Multi-Technique Diagnostic Investigation in View of the Restoration of “The Glory of St. Barbara” Painting by Mattia Preti
Abstract
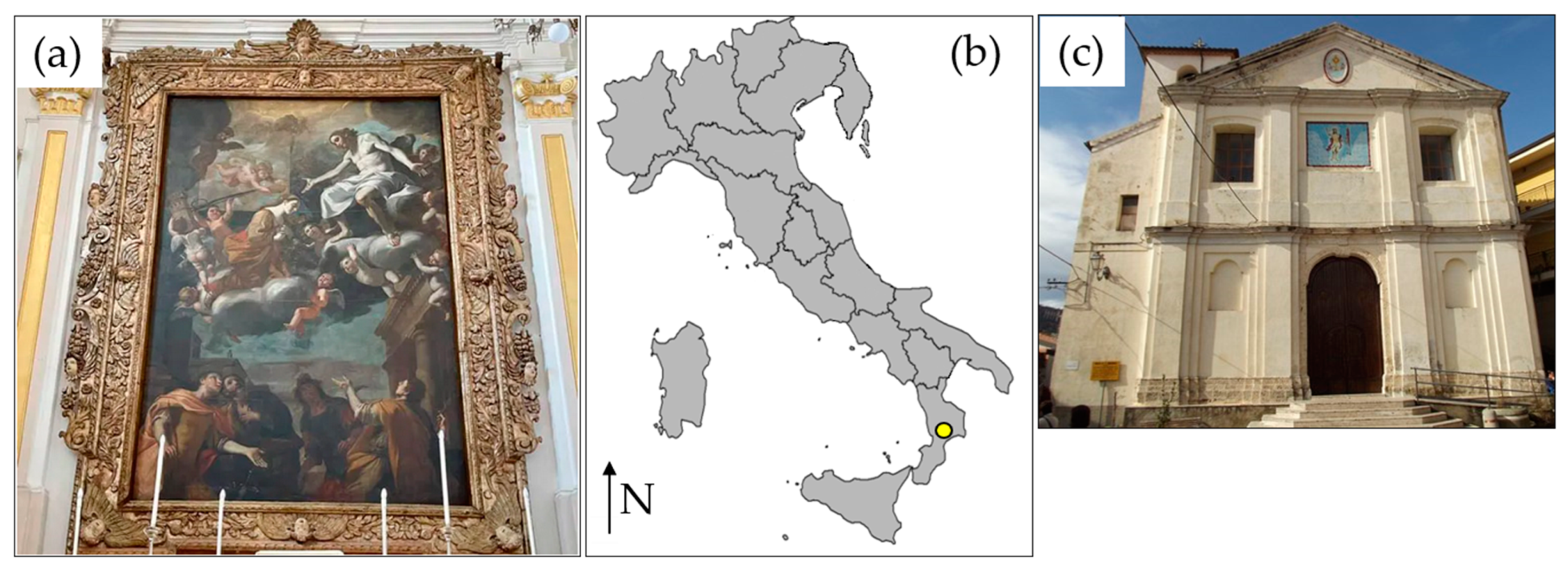
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. In Situ X-ray Fluorescence (XRF) Measurements
2.3. Laboratory Electron Probe Microanalyses–Energy-Dispersive Spectroscopy (EPMA-EDS) Measurements
2.4. Laboratory Micro-Raman Measurements
3. Results and Discussion
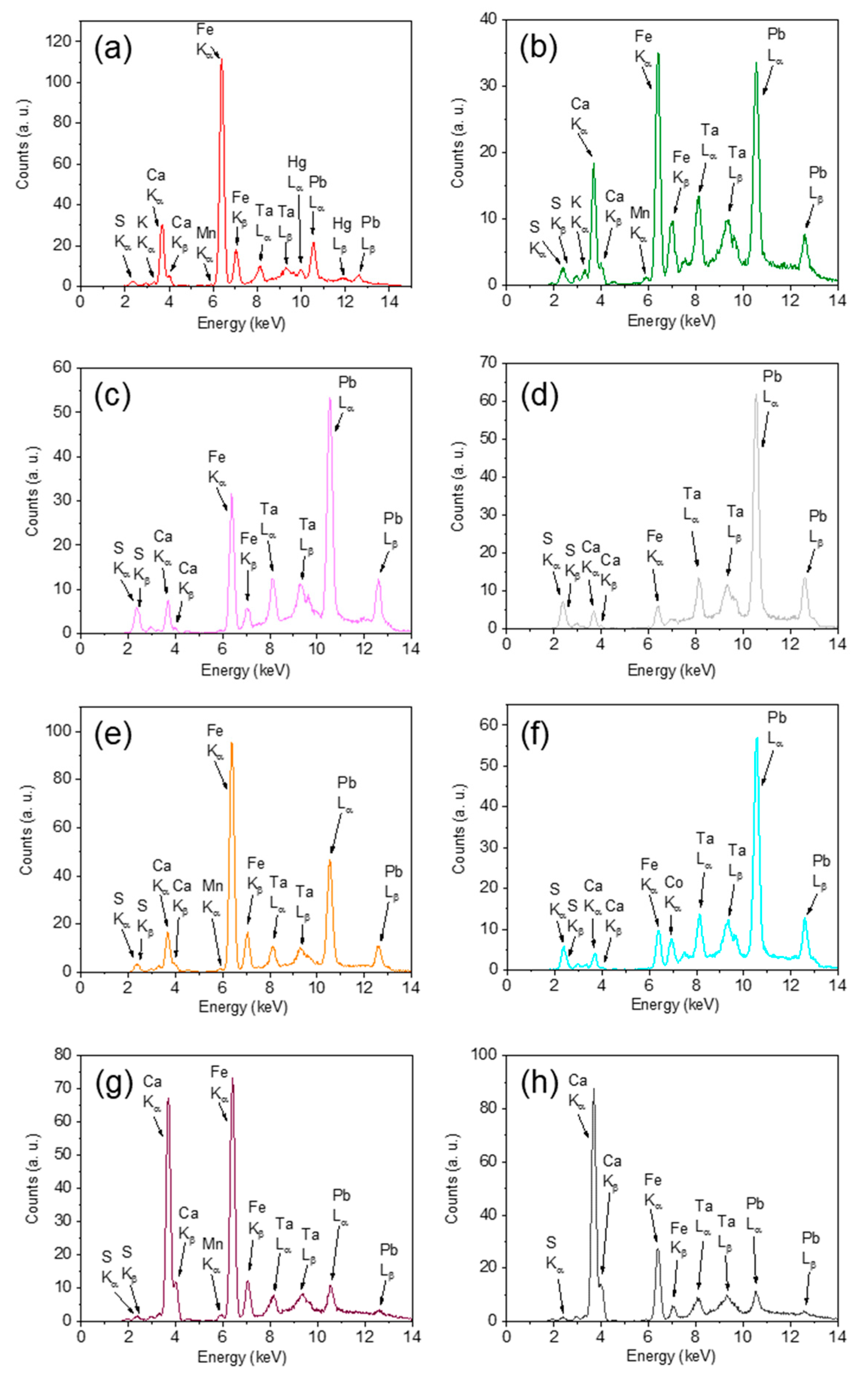
3.1. In Situ XRF Results
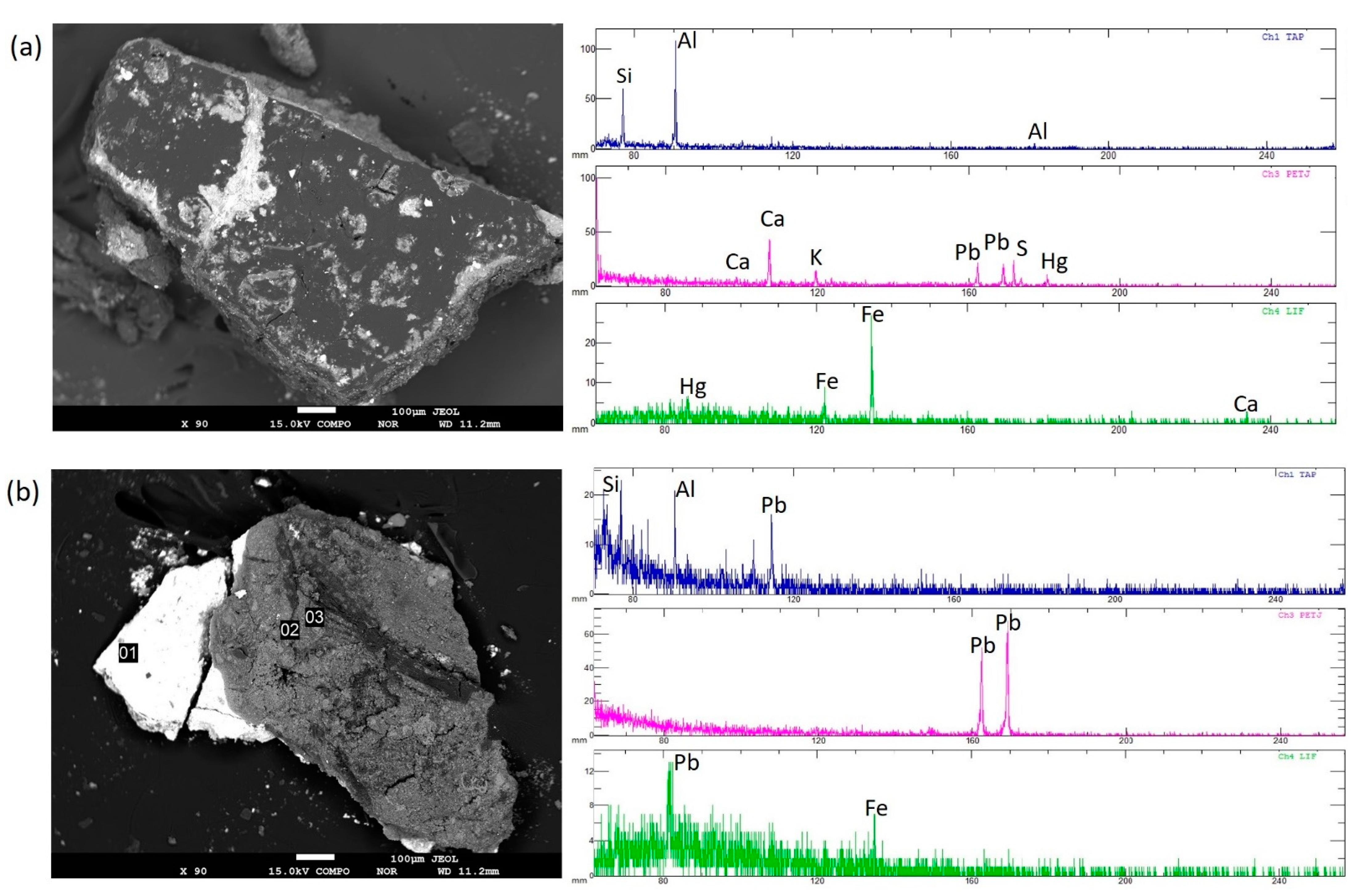
3.2. Laboratory EPMA-EDS Results
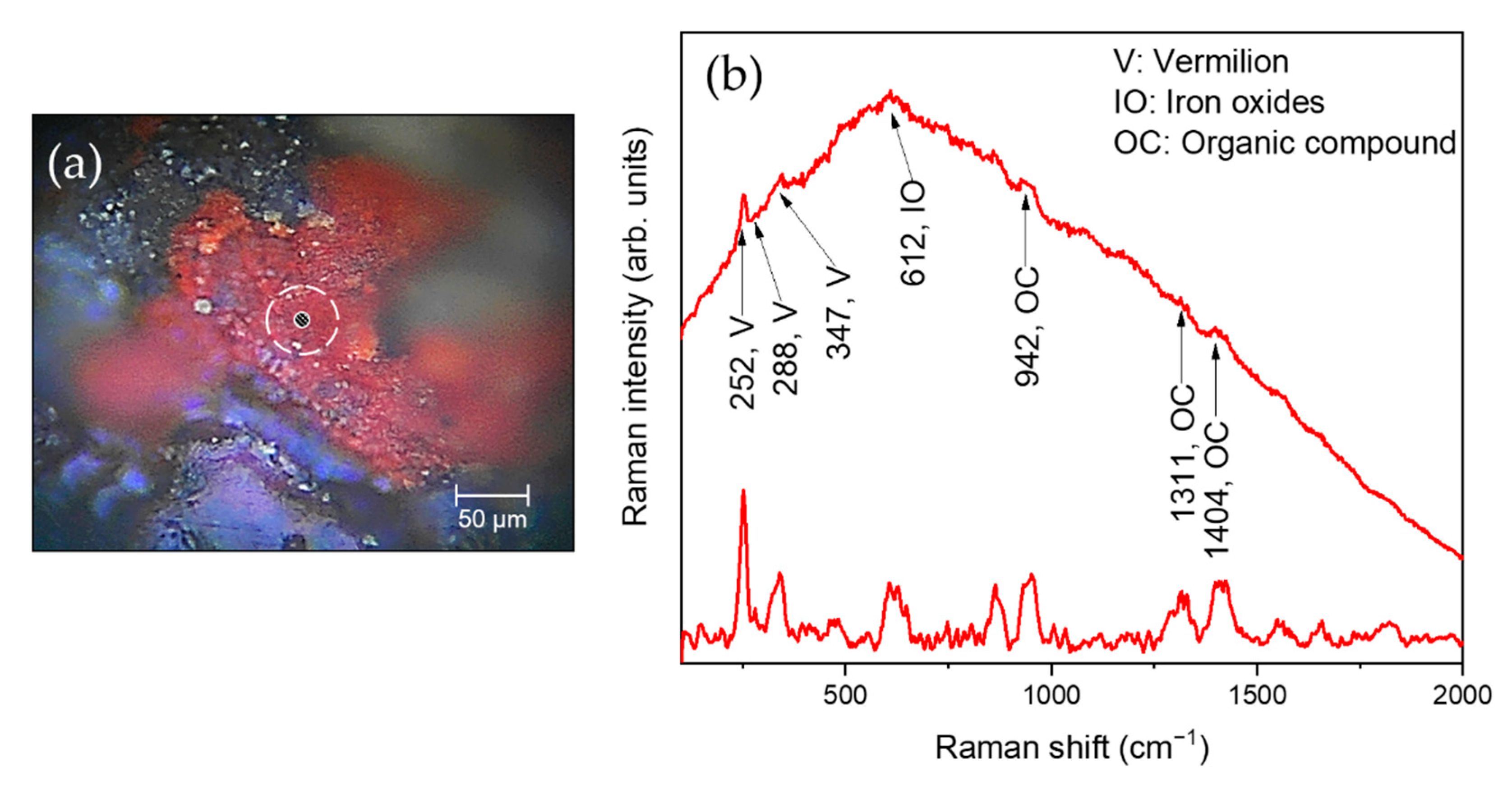
3.3. Laboratory Micro-Raman Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Debono, S.; Valentino, G. Mattia Preti: Faith and Humanity; Misdea Books Ltd.: Valletta, Malta, 2013; ISBN 9789993274360. [Google Scholar]
- King, G.G. Mattia Preti. Art Bull. 1936, 18, 371–386. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 1–30. ISBN 9783110417104. [Google Scholar]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (pre- ≈ 1850 AD). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker version 3.0, a handy set for conservation scientists: A free online Raman spectra database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Baraldi, P. The ‘slash of light’ in the late religious paintings of Mattia Preti technique and materials. Eur. J. Sci. Theol. 2018, 14, 151–160. [Google Scholar]
- Chiavari, G.; Prati, S.; Lanterna, G.; Lalli, C.; Cagnini, A. Diagnostic study of the materials and painting techniques in “The Dinner of Emmaus” by Gregorio (and Mattia?) Preti. Microchim. Acta 2007, 159, 357–362. [Google Scholar] [CrossRef]
- Ridolfi, S. Relazione Tecnico-Scientifica Delle Indagini Effettuate Sull’allegoria Dei Cinque Sensi di Mattia e Gregorio Preti; Ars Mensurae: Roma, Italy, 2019. [Google Scholar]
- D’Amico, S.; Comite, V.; Paladini, G.; Ricca, M.; Colica, E.; Galone, L.; Guido, S.; Mantella, G.; Crupi, V.; Majolino, D.; et al. Multitechnique diagnostic analysis and 3D surveying prior to the restoration of St. Michael defeating Evil painting by Mattia Preti. Environ. Sci. Pollut. Res. 2021; in press. [Google Scholar] [CrossRef]
- Hradil, D.; Hradilová, J.; Lanterna, G.; Galeotti, M.; Holcová, K.; Jaques, V.; Bezdička, P. Clay and alunite-rich materials in painting grounds of prominent Italian masters—Caravaggio and Mattia Preti. Appl. Clay Sci. 2020, 185, 105412. [Google Scholar] [CrossRef]
- Venuti, V.; Fazzari, B.; Crupi, V.; Majolino, D.; Paladini, G.; Morabito, G.; Certo, G.; Lamberto, S.; Giacobbe, L. In situ diagnostic analysis of the XVIII century Madonna della Lettera panel painting (Messina, Italy). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117822. [Google Scholar] [CrossRef]
- D’Amico, S.; Venuti, V.; Colica, E.; Crupi, V.; Paladini, G.; Guido, S.; Mantella, G.; Majolino, D. A combined 3D surveying, XRF and Raman in situ investigation on The Conversion of St Paul painting (Mdina, Malta) by Mattia Preti. ACTA IMEKO 2021, 10, 173–179. [Google Scholar] [CrossRef]
- D’Amico, S.; Venuti, V.; Colica, E.; Crupi, V.; Majolino, D.; Paladini, G.; Guido, S.; Mantella, G.; Zumbo, R. Scientific investigation of the Conversion of St Paul painting (Mdina, Malta). In Proceedings of the 2019 IMEKO TC4 International Conference on Metrology for Archaeology and Cultural Heritage, MetroArchaeo 2019, Florence, Italy, 4–6 December 2019; pp. 330–334. [Google Scholar]
- Lalli, C.G.; von Breska-Ficović, N.; Innocenti, F.; Kolić Pustić, M. Le due serie dei quattro “Evangelisti” di Mattia Preti a Dubrovnik: Un progetto multidisciplinare e di collaborazione tra il Laboratorio Scientifico dell’Opificio e l’Istituto Croato di RestauroSource: OPD Restauro. Cent. Di Della Edifimi SRL 2014, 26, 231–248. [Google Scholar]
- Feller, R.L. Artists’ Pigments: A Handbook of Their History and Characteristics; Feller, R.L., Ed.; Cambridge University Press: Cambridge, UK, 1986; Volume 1, ISBN 978-1-904982-74-6. [Google Scholar]
- Gettens, R.J.; Fitzhugh, E.W. Malachite and Green Verditer. In Artists’ Pigments: A Handbook of Their History and Characteristics; Roy, A., Ed.; Oxford University Press: Oxford, UK, 1993; Volume 2, ISBN 978-1-904982-75-3. [Google Scholar]
- Salvadó, N.; Butí, S.; Cotte, M.; Cinque, G.; Pradell, T. Shades of green in 15th century paintings: Combined microanalysis of the materials using synchrotron radiation XRD, FTIR and XRF. Appl. Phys. A 2013, 111, 47–57. [Google Scholar] [CrossRef]
- Genestar, C.; Pons, C. Earth pigments in painting: Characterisation and differentiation by means of FTIR spectroscopy and SEM-EDS microanalysis. Anal. Bioanal. Chem. 2005, 382, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Riccardi, M.P. Glass-based pigments in painting: Smalt blue and lead–tin yellow type II. Archaeol. Anthropol. Sci. 2021, 13, 199. [Google Scholar] [CrossRef]
- Zlámalová Cílová, Z.; Gelnar, M.; Randáková, S. Smalt production in the Ore Mountains: Characterization of samples related to the production of blue pigment in Bohemia. Archaeometry 2020, 62, 1202–1215. [Google Scholar] [CrossRef]
- Berrie, B.H. Rethinking the History of Artists’ Pigments Through Chemical Analysis. Annu. Rev. Anal. Chem. 2012, 5, 441–459. [Google Scholar] [CrossRef]
- Mühlethaler, B.; Thissen, J. Smalt. In Artists’ Pigments, Vol. 2; Roy, A., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 113–130. [Google Scholar]
- Sakellariou, K.; Miliani, C.; Morresi, A.; Ombelli, M. Spectroscopic investigation of yellow majolica glazes. J. Raman Spectrosc. 2004, 35, 61–67. [Google Scholar] [CrossRef]
- Borgia, I.; Brunetti, B.G.; Miliani, C.; Ricci, C.; Seccaroni, C.; Sgamellotti, A. The combined use of lead–tin yellow type I and II on a canvas painting by Pietro Perugino. J. Cult. Herit. 2007, 8, 65–68. [Google Scholar] [CrossRef]
- Welter, N.; Schüssler, U.; Kiefer, W. Characterisation of inorganic pigments in ancient glass beads by means of Raman microspectroscopy, microprobe analysis and X-ray diffractometry. J. Raman Spectrosc. 2007, 38, 113–121. [Google Scholar] [CrossRef]
- Venuti, V.; Crupi, V.; Fazio, B.; Paladini, G.; La Russa, M.F.; Ricca, M.; Rovella, N.; Macchia, A.; Khalilli, F.; Majolino, D. Investigation of glazed pottery fragments (XIX century A. D.) from Agsu site (Azerbaijan) by XRF and Raman techniques. EPJ Web Conf. 2020, 230, 00012. [Google Scholar] [CrossRef][Green Version]
- Efremov, E.V.; Ariese, F.; Gooijer, C. Achievements in resonance Raman spectroscopy. Anal. Chim. Acta 2008, 606, 119–134. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Grygar, T.; Bezdicka, P.; Brunetti, B.G.; Sgamellotti, A.; Burgio, L.; Seccaroni, C.; Miliani, C. Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situ characterisation of Renaissance plates by portable micro-Raman and XRF studies. J. Raman Spectrosc. 2011, 42, 407–414. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; López-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Coentro, S.; Mimoso, J.M.; Lima, A.M.; Silva, A.S.; Pais, A.N.; Muralha, V.S.F. Multi-analytical identification of pigments and pigment mixtures used in 17th century Portuguese azulejos. J. Eur. Ceram. Soc. 2012, 32, 37–48. [Google Scholar] [CrossRef][Green Version]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Cascales, C.; Alonso, J.A.; Rasines, I. The new pyrochlores Pb2(MSb)O6.5 (M = Ti, Zr, Sn, Hf). J. Mater. Sci. Lett. 1986, 5, 675–677. [Google Scholar] [CrossRef]
- Muralha, V.S.F.; Miguel, C.; Melo, M.J. Micro-Raman study of Medieval Cistercian 12–13th century manuscripts: Santa Maria de Alcobaça, Portugal. J. Raman Spectrosc. 2012, 43, 1737–1746. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H.; Firth, S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analyst 2001, 126, 222–227. [Google Scholar] [CrossRef]
- Baran, A.; Fiedler, A.; Schulz, H.; Baranska, M. In situ Raman and IR spectroscopic analysis of indigo dye. Anal. Methods 2010, 2, 1372–1376. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Moens, L. Micro-Raman spectroscopy of natural and synthetic indigo samples. Analyst 2003, 128, 187–193. [Google Scholar] [CrossRef]


| Analyzed Point | Sampling Area and Brief Description | Employed Techniques |
|---|---|---|
| S1 | Red micro-fragment from St. Clemente’s cloak | XRF, EPMA-EDS, µ-Raman |
| S2 | Red micro-fragment from St. Mercurio’s cloak | XRF, EPMA-EDS, µ-Raman |
| S3 | Orange micro-fragment from St. Vicario’s cloak | XRF, EPMA-EDS, µ-Raman |
| S4 | Green micro-fragment from St. Vicario’s cloak | XRF, EPMA-EDS, µ-Raman |
| S5 | Green micro-fragment from St. Vicario’s cloak | XRF, EPMA-EDS, µ-Raman |
| S6 | Flesh-toned micro-fragment from St. Vicario’s right hand | XRF, EPMA-EDS, µ-Raman |
| S7 | Salmon-orange micro-fragment from St. Clemente’s cloak | XRF, EPMA-EDS, µ-Raman |
| S8 | Green micro-fragment from St. Clemente’s cloak | XRF, EPMA-EDS, µ-Raman |
| S9 | Yellowish-green micro-fragment from St. Clemente’s halo | XRF, EPMA-EDS, µ-Raman |
| S10 | Flesh-toned micro-fragment from St. Clemente’s cheek | XRF, EPMA-EDS, µ-Raman |
| S11 | Blackish-brown micro-fragment from St. Barbara’s sandal | XRF, EPMA-EDS, µ-Raman |
| S12 | Grayish-white micro-fragment from the cloud under St. Barbara’s sandal | XRF, EPMA-EDS, µ-Raman |
| S13 | Yellow-orange micro-fragment from St. Barbara’s cloak | XRF, EPMA-EDS, µ-Raman |
| S14 | Grayish micro-fragment from the Angel’s wing | XRF, EPMA-EDS, µ-Raman |
| S15 | Light blue micro-fragment from the cerulean shades under the Angel’s feet | XRF, EPMA-EDS, µ-Raman |
| S16 | Blackish-brown micro-fragment, from the back of the Angel’s head | XRF, EPMA-EDS, µ-Raman |
| S17 | Grayish micro-fragment, from the top of the Angel’s sword | XRF, EPMA-EDS, µ-Raman |
| S18 | Grayish micro-fragment from the greyish shades under the Angel’s wing | XRF, EPMA-EDS, µ-Raman |
| S19 | Flesh-toned micro-fragment from the Angel’s belly | XRF, EPMA-EDS, µ-Raman |
| S20 | Blackish-brown micro-fragment from Christ’s hair | XRF, EPMA-EDS, µ-Raman |
| S21 | Light blue micro-fragment from behind Christ’s shoulders | XRF, EPMA-EDS, µ-Raman |
| S22 | Flesh-toned (whitish) micro-fragment from Christ’s arm | XRF, EPMA-EDS, µ-Raman |
| S23 | Flesh-toned micro-fragment from the Angel’s face, under Christ’s hand | XRF, EPMA-EDS, µ-Raman |
| S24 | Golden yellow micro-fragment from the Angel’s hair under Christ’s foot | XRF, EPMA-EDS, µ-Raman |
| S25 | Flesh-toned micro-fragment from the Angel’s leg under Christ’s foot | XRF, EPMA-EDS, µ-Raman |
| S26 | Yellow micro-fragment from St. Vicario’s cloak | XRF, EPMA-EDS, µ-Raman |
| S27 | Yellow micro-fragment from St. Vicario’s cloak | XRF, EPMA-EDS, µ-Raman |
| S28 | Left side of the canvas, bottom 1 | XRF |
| Analyzed Point | Elemental Composition by XRF |
|---|---|
| S1 | S, Ca, Pb, Fe, Hg, Cl, K, As, (Zn, Se, Mn, Ba, Sr, Rb, Cr, Mo, Zr) |
| S2 | Ca, S, Fe, K, Cl, Pb, (I, Hg, As, Ti, Mn, Zn, Ba, Sr, Cr, Mo, Zr, Rb, Se) |
| S3 | S, Pb, Ca, Cl, As, Fe, K, (Ti, Se, Hg, Mn, Cd, Zn, Sn, Ba, Rb, Sr) |
| S4 | S, Pb, Ca, Cl, As, K, Fe, Co, (Cu, Sb, Ti, Hg, Se, Ni, Mn, Zn, Sr, Rb, Ba, Cd, Cr, Mo) |
| S5 | S, Pb, Ca, Cl, As, K, Fe, Co, (Ni, Mn, Cu, Se, Hg, Zn, Sb, Rb, Ba, Sr, Cd, Mo) |
| S6 | S, Pb, Cl, As, Ca, Fe, K, Hg, (Se, Ti, Cd, Zn, Mn, Rb, Mo) |
| S7 | S, Pb, Ca, Cl, Fe, K, Ti, Fe, Zn, (Se, Sb, Mn, Ba, Cd, Rb, Sr) |
| S8 | S, Ca, Pb, Fe, Cl, As, K, Mn, (Hg, Ti, Zn, Se, Ba, Sr, Rb, Cr, Cu, Cd, Mo, Zr) |
| S9 | S, Pb, Ca, Cl, As, Fe, K, (Mn, Se, Ti, Zn, Hg, Cu, Rb, Ba, Sr, Cd, Mo) |
| S10 | S, Pb, Cl, As, Ca, Fe, Hg, K, (Se, Zn, Ti, Cd, Ba, Mn, Rb) |
| S11 | S, Pb, Ca, Cl, As, Fe, K, Hg, (Co, Mn, Se, Zn, Ti, Ni, Rb, Cd, Ba, Sr, Mo) |
| S12 | S, Pb, Cl, As, Ca, K, Co, (Fe, Se, Ti, Zn, Cd, Mn) |
| S13 | S, Pb, Ca, Fe, Cl, K, As, Sb, Hg, I, (Ti, Mn, Zn, Cd, Ba) |
| S14 | S, Pb, Cl, As, Ca, K, Hg, Fe, Co, (Zn, Cd, Sn, Ti, Mn) |
| S15 | S, Pb, Cl, As, Ca, K, Co, Fe, (Se, Zn, Ni, Cd, Rb, Mn, Ba) |
| S16 | S, Pb, Ca, Cl, As, K, Fe, Co, (Ti, Ni, Se, Cu, Zn, Ba, Mn, Rb) |
| S17 | S, Ca, Pb, Cl, As, K, Fe, (Hg, Se, Zn, Sr, Rb, Mn, Cd, Mo) |
| S18 | S, Pb, Ca, Cl, As, K, Fe, (Se, Zn, Ti, Cu, Rb, Cd, Sr, Mn, Ba) |
| S19 | S, Pb, Ca, Cl, As, Hg, Fe, K, (Ti, Se, Zn, Cu, Rb, Sb, Mn, Sr) |
| S20 | Ca, S, Fe, Pb, K, Cl, As, I, (Hg, Mn, Zn, Sr, Ba, Cu, Se, Rb, Mo, Zr) |
| S21 | S, Pb, Cl, As, Ca, K, (Fe, Se, Cd, Zn, Ti, Mn) |
| S22 | S, Pb, Cl, As, Ca, K, Sn, (Cd, Mn) |
| S23 | S, Pb, Cl, As, Ca, Hg, K, Fe, (Se, Zn, Cd, Rb, Mn) |
| S24 | S, Ca, Cl, Pb, K, Fe, As, (Hg, Mn, Se, Cu, Sb, Zn, Sr, Rb) |
| S25 | S, Pb, Cl, As, Ca, Hg, K, Fe, (Ti, Se, Cd, Zn, Mn, Ag) |
| S26 | S, Pb, Ca, Cl, K, As, Fe, Sb, Ti, (Zn, Ba, Se, Cr, Cd, Mn, Sr) |
| S27 | S, Ca, Pb, Cl, Fe, As, K, (Sb, Hg, Ti, Se, Mn, Zn, Cu, Sr, Rb, Cd, Mo) |
| S28 | Ca, S, Pb, K, Cl, Fe, As, (Zn, Sr, Mn, Cu, Se, Ba, Mo, Rb) |
| Analyzed Point | EDS Data |
|---|---|
| S1 | Hg, S, Pb, Fe, Ca, Si, Al, K |
| S2 | Hg, S, Pb, Fe, Ca, Si, Al, K, Ti |
| S3 | Pb, Fe, Ca, Si, Al, Mg, K |
| S4 | Si, Al, Fe, Cl, Mg, K, Na, Pb, Ca |
| S5 | Si, Al, Fe, Cl, Mg, K, Na, Pb, Ca |
| S6 | Pb, Fe, Ca, Si, Al, K, Na |
| S7 | Pb, Fe, Ca, Si, Al, K, Na |
| S8 | Si, Al, Fe, Cl, Mg, K, Na, Pb, Ca |
| S9 | Si, Al, Fe, Ca, Mg, K, Na, Ba, Pb, Ca |
| S10 | Pb, Fe, Ca, Si, Al, Mg, K |
| S11 | Si, Al, Mg, Na, Fe, K, S, Cl, Pb, Ca |
| S12 | Pb, Ca, Si, Al, Mg, Na, Fe, K, S, Ti |
| S13 | Pb, Sb, Si, Ca, Na, K, Al, Mg, S, Fe, Cl |
| S14 | Pb, Ca, Si, Al, Mg, Na, Fe, K, S, Ti |
| S15 | Si, Co, K, Ca, S, Fe, Al, K, Ti, Pb, Ca |
| S16 | Si, Al, Mg, Na, Fe, K, S, Cl, Pb, Ca |
| S17 | Pb, Ca, Si, Al, Mg, Na, Fe, K, S, Ti |
| S18 | Pb, Ca, Si, Al, Mg, Na, Fe, K, S, Ti |
| S19 | Pb, Fe, Ca, Si, Al, Mg, K, Ti |
| S20 | Pb, Ca, Si, Al, Mg, Fe, Mn, P, Na, K, Ti |
| S21 | Si, Al, Fe, Cu, K, Mg, Fe, Na, Pb, Ca |
| S22 | Pb, Fe, Ca, Si, Al, Mg, K, Na |
| S23 | Pb, Ca, Si, Al, Mg, K, Fe, S, Na, Hg |
| S24 | Pb, Hg, Sb, S, Ca, Si, Na, Al, Mg, K, Cl |
| S25 | Pb, Fe, Ca, Si, Al, Mg, K |
| S26 | Pb, Sb, Si, Ca, Na, K, Al, Mg, S, Fe, Cl, Ti |
| S27 | Pb, Sb, Si, Ca, Na, K, Al, Mg, S, Fe, Cl, Ti |
| Point of Analysis | Color | Used Pigments and Other Materials |
|---|---|---|
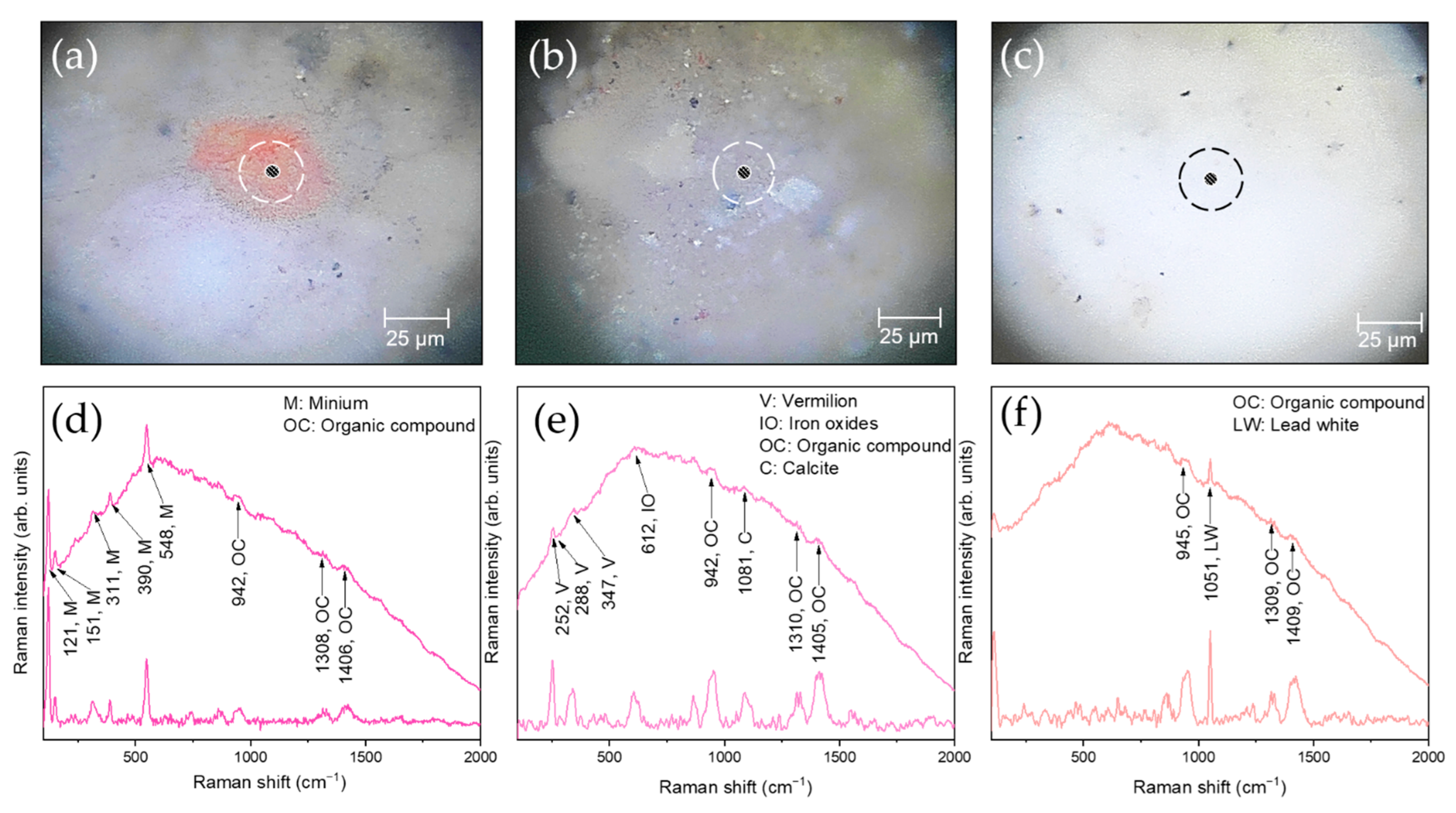
| S1, S2 | Red | Vermilion, iron oxides, Pb-based compounds, Ti-based compounds, organic compounds |
| S3, S7, S13, S24, S26, S27 | Orange/yellow-orange/yellow | Naples yellow, Fe-based compounds, Hg-based compounds, organic compounds |
| S4, S5, S8, S9 | Green | Fe-based compounds |
| S6, S10, S19, S22, S23, S25 | Flesh tones | Minium, vermilion, iron oxides, calcite, lead white, organic compounds |
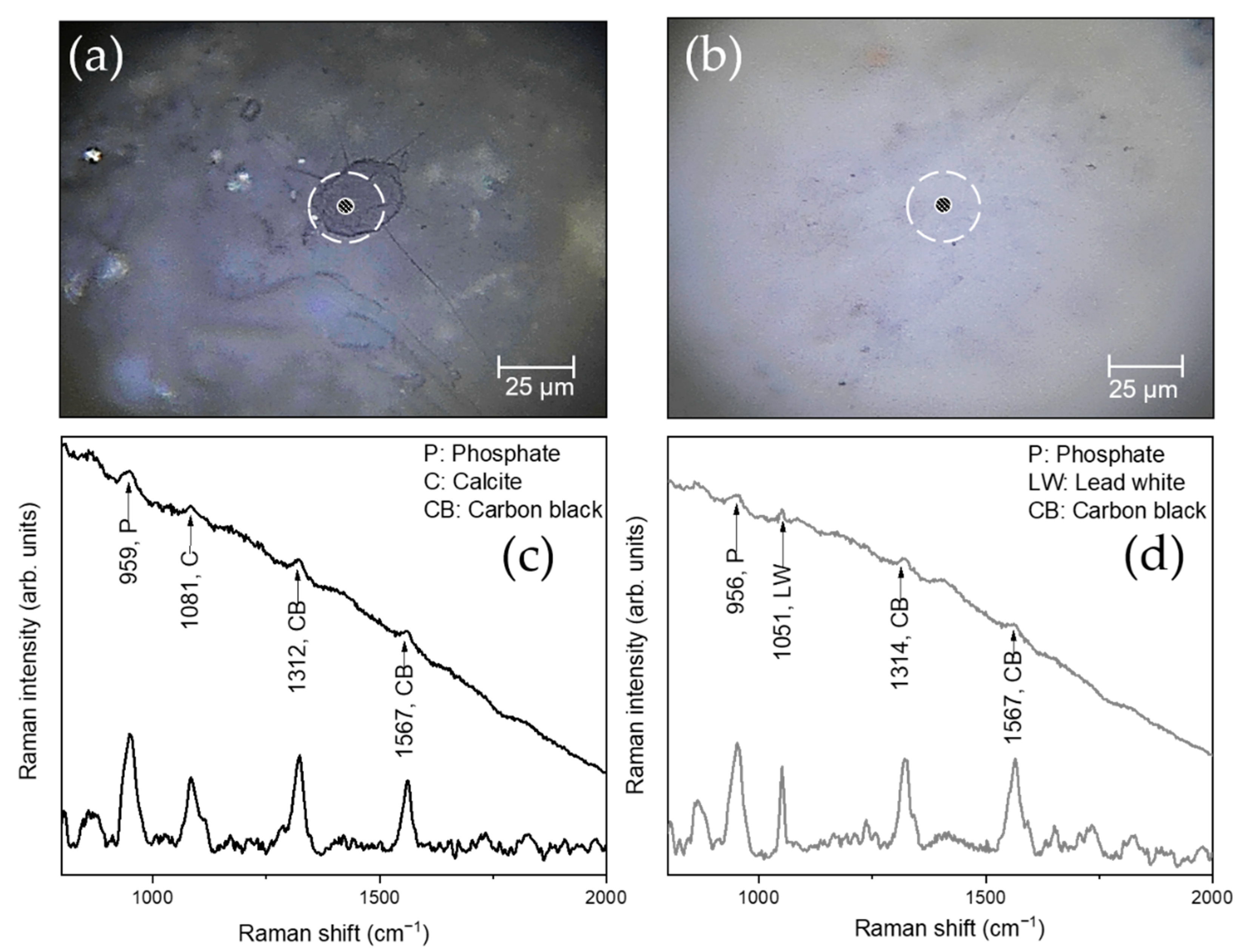
| S11, S12, S14, S16, S17, S18, S20 | Blackish-brown/grayish | Carbon black (bone black or ivory black), calcite, lead white, Fe-based compounds, Mg-based compounds |
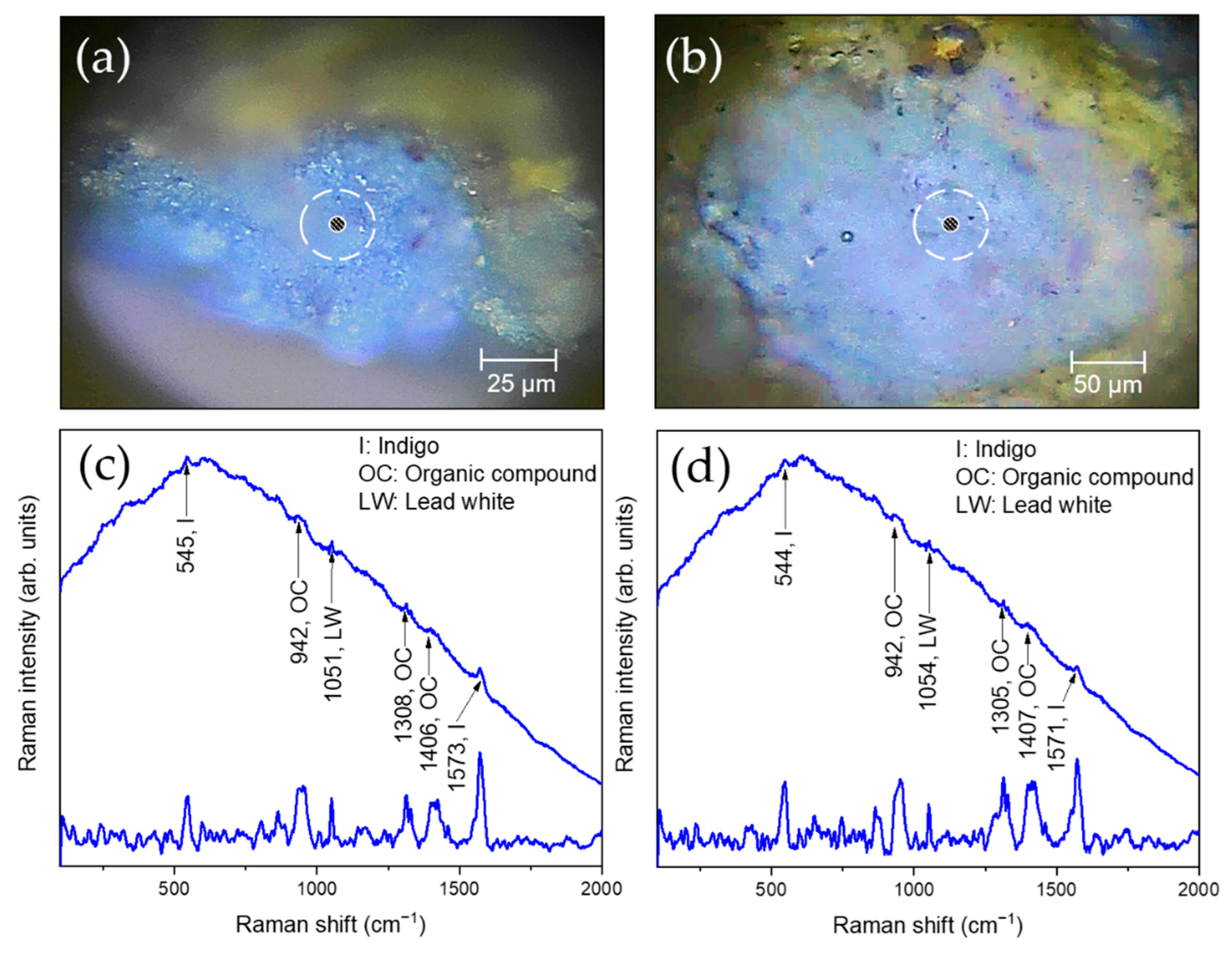
| S15, S21 | Blue | Indigo, lead white, Co-based compounds, organic compounds |
| S28 | Canvas | Ca-based compounds, S-based compounds, Pb-based compounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caridi, F.; Ricca, M.; Paladini, G.; Crupi, V.; Majolino, D.; Donato, A.; Guido, S.; Mantella, G.; Randazzo, L.; La Russa, M.F.; et al. Multi-Technique Diagnostic Investigation in View of the Restoration of “The Glory of St. Barbara” Painting by Mattia Preti. Appl. Sci. 2022, 12, 1385. https://doi.org/10.3390/app12031385
Caridi F, Ricca M, Paladini G, Crupi V, Majolino D, Donato A, Guido S, Mantella G, Randazzo L, La Russa MF, et al. Multi-Technique Diagnostic Investigation in View of the Restoration of “The Glory of St. Barbara” Painting by Mattia Preti. Applied Sciences. 2022; 12(3):1385. https://doi.org/10.3390/app12031385
Chicago/Turabian StyleCaridi, Francesco, Michela Ricca, Giuseppe Paladini, Vincenza Crupi, Domenico Majolino, Antonio Donato, Sante Guido, Giuseppe Mantella, Luciana Randazzo, Mauro Francesco La Russa, and et al. 2022. "Multi-Technique Diagnostic Investigation in View of the Restoration of “The Glory of St. Barbara” Painting by Mattia Preti" Applied Sciences 12, no. 3: 1385. https://doi.org/10.3390/app12031385
APA StyleCaridi, F., Ricca, M., Paladini, G., Crupi, V., Majolino, D., Donato, A., Guido, S., Mantella, G., Randazzo, L., La Russa, M. F., & Venuti, V. (2022). Multi-Technique Diagnostic Investigation in View of the Restoration of “The Glory of St. Barbara” Painting by Mattia Preti. Applied Sciences, 12(3), 1385. https://doi.org/10.3390/app12031385













