Ridge Preservation in the Esthetic Zone. Periodontology 2000 2018, 77, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical Protocols for Ridge Preservation after Tooth Extraction. A Systematic Review. Clin. Oral Implants Res. 2012, 23 (Suppl. S5), 22–38. [Google Scholar] [CrossRef]
- Vittorini Orgeas, G.; Clementini, M.; De Risi, V.; de Sanctis, M. Surgical Techniques for Alveolar Socket Preservation: A Systematic Review. Int. J. Oral Maxillofac. Implants 2013, 28, 1049–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Laino, L.; Cicciù, M.; Lo Russo, L. Combination of Bone Graft and Resorbable Membrane for Alveolar Ridge Preservation: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. J. Periodontol. 2018, 89, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Hedbom, E.; Saulacic, N.; Zhang, Y.; Sculean, A.; Bosshardt, D.D.; Buser, D. Osteogenic Potential of Autogenous Bone Grafts Harvested with Four Different Surgical Techniques. J. Dent. Res. 2011, 90, 1428–1433. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Zara, S.; Tetè, G.; Vinci, R.; Gherlone, E.; Cataldi, A. Biologic and Clinical Aspects of Integration of Different Bone Substitutes in Oral Surgery: A Literature Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 392–402. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front. Biosci. Elite Ed. 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-Derived Bone Graft Material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.I.T.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone Regeneration Using Dentin Matrix Depends on the Degree of Demineralization and Particle Size. PLoS ONE 2016, 11, e0147235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-K.; Kim, S.-G.; Byeon, J.-H.; Lee, H.-J.; Um, I.-U.; Lim, S.-C.; Kim, S.-Y. Development of a Novel Bone Grafting Material Using Autogenous Teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Bae, J.-H.; Um, I.-W.; Oh, J.-S.; Jeong, K.-I. Guided Bone Regeneration Using Autogenous Tooth Bone Graft in Implant Therapy: Case Series. Implant Dent. 2014, 23, 138–143. [Google Scholar] [CrossRef]
- Pang, K.-M.; Um, I.-W.; Kim, Y.-K.; Woo, J.-M.; Kim, S.-M.; Lee, J.-H. Autogenous Demineralized Dentin Matrix from Extracted Tooth for the Augmentation of Alveolar Bone Defect: A Prospective Randomized Clinical Trial in Comparison with Anorganic Bovine Bone. Clin. Oral Implants Res. 2017, 28, 809–815. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, S.-G.; Yun, P.-Y.; Yeo, I.-S.; Jin, S.-C.; Oh, J.-S.; Kim, H.-J.; Yu, S.-K.; Lee, S.-Y.; Kim, J.-S.; et al. Autogenous Teeth Used for Bone Grafting: A Comparison with Traditional Grafting Materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e39–e45. [Google Scholar] [CrossRef]
- Tabatabaei, F.S.; Tatari, S.; Samadi, R.; Moharamzadeh, K. Different Methods of Dentin Processing for Application in Bone Tissue Engineering: A Systematic Review. J. Biomed. Mater. Res. A 2016, 104, 2616–2627. [Google Scholar] [CrossRef]
- Tabatabaei, F.S.; Tatari, S.; Samadi, R.; Torshabi, M. Surface Characterization and Biological Properties of Regular Dentin, Demineralized Dentin, and Deproteinized Dentin. J. Mater. Sci. Mater. Med. 2016, 27, 164. [Google Scholar] [CrossRef]
- Kolmas, J.; Kalinowski, E.; Wojtowicz, A.; Kolodziejski, W. Mid-Infrared Reflectance Microspectroscopy of Human Molars: Chemical Comparison of the Dentin–Enamel Junction with the Adjacent Tissues. J. Mol. Struct. 2010, 966, 113–121. [Google Scholar] [CrossRef]
- Iafisco, M.; Palazzo, B.; Martra, G.; Margiotta, N.; Piccinonna, S.; Natile, G.; Gandin, V.; Marzano, C.; Roveri, N. Nanocrystalline Carbonate-Apatites: Role of Ca/P Ratio on the Upload and Release of Anticancer Platinum Bisphosphonates. Nanoscale 2011, 4, 206–217. [Google Scholar] [CrossRef]
- Lopes, C. de C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier Transform Infrared Spectroscopy (FTIR) Application Chemical Characterization of Enamel, Dentin and Bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Janicki, P.; Schmidmaier, G. What Should Be the Characteristics of the Ideal Bone Graft Substitute? Combining Scaffolds with Growth Factors and/or Stem Cells. Injury 2011, 42 (Suppl. S2), S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Um, I.-W.; Kim, K.-W.; Kim, Y.-K. Human Dentin as Novel Biomaterial for Bone Regeneration; IntechOpen: London, UK, 2011; ISBN 978-953-307-418-4. [Google Scholar]
- Elfana, A.; El-Kholy, S.; Saleh, H.A.; Fawzy El-Sayed, K. Alveolar Ridge Preservation Using Autogenous Whole-Tooth versus Demineralized Dentin Grafts: A Randomized Controlled Clinical Trial. Clin. Oral Implants Res. 2021, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Shapoff, C.A.; Bowers, G.M.; Levy, B.; Mellonig, J.T.; Yukna, R.A. The Effect of Particle Size on the Osteogenic Activity of Composite Grafts of Allogeneic Freeze-Dried Bone and Autogenous Marrow. J. Periodontol. 1980, 51, 625–630. [Google Scholar] [CrossRef]
- Nam, J.-W.; Kim, M.-Y.; Han, S.-J. Cranial Bone Regeneration According to Different Particle Sizes and Densities of Demineralized Dentin Matrix in the Rabbit Model. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 27. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, G.S.; Miziara, M.N.; da Silva, E.R.; Ferreira, E.L.; Biulchi, A.P.F.; Alves, J.B. Enhanced Bone Formation during Healing Process of Tooth Sockets Filled with Demineralized Human Dentine Matrix. Aust. Dent. J. 2013, 58, 326–332. [Google Scholar] [CrossRef]
- Tanoue, R.; Ohta, K.; Miyazono, Y.; Iwanaga, J.; Koba, A.; Natori, T.; Iwamoto, O.; Nakamura, K.; Kusukawa, J. Three-Dimensional Ultrastructural Analysis of the Interface between an Implanted Demineralised Dentin Matrix and the Surrounding Newly Formed Bone. Sci. Rep. 2018, 8, 2858. [Google Scholar] [CrossRef]
- Inoue, T.; Deporter, D.A.; Melcher, A.H. Induction of Cartilage and Bone by Dentin Demineralized in Citric Acid. J. Periodontal Res. 1986, 21, 243–255. [Google Scholar] [CrossRef]
- Ike, M.; Urist, M.R. Recycled Dentin Root Matrix for a Carrier of Recombinant Human Bone Morphogenetic Protein. J. Oral Implantol. 1998, 24, 124–132. [Google Scholar] [CrossRef]
- Murata, M.; Sato, D.; Hino, J.; Akazawa, T.; Tazaki, J.; Ito, K.; Arisue, M. Acid-Insoluble Human Dentin as Carrier Material for Recombinant Human BMP-2. J. Biomed. Mater. Res. A 2012, 100, 571–577. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V.; Thies, R.S. The BMP Proteins in Bone Formation and Repair. Trends Genet. TIG 1992, 8, 97–102. [Google Scholar] [CrossRef]
- Cao, X.; Chen, D. The BMP Signaling and in Vivo Bone Formation. Gene 2005, 357, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Urist, M.R. Bovine Tooth-Derived Bone Morphogenetic Protein. J. Dent. Res. 1989, 68, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Bessho, K.; Tagawa, T.; Murata, M. Purification of Rabbit Bone Morphogenetic Protein Derived from Bone, Dentin, and Wound Tissue after Tooth Extraction. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1990, 48, 162–169. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human Dentin-Matrix-Derived Bone Morphogenetic Protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Serper, A.; Calt, S. The Demineralizing Effects of EDTA at Different Concentrations and PH. J. Endod. 2002, 28, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.H.; Ritchie, D.G.; Wang, L.H. Six Decades of Dentinogenesis Research. Historical and Prospective Views on Phosphophoryn and Dentin Sialoprotein. Eur. J. Oral Sci. 1998, 106 (Suppl. S1), 211–220. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, Y.-K. Retrospective Cohort Study of Autogenous Tooth Bone Graft. Oral Biol. Res. 2012, 36, 39–43. [Google Scholar]
- Kim, Y.-K.; Bang, K.-M.; Murata, M.; Mitsugi, M.; Um, I.-W. Retrospective Clinical Study of Allogenic Demineralized Dentin Matrix for Alveolar Bone Repair. J. Hard Tissue Biol. 2017, 26, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Nampo, T.; Watahiki, J.; Enomoto, A.; Taguchi, T.; Ono, M.; Nakano, H.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Maki, K. A New Method for Alveolar Bone Repair Using Extracted Teeth for the Graft Material. J. Periodontol. 2010, 81, 1264–1272. [Google Scholar] [CrossRef]
- Santos, A.; Botelho, J.; Machado, V.; Borrecho, G.; Proença, L.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G. Autogenous Mineralized Dentin versus Xenograft Granules in Ridge Preservation for Delayed Implantation in Post-Extraction Sites: A Randomized Controlled Clinical Trial with an 18 Months Follow-Up. Clin. Oral Implants Res. 2021, 32, 905–915. [Google Scholar] [CrossRef]
| Nomenclature | Demineralization Process |
|---|---|
| T0 | not-demineralized |
| T2 | 2 min in 12% EDTA dentin particles |
| T5 | 5 min in 12% EDTA dentin particles |
| T10 | 10 min in 12% EDTA dentin particles |
| T60 | 60 min in 12% EDTA dentin particles |
| Specimen | T2 | T5 | T10 | T60 |
|---|---|---|---|---|
| D1 | 0.075 g | 0.075 g | 0.075 g | 0.075 g |
| D2 | 0.093 g | 0.081 g | 0.051 g | 0.024 g |
| D3 | 0.061 g | 0.057 g | 0.034 g | 0.014 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memè, L.; Strappa, E.M.; Monterubbianesi, R.; Bambini, F.; Mummolo, S. SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Appl. Sci. 2022, 12, 1480. https://doi.org/10.3390/app12031480
Memè L, Strappa EM, Monterubbianesi R, Bambini F, Mummolo S. SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Applied Sciences. 2022; 12(3):1480. https://doi.org/10.3390/app12031480
Chicago/Turabian StyleMemè, Lucia, Enrico M. Strappa, Riccardo Monterubbianesi, Fabrizio Bambini, and Stefano Mummolo. 2022. "SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study" Applied Sciences 12, no. 3: 1480. https://doi.org/10.3390/app12031480
APA StyleMemè, L., Strappa, E. M., Monterubbianesi, R., Bambini, F., & Mummolo, S. (2022). SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Applied Sciences, 12(3), 1480. https://doi.org/10.3390/app12031480








