Periodontal Tissue Reaction Consecutive Implantation of Endodontic Materials and Subsequent Integration of Complex Oral Rehabilitation Treatments
Abstract
1. Introduction
Aim of Study
2. Materials and Methods
2.1. The Experimental Procedures
- MTA (Mineral Trioxide Aggregate, Dentsply, Tulsa Dental, Johnson City, TN, USA), an alkaline powder consisting of fine hydrophilic particles that set in the presence of moisture, promotes bone formation, and facilitates the regeneration of the periodontal ligament, with a high antibacterial effect [15,16,17]. It is made of calcium hydroxide, bismuth oxide (Bi2O3), calcium sulfate (CaSO4), tricalcium silicate ((CaO)3·SiO2), dicalcium silicate ((CaO)2·SiO2), and tricalcium aluminate ((CaO)3·Al2O3).
- DiaRoot BioAggregate (Innovative BioCeramix, Inc., Vancouver, BC, Canada) is a material similar in structure to MTA that additionally contains ceramic nanoparticles. It has proven antiseptic proprieties and at the same time stimulates cementogenesis [18,19]. The chemical composition includes calcium silicate, calcium hydroxide, hydroxyapatite, and tantalum oxygen (Ta2O5).
- Sealapex (Kerr, Switzerland) is a calcium hydroxide-based cement with good compatibility used for root canal sealing [20], but with a weak leakage resistance [21]. It has the following chemical composition: barium sulfate, titanium dioxide, zinc oxide, calcium hydroxide, butylbenzene, sulfonamide, zinc stearate.
2.1.1. In Vivo Experiment
- Group 1—9 rabbits—receiving MTA implants;
- Group 2—9 rabbits—receiving DiaRoot implants;
- Group 3—9 rabbits—receiving Sealapex implants;
- Group 4 (control)—3 rabbits—receiving empty polyethylene tube implants.
Surgical and Post-Operative Protocol
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | stomatognathic system |
| ZOE | zinc oxide eugenol |
| Super-EBA | zinc oxide eugenol cement reinforced with ethoxy benzoic acid (EBA) |
| IRM | zinc oxide eugenol-based cement reinforced with polymethacrylate |
| SDSS/TMDs | dysfunctional syndrome of the stomatognathic system |
| TMJ | temporo-mandibular joint |
| PMMA | Poly(methyl methacrylate) |
| Ca(OH)2 | calcium dioxide |
| BA | material similar in structure to MTA (mineral trioxide aggregate), that additionally contains ceramic nanoparticles. |
| MTA | mineral trioxide aggregate, |
| Sealapex | calcium hydroxide-based cement |
| SiO2 | silicon dioxide |
References
- Mohapatra, S.; Patro, S.; Mishra, S. Bioactive Materials in Endodontics: An Evolving Component of Clinical Dentistry. Compend. Contin. Educ. Dent. 2016, 38, 376–381, quiz 382. [Google Scholar] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Di Alberti, L.; Rossetto, A.; Albanese, M.; D’Agostino, A.; De Santis, D.; Bertossi, D.; Nocini, P.F. Expression of Vascular Endothelial Growth Factor (VEGF) mRNA in healthy bone tissue around implants and in peri-implantitis. Minerva Stomatol. 2013, 62 (Suppl. 1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Favaloro, A.; Milardi, D.; Vermiglio, G.; Vita, G.; Cordasco, G.; Cutroneo, G. Immunohistochemical analysis of TGF-β1 and VEGF in gingival and periodontal tissues: A role of these biomarkers in the pathogenesis of scleroderma and periodontal disease. Int. J. Mol. Med. 2012, 30, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef]
- Eliyas, S.; Jalili, J.; Martin, N. Restoration of the root canal treated tooth. Br. Dent. J. 2015, 218, 2–7. [Google Scholar] [CrossRef]
- Gupta, S.K. A study to assess the methodological quality of in vivo animal experiments published in Indian journal of pharmacology: A retrospective, cross-sectional, observational study. Indian J. Pharmacol. 2019, 51, 11–16. [Google Scholar] [CrossRef]
- De-Deus, G.; Canabarro, A.; Alves, G.; Linhares, A.; Isabel Senne, M.; Granjeiro, J.M. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J. Endod. 2009, 35, 1387–1390. [Google Scholar] [CrossRef]
- Camps, J.; About, I. Cytotoxicity testing of endodontic sealers: A new method. J. Endod. 2003, 29, 583–586. [Google Scholar] [CrossRef]
- Banomyong, D.; Ongchavalit, L.; Yanpiset, K. Cytotoxicity evaluatin of newly developed bi-functional, oligomer-based sealers and a methacrylate resin-based root canal sealer. M Dent. J. 2016, 36, 89–98. [Google Scholar]
- Estrela, C.; Bammann, L.L.; Estrela, C.R.d.A.; Silva, R.S.d.; Pecora, J.D. Antimicrobial and Chemical Study of MTA, Portland Cement, Calcium Hydroxide Paste, Sealapex and Dycal. Braz. Dent. J. 2000, 11, 3–9. [Google Scholar] [PubMed]
- Teixeira, L.; Gonçalves Basso, F.; Hebling, J.; Costa, C.A.; Garrido Mori, G.; Yara Silva-Sousa, T.C.; Oliveira, C.F. Cytotoxicity Evaluation of Root Canal Sealers Using an In Vitro Experimental Model with Roots. Braz. Dent. J. 2017, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Council of the European Union. Caring for animals aiming for better science. Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79. [Google Scholar]
- Kopper, P.M. Evaluation of bone tissue response to a sealer containing mineral trioxide aggregate. J. Endod. 2015, 41, 62–66. [Google Scholar]
- Roberts, H.W.; Toth, J.M.; Berzins, D.W.; Charlton, D.G. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent. Mater. 2008, 24, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; de Souza, V.; Nery, M.J.; Otoboni Filho, J.A.; Bernabe, P.F.; Dezan Junior, E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J. Endod. 1999, 25, 161–166. [Google Scholar] [CrossRef]
- Chacko, V.; Kurikose, S. Human pulpal response to mineral trioxide aggregate (MTA): A histologic study. J. Clin. Pediatr. Dent. 2006, 30, 203–209. [Google Scholar] [CrossRef]
- Khalil, W.A.; Eid, N.F. Biocompatibility of BioAggregate and mineral trioxide aggregate on the liver and kidney. Int. Endod. J. 2013, 46, 730–737. [Google Scholar] [CrossRef]
- Assadian, H.; Hamzelouei Moghaddam, E.; Amini, A.; Nazari Moghaddam, K.; Hashemzehi, M. A Review of Endodontic Bioceramics. J. Islamic Dent. Assoc. Iran. 2016, 28, 20–33. [Google Scholar] [CrossRef][Green Version]
- Chang, S.-W.; Lee, S.-Y.; Kang, S.-K.; Kum, K.-Y.; Kim, E.-C. In Vitro Biocompatibility, Inflammatory Response, and Osteogenic Potential of 4 Root Canal Sealers: Sealapex, Sankin Apatite Root Sealer, MTA Fillapex, and iRoot SP Root Canal Sealer. J. Endod. 2014, 40, 1642–1648. [Google Scholar] [CrossRef]
- Huang, F.M.; Tai, K.W.; Chou, M.Y.; Chang, Y.C. Cytotoxicity of resin-, zinc oxide-eugenol, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int. Endod. J. 2002, 35, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, H.; Filser, F.; Loeffel, O.; Schumacher, M.; Gauckler, L.J.; Hämmerle, C.H. Strength and reliability of four-unit all-ceramic posterior bridges. Dent. Mater. 2005, 21, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Groten, M.; Huttig, F. The performance of zirconium dioxide crowns: A clinical follow-up. Int. J. Prosthodont. 2010, 23, 429–431. [Google Scholar]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. NANOmetric BIO-Banked MSC-Derived Exosome (NANOBIOME) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Conrad, H.J.; Seong, W.J.; Pesun, I.J. Current ceramic materials and systems with clinical recommendations: A systematic review. J. Prosthet. Dent. 2007, 98, 389–404. [Google Scholar] [CrossRef]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, A.C.; Martu, I.; Teslaru, S.; Kappenberg-Nitescu, D.C.; Goriuc, A.; Luchian, I.; Martu, M.A.; Solomon, S.M.; Mârțu, S. Assessment of salivary levels of RANKL and OPG in aggressive versus chronic periodontitis. J. Immunol. Res. 2019, 28, 2019. [Google Scholar] [CrossRef]
- Gabbert, O.; Ohlmann, B.; Schmitter, M.; Gilde, H.; Ruef, T.; Rammelsberg, P. Fracture behavior of zirconia ceramic cantilever fixed dental prostheses in vitro. Acta Odontol. Scand. 2008, 66, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Butler, D.S. Fifteen Years of Explaining Pain: The Past, Present, and Future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef]
- Van der Meer, H.A.; Speksnijder, C.M.; Engelbert, R.H.H.; Lobbezoo, F.; Nijhuis-van der Sanden, M.W.G.; Visscher, C.M. The association between headaches and temporomandibular disorders is confounded by bruxism and somatic complaints. Clin. J. Pain 2017, 33, 835–843. [Google Scholar] [CrossRef]
- Jenei, Á.; Sándor, J.; Hegedűs, C.; Bágyi, K.; Nagy, L.; Kiss, C.; Szabó, G.; Márton, I.J. Oral health-related quality of life after prosthetic rehabilitation: A longitudinal study with the OHIP questionnaire. Health Qual. Life Outcomes 2015, 13, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, K.F.; Al-Khabbaz, A.K.; Al-Ansari, J.M.; Neiva, R.; Wang, H.L. Risk indicators for tooth loss due to periodontal disease. J. Periodontol. 2005, 76, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C.; Cullinan, M.P. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontology 2000 2010, 53, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Thyvalikakath, T.; Song, M.; Schleyer, T. Perceptions and attitudes toward performing risk assessment for periodontal disease: A focus group exploration. BMC Oral Health 2018, 18, 90–95. [Google Scholar] [CrossRef]
- Hategan, S.I.; Kamer, A.R.; Sinescu, C.; Craig, R.G.; Jivanescu, A.; Gavrilovici, A.M.; Negrutiu, M.-L. Periodontal disease in a young Romanian convenience sample: Radiographic assessment. BMC Oral Health 2019, 19, 94–98. [Google Scholar] [CrossRef]
- Satheesh Kumar, P.; Satheesh, K.K.; John, J.; Patil, G.; Patel, R. Force Transfer and Stress Distribution in an Implant-Supported Overdenture Retained with a Hader Bar Attachment: A Finite Element Analysis. ISRN Dent. 2013, 2013, 21–27. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Baino, F.; Tallia, F.; Gervasio, C.; Verné, E. Bioactive glass-derived trabecular coating: A smart solution for enhancing osteointegration of prosthetic elements. J. Mater. Sci. Mater. Med. 2012, 23, 2369–2380. [Google Scholar] [CrossRef]
- Bernabé, P.F.E.; Holland, R.; Morandi, R.; Souza, V.d.; Nery, M.J.; Otoboni Filho, J.A.; Dezan Junior, E.; Gomes-Filho, J.E. Comparative Study of MTA and other materials in retrofilling of pulpless dogs’ teeth. Braz. Dent. J. 2005, 16, 151–155. [Google Scholar] [CrossRef]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene is the new Graphene in Biomedical Applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef]
- Scalera, F.; Esposito Corcione, C.; Montagna, F.; Sannino, A.; Maffezzoli, A. Development and characterization of UV curable epoxy/hydroxyapatite suspensions for stereolithography applied to bone tissue engineering. Ceram. Int. 2014, 40, 15455–15462. [Google Scholar] [CrossRef]
- Solomon, S.M.; Timpu, D.; Forna, D.A.; Stefanache, M.A.M.; Martu, S.; Stoleriu, S. AFM comparative study of root surface morphology after three methods of scaling. Mater. Plast. 2016, 53, 546–549. [Google Scholar]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.G.; Luchian, I.; Ioanid, N.; Goriuc, A.; Martu, I.; Bosinceanu, D.; Martu, M.A.; Tirca, T.; Martu, S. ELISA Evaluation of RANKL Levels in Gingival Fluid in Patients with Periodontitis and Occlusal Trauma. Rev. Chim. 2018, 69, 1578–1580. [Google Scholar] [CrossRef]
- Nicolae, V.; Neamtu, B.; Picu, O.; Stefanache, M.A.M.; Cioranu, V.S.I. The comparitive evaluation of salivary biomarkers (calcium, phosphate, salivary pH) in the cario-resistance versus cario-activity. Rev. Chim. 2016, 68, 821–824. [Google Scholar]
- Taraboanta, I.; Stoleriu, S.; Nica, I.; Georgescu, A.; Gamen, A.C.; Maftei, G.A.; Andrian, S. Roughness variation of a nonhybrid composite resin submitted to acid and abrasive challenges. Int. J. Med. Dent. 2020, 24, 182–187. [Google Scholar]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Popa, C.; Filioreanu, A.M.; Tatarciuc, D.; Nichitean, G.; Hurjui, L.-L.; Foia, L.-G. Wound healing of periodontal and oral tissues: Part II—Patho-phisiological conditions and metabolic diseases. Rom. J. Oral Rehabil. 2020, 12, 30–40. [Google Scholar]
- Doyle, S.L.; Hodges, J.S.; Pesun, I.J.; Law, A.S.; Bowles, W.R. Retrospective cross sectional comparison of initial nonsurgical endodontic treatment and single-tooth implants. J. Endod. 2006, 32, 822–827. [Google Scholar] [CrossRef]
- Iqbal, M.K.; Kim, S. For teeth requiring endodontic treatment, what are the differences in outcomes of restored endodontically treated teeth compared to implant-supported restorations? Int. J. Oral Maxillofac. Implant 2007, 22, 96–116. [Google Scholar]
- Filip, A.; Badulescu, O.V.; Sirbu, P.D.; Cojocaru, E.; Filip, N.; Puha, G.; Trandafir, L.; Iancu, C.; Trandafirescu, M.F.; Alexa, O. Serum Homocysteine and Reactive Species Levels in Fragility Fractures of the Pelvis. Rev. Chim. 2019, 70, 3216–3219. [Google Scholar] [CrossRef]
- Cojocaru, E.; Mihaila, D.; Leon-Constantin, M.M.; Cobzaru, R.G.; Ripa, C.V.; Trandafirescu, M.F.; Trandafir, L.M. Update in pediatric primary brain tumors—From histology to genetically defined tumors. Rom. J. Morphol. Embryol. 2019, 60, 761–767. [Google Scholar] [PubMed]
- Filip, A.; Alexa, O.; Sirbu, P.D.; Filip, C.; Cojocaru, E.; Puha, G.; Trandafirescu, M.F.; Badulescu, O.V. Calcium and vitamin D involvement in the fragility fracture of the pelvis. Rev. Chim. 2019, 70, 3674–3677. [Google Scholar] [CrossRef]
- Luca, A.C.; Miron, I.C.; Trandafir, L.M.; Cojocaru, E.; Pădureț, I.A.; Trandafirescu, M.F.; Iordache, A.C.; Țarcă, E. Morphological, genetic and clinical correlations in infantile hemangiomas and their mimics. Rom. J. Morphol. Embryol. 2020, 61, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, L.M.; Frăsinariu, O.; Leon-Constantin, M.M.; Chiriac, S.; Trandafirescu, M.F.; Miron, I.C.; Luca, A.C.; Iordache, A.C.; Cojocaru, E. Pediatric nonalcoholic fatty liver disease—A changing diagnostic paradigm. Rom. J. Morphol. Embryol. 2020, 61, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Martu, M.A.; Solomon, S.M.; Toma, V.; Maftei, G.A.; Iovan, A.; Gamen, A.; Hurjui, L.; Rezus, E.; Foia, L.; Forna, N.C. The importance of cytokines in periodontal disease and rheumatoid arthritis. Review. Rom. J. Oral Rehabil. 2019, 11, 220–240. [Google Scholar]
- Cojocaru, E.; Magdalena Leon-Constantin, M.; Ungureanu, C.; Trandafirescu, M.F.; Maștaleru, A.; Mihaela Trandafir, L.; Dumitru Petrariu, F.; Viola Bădulescu, O.; Filip, N. Hypolipemiant Actions and Possible Cardioprotective Effects of Valine and Leucine: An Experimental Study. Medicina 2021, 57, 239. [Google Scholar] [CrossRef] [PubMed]
- Trandafirescu, M.F.; Agop-Forna, D.; Clim, A.; Bețiu, C.; Clim, I.A.; Cojocariu, S.; Pînzariu, A.C. Oral manifestations and management of patients with chronic kidney disease. Rom. J. Oral Rehabil. 2020, 12, 212–219. [Google Scholar]
- Nam, J.; Tokutomi, H. Using zirconia-based prosthesis in a complete-mouth reconstruction treatment for worn dentition with the altered vertical dimension of occlusion. J. Prosthet. Dent. 2015, 113, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Tatarciuc, M.; Maftei, G.A.; Vitalariu, A.; Luchian, I.; Martu, I.; Diaconu-Popa, D. Inlay-Retained Dental Bridges—A Finite Element Analysis. Appl. Sci. 2021, 11, 3770. [Google Scholar] [CrossRef]
- Singh, R.G.; Sinha, P. Functional and Aesthetic Full Mouth Rehabilitation of a Severely Worn Dentition to Restore Vertical Dimension: A Case Report. J. Indian Prosthodont. Soc. 2014, 14, 210–214. [Google Scholar] [CrossRef]
- Şahin, O.; Doğan, D.Ö.; Fatih, S. Aesthetic full-mouth oral rehabilitation of a young adult with amelogenesis imperfecta: A clinical report. Cumhur. Dent. J. 2014, 17, 308–315. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic Profiles of Patients with Dental Prosthetic Treatment and Periodontitis before and after Photoactivation Therapy—Randomized Clinical Trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef] [PubMed]
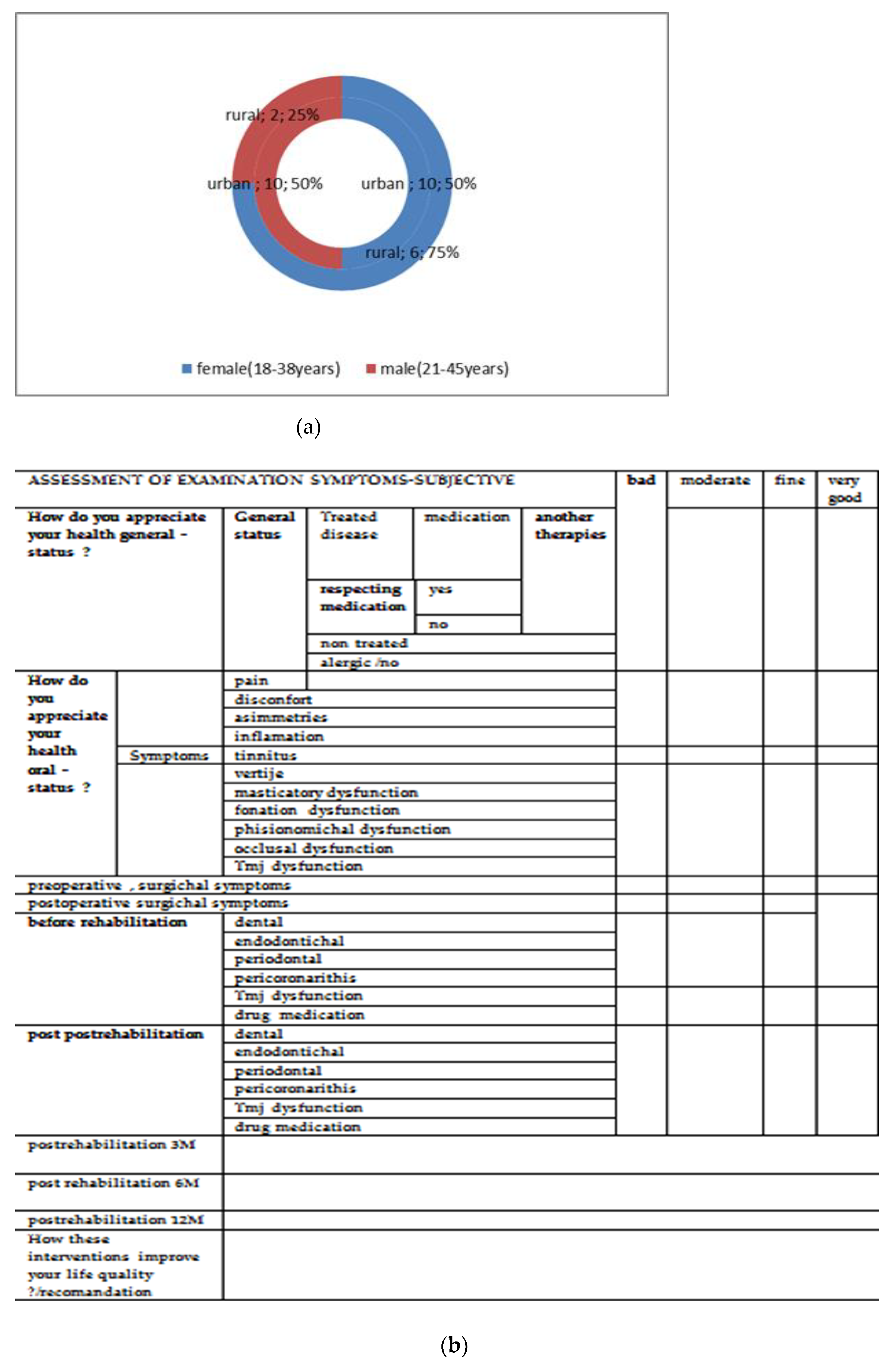
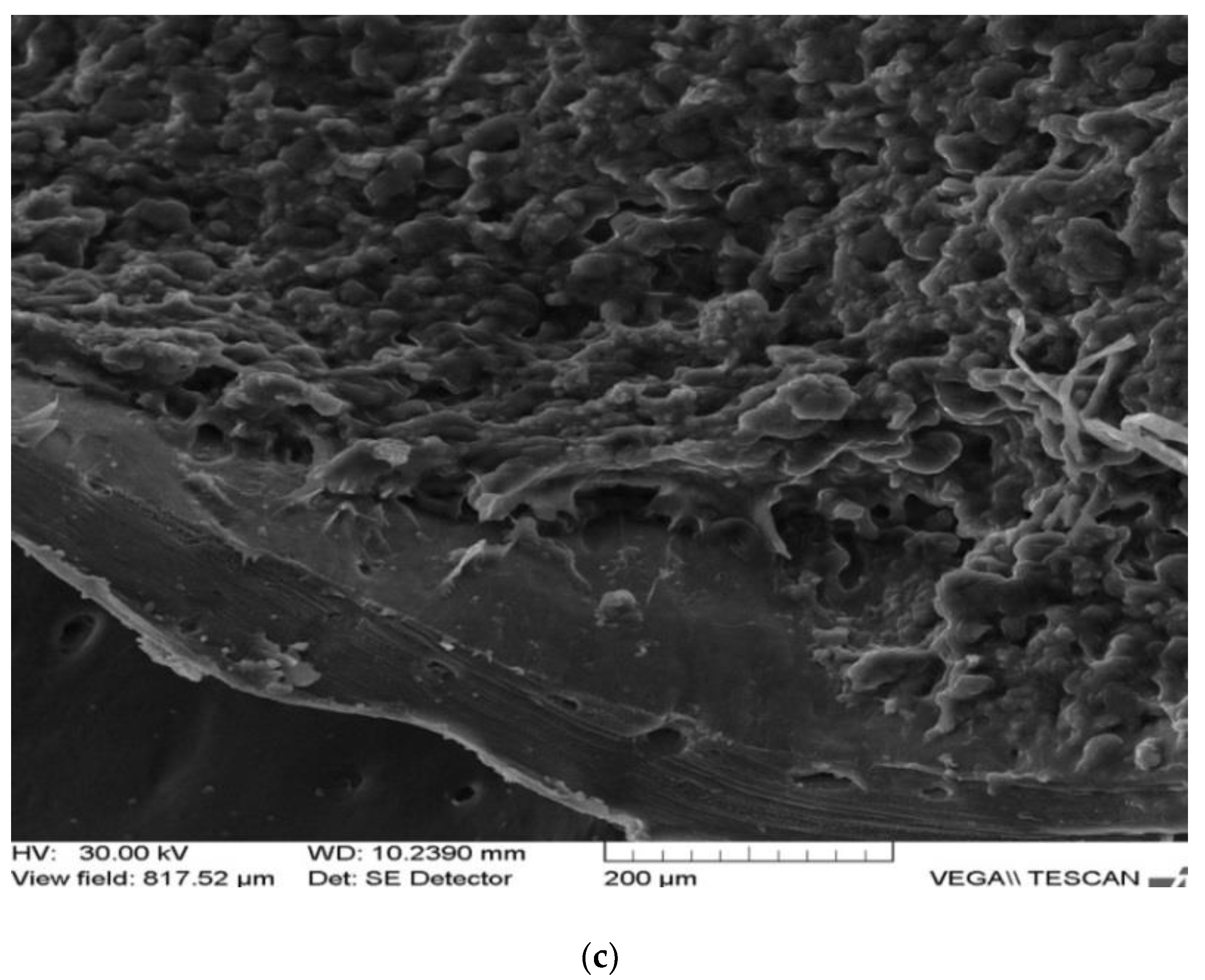

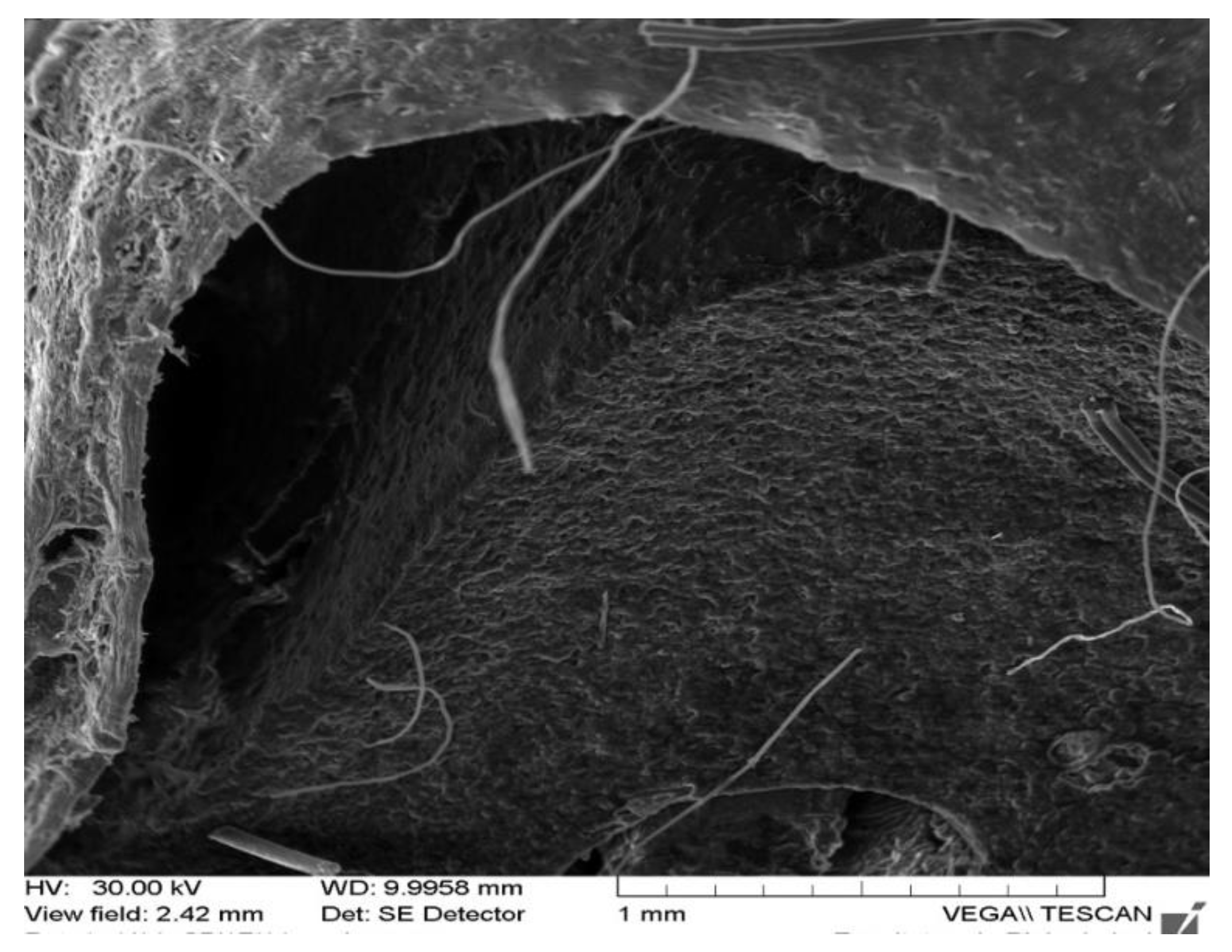
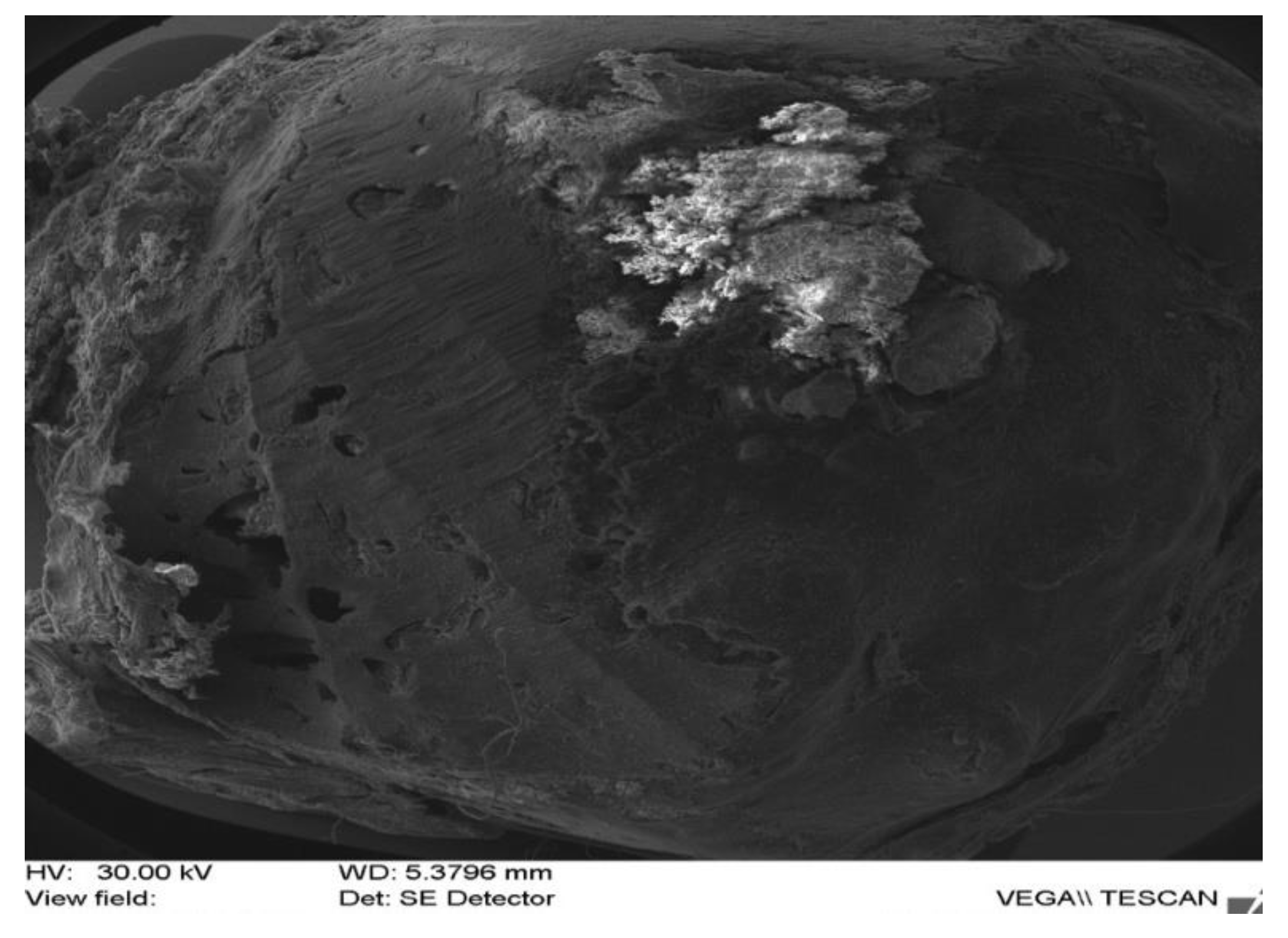

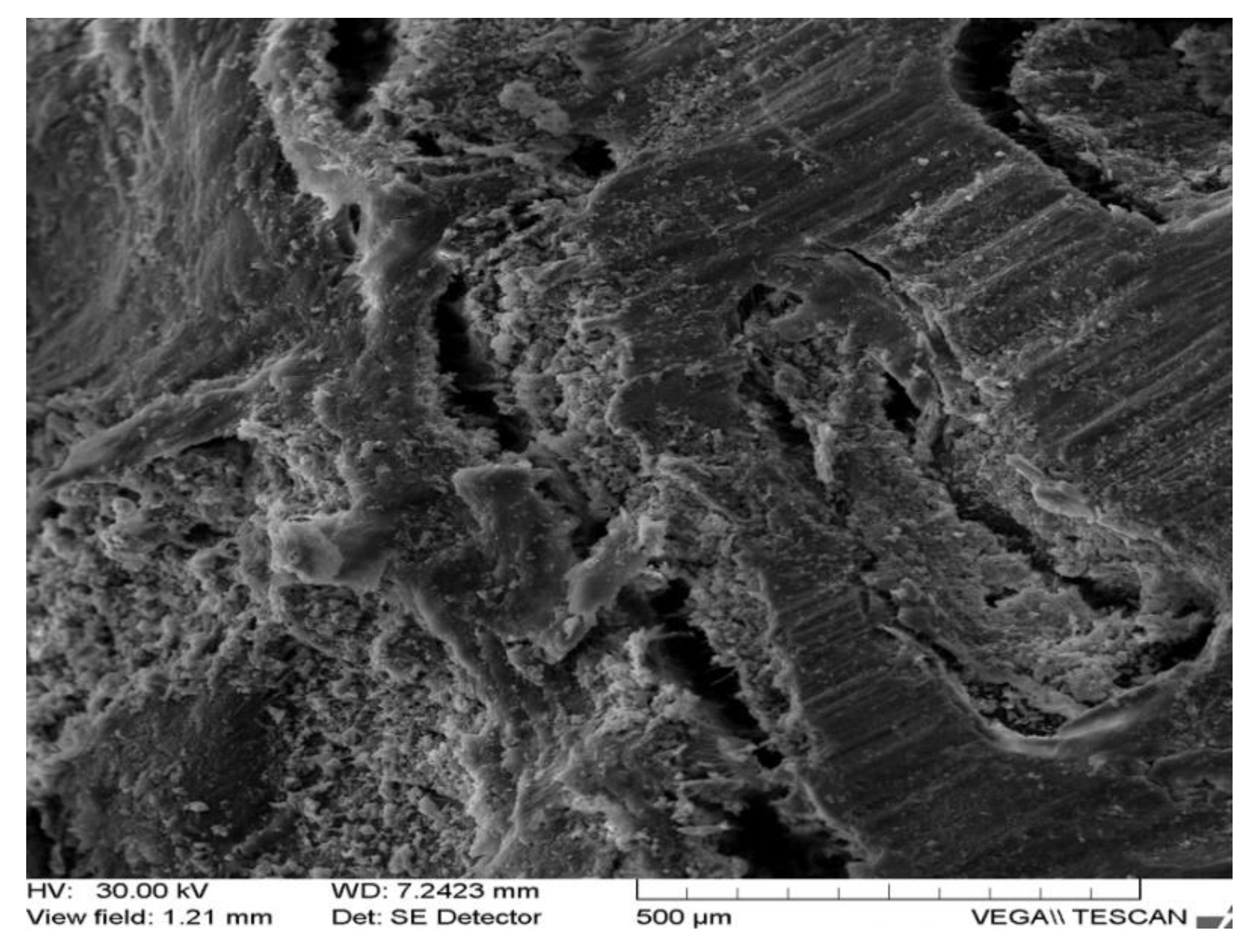

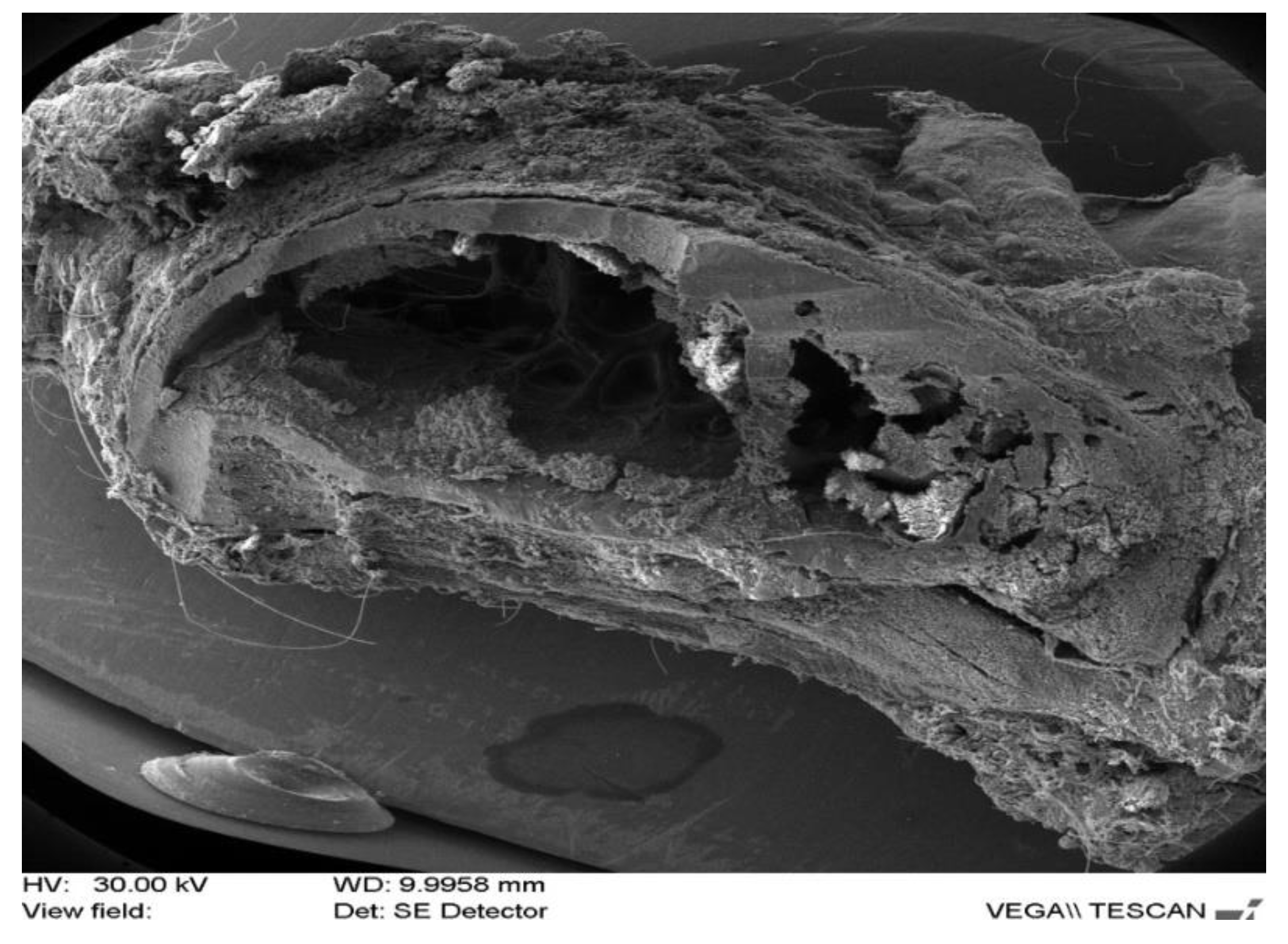





| Rabbit No. | Control Rabbit | Implanted Material | ||
|---|---|---|---|---|
| Sealapex | MTA | BioAggregate | ||
| 1 | 163.6 | 164.3 | 176.5 | 182.4 |
| 2 | 168.8 | 178.4 | 180.1 | |
| 3 | 165.6 | 181.3 | 179.8 | |
| 4 | 167.4 | 179.5 | 182.3 | |
| 5 | 169.1 | 180.2 | 183.2 | |
| 6 | 168.3 | 177.8 | 183.8 | |
| 7 | 167.6 | 175.6 | 179.6 | |
| 8 | 165.7 | 176.6 | 181.5 | |
| 9 | 168.7 | 180.2 | 182.4 | |
| Rabbit No. | Control Rabbit | Implanted Material | ||
|---|---|---|---|---|
| Sealapex | MTA | BioAggregate | ||
| 1 | 163.6 | 181.3 | 196.5 | 199.4 |
| 2 | 173.9 | 198.4 | 193.1 | |
| 3 | 180.6 | 189.8 | 196.8 | |
| 4 | 188.9 | 190.5 | 195.3 | |
| 5 | 182.3 | 191.3 | 196.2 | |
| 6 | 181.2 | 193.5 | 190.2 | |
| Rabbit No. | Control Rabbit | Implanted Material | ||
|---|---|---|---|---|
| Sealapex | MTA | BioAggregate | ||
| 1 | 163.6 | 162.8 | 164.2 | 164.8 |
| 2 | 163.4 | 165.4 | 164.1 | |
| 3 | 165.2 | 167.5 | 166.4 | |
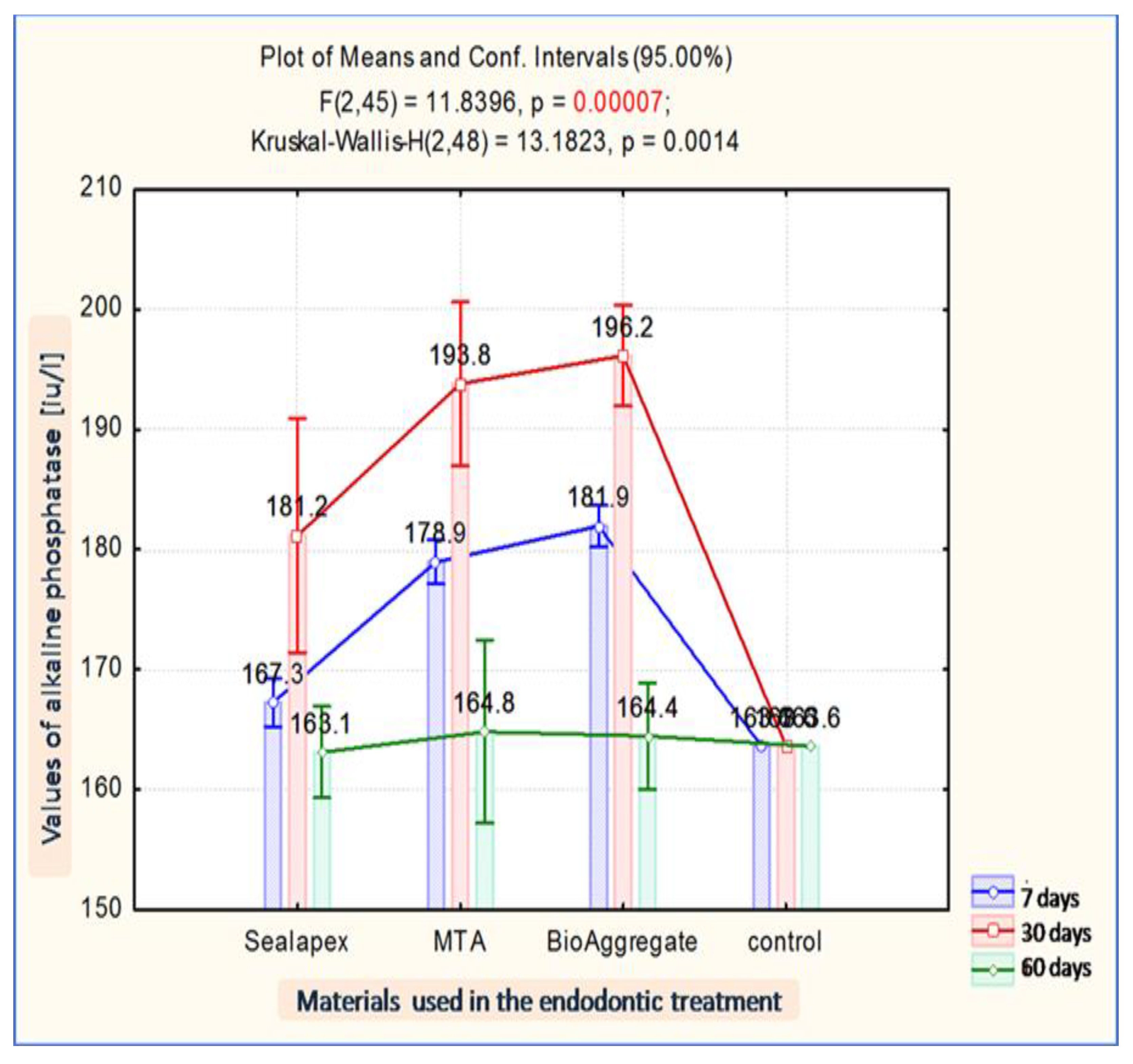
| Implanted Material | Evaluation Moment | Mean FA | Average | Std Dev. | Std Er. | Min. | Max. | Median | |
|---|---|---|---|---|---|---|---|---|---|
| −95% | +95% | ||||||||
| Sealapex | 7 days | 167.25 | 165.24 | 169.26 | 1.92 | 0.78 | 164.30 | 169.10 | 167.85 |
| Sealapex | 30 days | 181.18 | 171.41 | 190.94 | 6.14 | 3.07 | 173.90 | 188.90 | 180.95 |
| Sealapex | 60 days | 163.10 | 159.29 | 166.91 | 0.42 | 0.30 | 162.80 | 163.40 | 163.10 |
| MTA | 7 days | 178.95 | 177.13 | 180.77 | 1.73 | 0.71 | 176.50 | 181.30 | 178.95 |
| MTA | 30 days | 193.80 | 186.97 | 200.63 | 4.29 | 2.15 | 189.80 | 198.40 | 193.50 |
| MTA | 60 days | 164.80 | 157.18 | 172.42 | 0.85 | 0.60 | 164.20 | 165.40 | 164.80 |
| BioAggregate | 7 days | 181.93 | 180.22 | 183.65 | 1.63 | 0.67 | 179.80 | 183.80 | 182.35 |
| BioAggregate | 30 days | 196.15 | 191.94 | 200.36 | 2.65 | 1.32 | 193.10 | 199.40 | 196.05 |
| BioAggregate | 60 days | 164.45 | 160.00 | 168.90 | 0.49 | 0.35 | 164.10 | 164.80 | 164.45 |
| control | 163.60 | 163.60 | 163.60 | 163.60 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminov, L.; Pasca, A.S.; Sindilar, E.V.; Beldiman, M.A.; Bulancea, B.P.; Stamatin, O.; Lupu, I.C.; Croitoru, I.; Teslaru, S.; Solomon, S.M.; et al. Periodontal Tissue Reaction Consecutive Implantation of Endodontic Materials and Subsequent Integration of Complex Oral Rehabilitation Treatments. Appl. Sci. 2022, 12, 1464. https://doi.org/10.3390/app12031464
Aminov L, Pasca AS, Sindilar EV, Beldiman MA, Bulancea BP, Stamatin O, Lupu IC, Croitoru I, Teslaru S, Solomon SM, et al. Periodontal Tissue Reaction Consecutive Implantation of Endodontic Materials and Subsequent Integration of Complex Oral Rehabilitation Treatments. Applied Sciences. 2022; 12(3):1464. https://doi.org/10.3390/app12031464
Chicago/Turabian StyleAminov, Liana, Aurelian Sorin Pasca, Eusebiu Viorel Sindilar, Maria Antonela Beldiman, Bogdan Petru Bulancea, Ovidiu Stamatin, Iulian Costin Lupu, Irina Croitoru, Silvia Teslaru, Sorina Mihaela Solomon, and et al. 2022. "Periodontal Tissue Reaction Consecutive Implantation of Endodontic Materials and Subsequent Integration of Complex Oral Rehabilitation Treatments" Applied Sciences 12, no. 3: 1464. https://doi.org/10.3390/app12031464
APA StyleAminov, L., Pasca, A. S., Sindilar, E. V., Beldiman, M. A., Bulancea, B. P., Stamatin, O., Lupu, I. C., Croitoru, I., Teslaru, S., Solomon, S. M., Foia, G. L., & Checherita, L. E. (2022). Periodontal Tissue Reaction Consecutive Implantation of Endodontic Materials and Subsequent Integration of Complex Oral Rehabilitation Treatments. Applied Sciences, 12(3), 1464. https://doi.org/10.3390/app12031464







