Abstract
(1) Aims: To test a newly designed helical-wire hook electrode implanted in the bladder wall to induce contraction and promote voiding. (2) Methods: In three minipigs with a created lesion of the sacral spinal cord, four electrodes were implanted in the bladder wall, ventral to the trigone. Stimulation tests were conducted initially in conscious pigs, and later after general anesthesia. (3) Results: Electrical stimulation in the conscious animals on postoperative days 4 and 7 at 40 Hz was limited to 10 mA, because of abdominal, leg, and anal contractions with animal discomfort; bladder contractions were not induced. Electrical stimulation on postoperative days 9 and 28 at 60 mA under anesthesia induced sustained vesical wall contractions with bladder pressure variations, but without voiding. Simultaneous abdominal contractions occurred, with strong leg and anal contractions. Subsequent stimulation with a single set of electrodes or at 20 Hz induced less vesical pressure response. At autopsy, the electrodes had not migrated, and extraction forces were high, at 7.9 ± 0.9 Newtons (n = 12). (4) Conclusions: Our 28-day study has confirmed the utility of the new electrode design, preventing migration from the bladder wall and making it suitable for long-term electrode implants.
1. Background
The use of catheters for neurogenic bladder management is a source of significant morbidity, so different alternatives such as neuroprosthetic devices using electrical stimulation were developed and put into clinical use [1,2,3,4]. For complete suprasacral spinal cord lesions, the Brindley sacral anterior root stimulator is commercially available [5]. For complete sacral cord lesions resulting in a decentralized bladder and sphincter (“second neuron damage”), direct bladder wall stimulation (DBWS) is still in development, as post-ganglionic nerves located in the bladder wall (intramural nerve network) are viable. Starting in 1958, several study teams demonstrated the feasibility of DBWS, with promising results [6,7,8,9,10,11].
More recent studies were published by Merrill (1975) and by Jonas and Hohenfellner (1978), using the Mentor™ bladder stimulator in patients with “urinary vesical hypotonia”, with a few good long-term results [12,13]. The most recent clinical studies were conducted by Magasi and Simon (1986) [14]. They concluded that the best location for electrode implantation was ventral to the ureters and trigone area, which was in line with prior results [14].
With the increasing use of intermittent catheterization for the management of neurogenic bladder, DBWS was considered less interesting for clinicians [3]. However, experimental studies in cats and dogs have continued [5,15,16,17]. In one chronic study, a suture electrode composed of multistranded stainless steel wire electrodes with a needle attached to the tip was sutured into the bladder wall, and effective bladder contractions were induced with voiding; however, electrode migration was a problem. The most recent DBWS study (2012) used Permaloc® stainless steel wire electrodes that had two stimulating surfaces on a single lead [18]. An anesthetized swine model was used, and only limited detrusor contraction was produced, with no voiding.
2. Aim of the Study
We have tested a new electrode design, composed of a monopolar multistranded stainless-steel wire, which we expected to be less prone to migration and to allow for wider separation of the stimulating tips. The tests were conducted with the electrodes implanted in the bladder wall to induce detrusor contraction and promote voiding in minipigs with an induced chronic sacral spinal cord lesion.
3. Materials and Methods
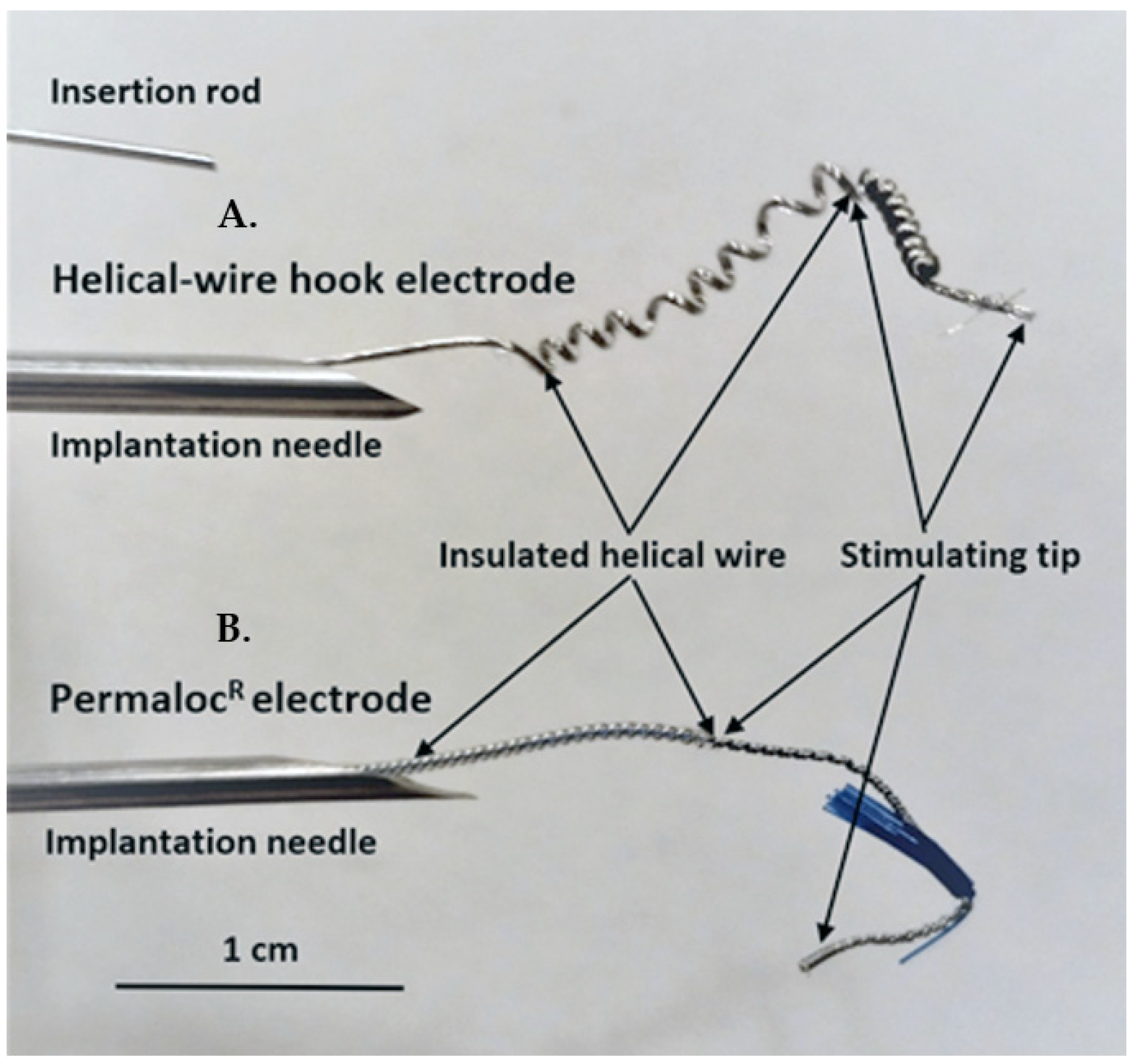
Helical-wire hook electrodes were made from sixty-five strands of thin 316LVM stainless-steel wires (0.001 inches in diameter each) insulated with Teflon (Med-wire 65, Cooner Wire Co, Chatsworth, CA, USA). A lead was formed into a helix near the stimulating tip by wrapping the lead 10 times around a 21-gauge needle with a tension of 2 to 3 kg (Figure 1A). The stimulating tip consisted of one and a half cm of exposed wire, which was also wrapped into a helix and bent back to form a hook. For comparison, the Permaloc® intramuscular electrode, which is used for phrenic nerve stimulation and respiratory management in SCI patients, is shown (Figure 1B). The insertion rod is used with both types of electrodes during the needle insertion. The rod is put into the needle and advanced to the helical area of the electrodes. During insertion, the rod ensures that the electrode tip stays where it is placed in the needle; after insertion, the rod can be held in place and the needle removed without the electrode tip moving.
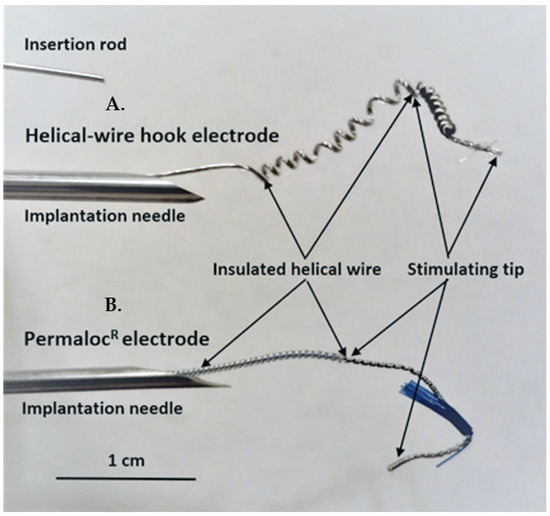
Figure 1.
Photograph comparing the helical-wire hook electrode used in this study to the commercial Permaloc® electrode used for respiratory pacing in SCI patients. (A) The lead of the helical-wire hook electrode is shown in a 14-gauge needle. The needle would be advanced up to the exposed hook of the stimulating tip prior to insertion. Needle insertion was used in the first minipig and implantation with imbrication on the bladder wall was used in the last two animals. An insertion rod is shown above the needles that is used to ensure accurate deployment of the electrode during needle insertion. (B) The Permaloc® electrode was not used in this study but is shown for comparison. The constructing materials are the same for both electrodes.
Two isolated electrical stimulators (Model SD-9, Grass Inc, Quincy, MA, USA) were used with 400 µs pulse durations, as the 400 µs duration was reported to be the most effective in a current-response study of DBWS; also, a high frequency of 40 Hz was used as previously reported [6,14,15,16,17,18]. The stimulators were synchronized for simultaneous pulses, and they were connected to the implanted electrodes as two bilateral sets of bipolar electrodes. Stimulation tests consisted of either increasing the current every few seconds or using a single current for 10 to 20 s [18].
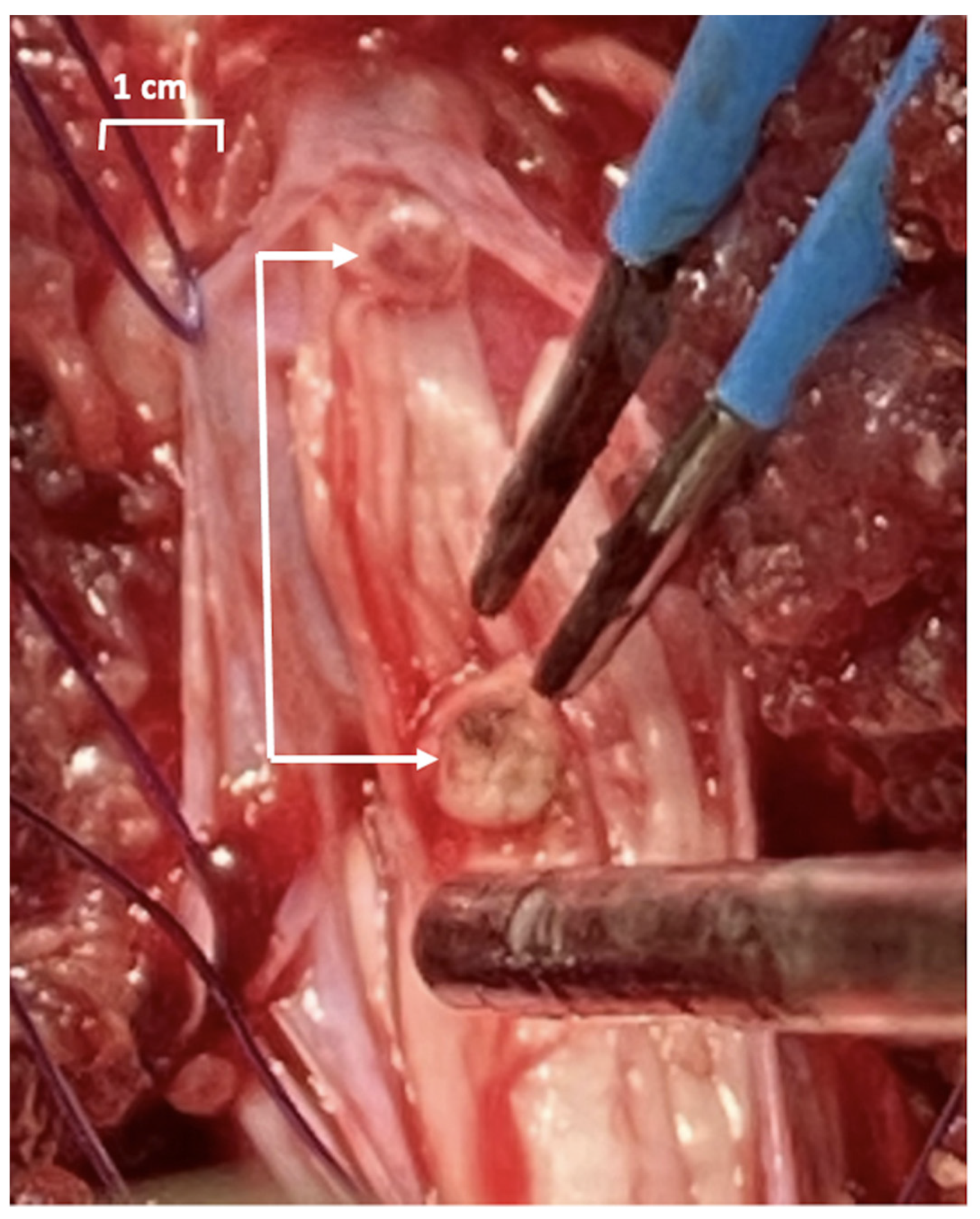
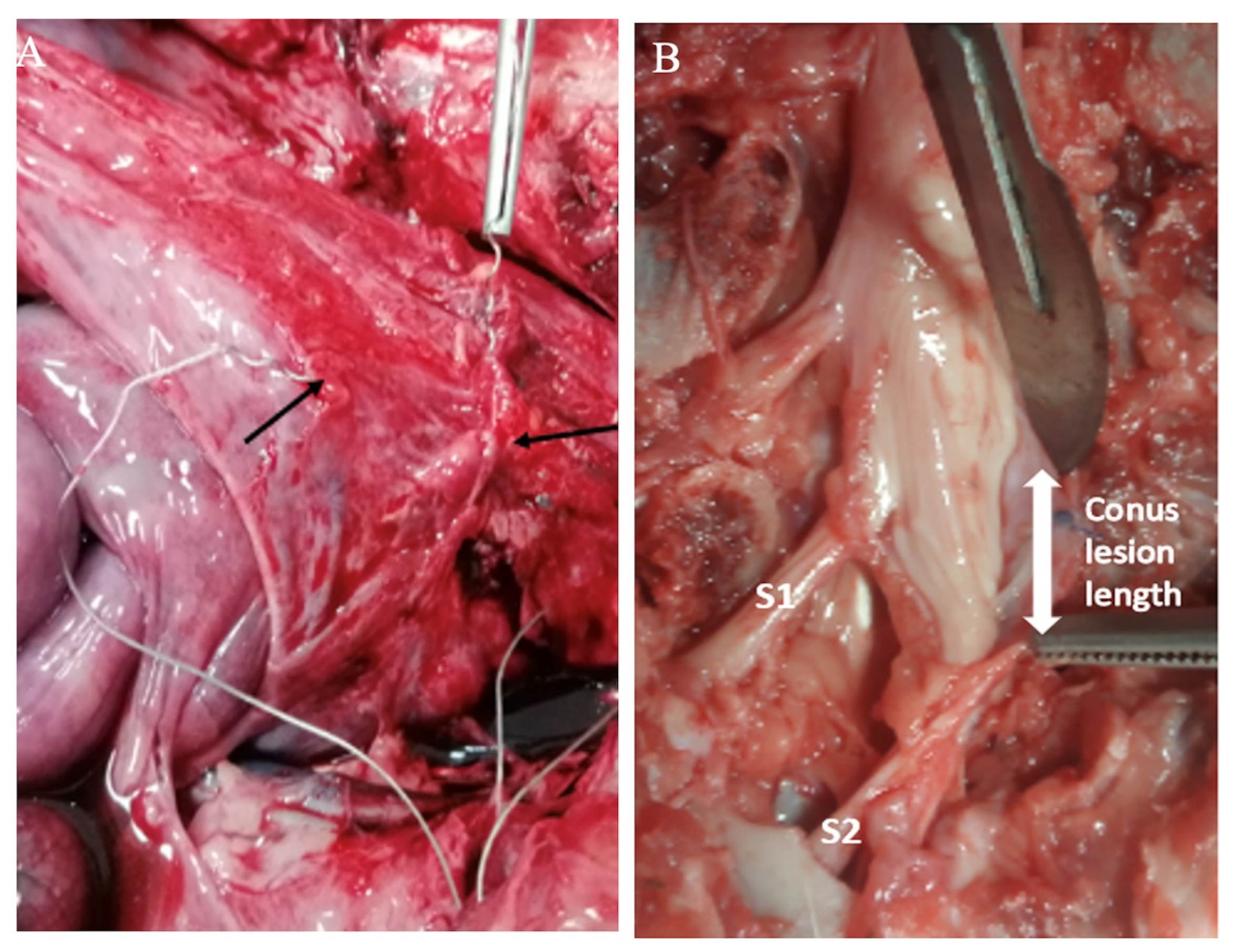
The protocol was approved by the Ethics Committee for Research on Animals of the Banat University of Agricultural Sciences and Veterinary Medicine, Timisoara, Romania (Ethics Committee Approval No. 73/30 January 2019, and Project Approval from Romanian Veterinarian Authority No. 5/5 February 2020). Three healthy Gottingen Minipigs (females, 8 months of age, 20–30 kg; Ellegaard Minipigs, Dalmose, Denmark) were used. Prior to surgery, the minipigs were acclimated for six months to remain in a study cage. General anesthesia was induced as previously described, and a Foley catheter (Ch10, Coloplast, Humlebaek, Denmark was placed [19]. For creating a defined sacral spinal cord lesion, the pigs were placed prone, and after a 15 cm midline incision with the preparation of the dorsal spinal elements, a two-level-laminectomy (L5, L6 and partially S1) was performed, the dura was opened longitudinally and the spinal cord with the lumbosacral roots was exposed. The plan was to achieve a complete sacral spinal cord lesion by resecting the conus medullaris (Figure 2), but it was difficult to assess intraoperatively where the conus medullaris ended and the terminus filum (terminal thread) started.
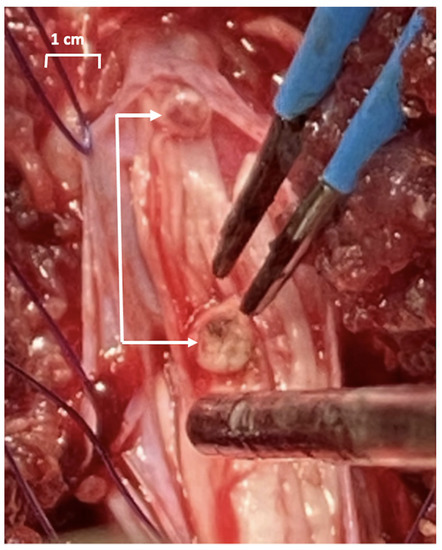
Figure 2.
Photograph of the sacral spinal lesion during surgery. The sectioned part of the sacral levels of lesion are marked with arrows. The cauterizing electrodes (blue) and a suction tube are also shown.
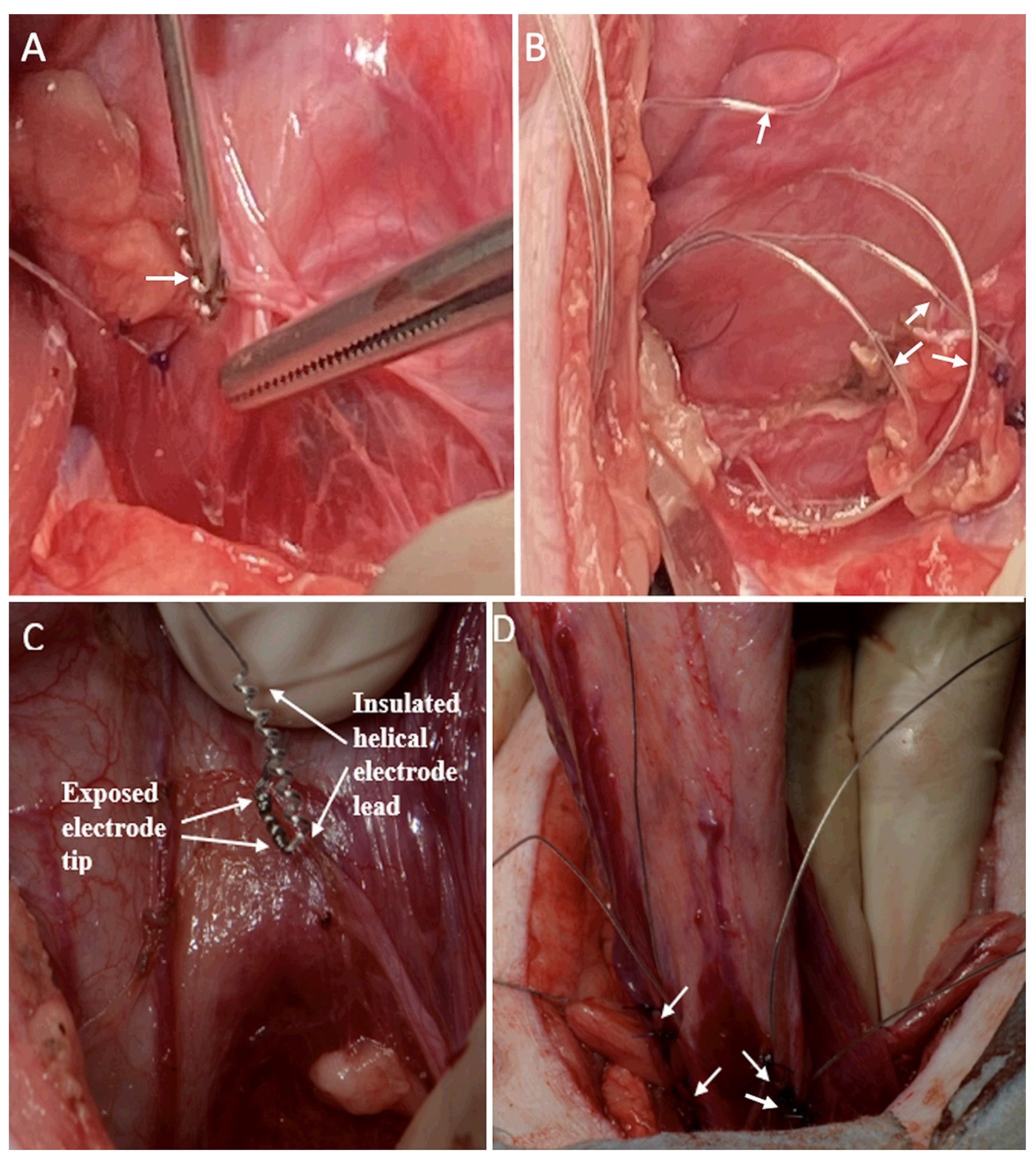
For placement of the bladder wall electrodes, a subumbilical midline abdominal incision was performed with the abdominal rectus muscle separated along the midline, and the peritoneum was incised. The ureters were identified in the posterior parietal peritoneum, just over the common iliac artery, and dissected down to their insertion into the bladder wall. Two previously sterilized bilateral sets of bipolar electrodes were implanted, using sites already described by other authors [14,15,16]. A caudal set was implanted adjacent to the bladder neurovascular bundle and 3 mm ventral to the ureters. The second bipolar set was implanted 1.5 cm directly rostral to the first set [14,15,16]. In the first animal, the electrodes were implanted in the superficial bladder wall with a 14-gauge needle and were further secured with two 3-0 polypropylene sutures (Figure 3A). The electrode leads were looped in the abdominal cavity to reduce the risk of tension on the electrode tip (Figure 3B). A risk of perforating the thin bladder wall was determined in this animal; therefore, the remaining two animals were implanted in the same locations, but with imbrication of the serosa around the electrode using sutures (Figure 3C, D). A trocar was used to extend the electrode leads under the skin to an exit site on the back of the neck. The external leads were sutured to the skin, and a protective backpack was used with a securing belt placed under the animal. One to four knots on the ends of the leads identified the implant locations. All incisions were closed.
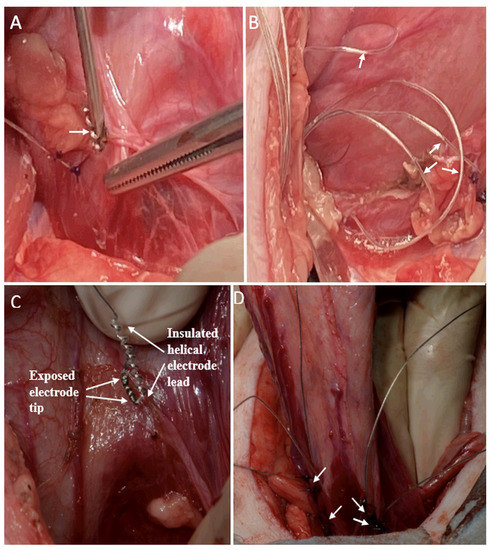
Figure 3.
Helical-wire hook electrode implantation in the first minipig with a needle and sutures, and in the second and third minipigs with imbrication and sutures. (A) In the first minipig, one electrode is shown after implantation with sutures in place and a second electrode is being implanted with a needle in a more medial–dorsal location (white arrow). (B) Looping of four electrode leads for strain relief is shown in the abdominal cavity. (C) The imbrication method of electrode implantation is shown for the last two animals. The electrode is over the bladder wall prior to implantation. (D) The area of the bladder wall ventral to the trigone is implanted with the four imbricated electrodes (white arrows).
Twenty-four-hour surveillance was used to monitor the health of the animals during four weeks of follow-up. For post-operative bladder emptying, a permanent Foley catheter and a urine bag were used, with the catheter tube taped to the tail. Intermittent catheterization could not be used due to preserved pain sensation.
On postoperative days 4 and 7 (early period), bladder pressure was assessed in the conscious minipigs by connecting the Foley catheter to the pressure transducer of the urodynamics machine (Andromeda Ellipse, Laborie, Potzham, Germany) [20]. On postoperative days 9 and 28 (late period), the animals underwent surgical-level anesthesia (using IV Propofol) and 2-channel cystometry bladder catheters were used. DBWS was conducted with the bladder previously filled with 150–250 mL of saline solution.
During autopsy, the extent of the sacral spinal lesion and electrodes were evaluated. Electrode extraction force was measured in Newtons with a beaker tied to the lead and by the weight of water added to cause dislodgement. Results are presented as the mean ± SEM. With testing limited to three animals, further statistical analysis was not warranted. See Supplementary Materials.
4. Results
The animals quickly recovered from surgery and began walking on all four legs. The bulbocavernosus reflex test conducted with a pull on the Foley catheter produced a moderate anal contraction.
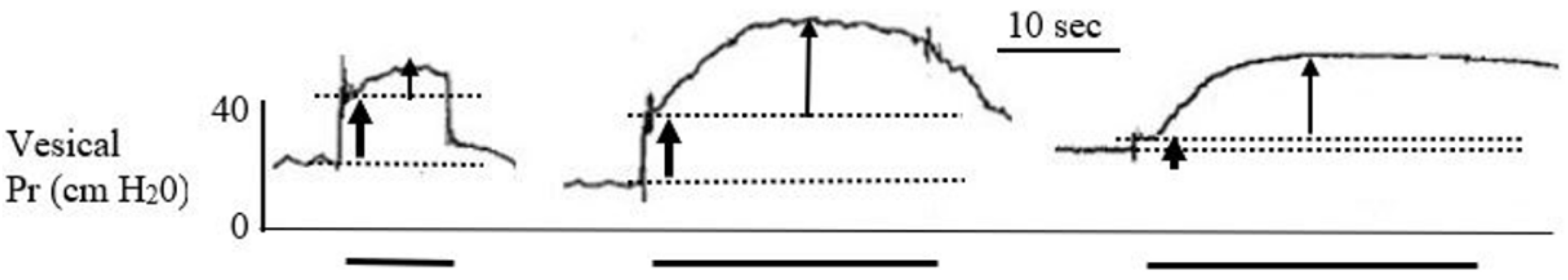
DBWS was conducted during the early and late testing periods. Early tests in the conscious animal used the two bipolar sets of electrodes with simultaneous pulses at 40 Hz. The stimulating current, however, was limited to 10 mA due to abdominal, leg, and anal contractions, and animal discomfort; bladder contractions were not induced. Therefore, general anesthesia was used during testing in the late period to prevent any animal discomfort. Responses to stimulation at 10 to 40 mA induced stronger skeletal muscle contraction and no or slight vesical pressure responses. Representative records for the 60 mA stimulation current for each of the three animals on PO Day 9 are shown in Figure 4: vesical pressure increased rapidly at the start of stimulation, and this was attributed to abdominal pressure changes caused by abdominal wall contractions. Continued stimulation was associated with a slow but significant increase in the vesical pressure to a plateau value, which was attributed to vesical wall contraction. The 60 mA current also caused strong contraction of the legs and anal sphincter.
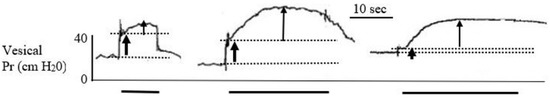
Figure 4.
Bladder pressure responses to DBWS from the three minipigs after general anesthesia and using the single-current protocol of 60 mA, 40 Hz and 10–20 s stimulation (PO day 9). The pressure records demonstrate the initial abdominal wall contraction as a sharp rise in pressure, marked between the two guidelines by the lower thick arrow. The vesical wall contractions are marked by the narrow upper arrow. The continuous stimulation period is marked below the records by the thick solid lines.
Representative responses for all stimulation tests conducted at 40 Hz using all four electrodes, at an average filling volume of 188 mL (±29 mL), are shown in Table 1. Moderate vesical wall contractions were observed only during the late period, when anesthesia and high stimulating currents were used. Use of only a single set of electrodes or stimulation at 20 Hz produced less vesical wall response (results not shown in Table 1). These tests, however, were conducted at the end of the protocol and bladder fatigue may have been a factor in decreased responses. None of the stimulation tests induced voiding, even after removal of the Foley catheter, so the voiding efficiency was 0%.

Table 1.
Responses to 40 Hz four electrode stimulation at 10 mA in the conscious animal during early testing days (average values from postoperative days 4 and 7) and at 60 mA under anesthesia during the late testing days (average values from postoperative days 9 and 28) from the three minipigs. The important vesical wall contraction increases in pressure are highlighted in red.
Daily animal care included disposal of copious amounts of urine from the Foley bag, and dipstick tests indicated no signs of urinary tract infection. Animals were sacrificed on postoperative day 28 and the autopsy revealed that all the electrodes were in the positions where they had been implanted. A thin film of connective tissue covered the implants leads next to the bladder wall and in the abdomen, as shown in Figure 5A. The leads were noted to easily slip inside their connective tissue covering, which is important for avoiding lead pulling on the electrode tip. Electrode extraction forces were similar between the three animals, amounting to 7.9 ± 0.9 Newtons (n = 12), which demonstrates good security. Dissection of the spinal cord revealed that the segments S1 and S2 of the conus were resected, but there was sparing of lower sacral levels, S3 to S5 (Figure 5B).
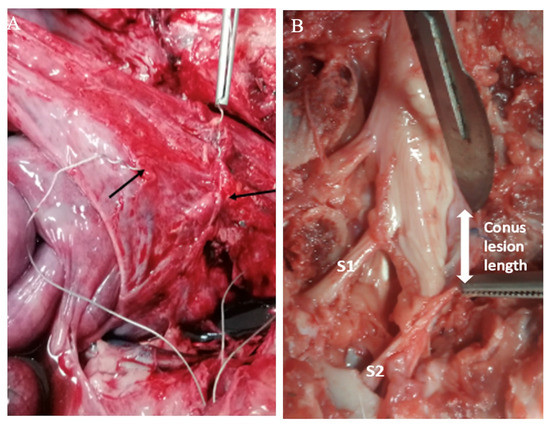
Figure 5.
Autopsy photographs of bladder wall electrodes and the sacral cord lesion. (A) The leads of two helical-wire hook electrodes are shown where they had been implanted on the right side of the bladder wall (black arrows); a forceps is attached to one electrode lead. Looping of the four electrode leads in the suprapubic area is also shown. (B) Sacral spine showing S1 and S2 levels of the lesion. The lower sacral levels, S3, S4, S5, were partially spared.
5. Discussion
Several prior DBWS clinical studies have reported effective voiding; however, some patients have had side effects of pain, abdominal muscles contraction, urethral sphincter contractions preventing voiding, and limited detrusor responses [11,12,13,14]. Our current test results highlight these potential problems.
The primary limitation encountered in the current protocol was animal discomfort and aversive skeletal muscle contractions at levels of stimulating currents which were lower than necessary for inducing vesical wall contractions. Only with the administration of general anesthesia and the use of a high stimulating current (60 mA) were we able to induce moderate but reproducible bladder contractions. Several factors may have contributed to the adverse effects and the need for higher stimulating currents. The sacral spinal cord lesion was incomplete below the S2 level, and spinal sacral activity persisted at levels S3-S5 due to surgical difficulties. During the conus medullaris resection, it was observed to be at the level of the S2 vertebra. Using a two-level laminectomy, first in the lumbar and then in the sacral areas, it was difficult to resect the whole length of the conus, which reached almost the end of the dural sac; thus, there was remaining lower sacral reflex function. This was confirmed postoperatively by a positive bulbocavernosus reflex and discomfort to passing a rectal catheter [21]. Moreover, we know from studies of the Brindley implant that it can only be applied in patients with complete spinal lesions, because electrostimulation causes pain in patients with preserved sensation [5]. Stimulating currents as low as 10 mA were causing the abdominal and leg muscles to contract, which may have been painful; however, all stimulation was stopped if there was any indication of animal discomfort.
Several additional factors may have contributed to the poor detrusor responses to stimulation. First, the porcine models may have low detrusor contractility; for example, the empty bladder was observed as large and flaccid, being previously reported as a reason for non-response to DBWS in swine [18]. Other species, including cats, dogs, and humans, have shown higher detrusor responses to DBWS [15,16,17,22]. Second, the use of general anesthesia with Propofol may have limited the maximal vesical responses to DBWS; however, higher vesical pressures have been induced during anesthesia in other species [15,16,17]. Third, this was a pilot study with the results limited to three animals.
The lack of voiding during stimulation was another limitation encountered in this study. The high current which induced moderate vesical pressure increases could have produced voiding. In addition, resting vesical pressure before stimulation was moderately high, which could have resulted in voiding. Several mechanisms should be considered for the observed lack of voiding. First, detrusor sphincter dyssynergia, or detrusor bladder neck dyssynergia: the incomplete sacral lesion could have resulted in dyssynergia, which produces urethral sphincter contractions during bladder contraction. Moreover, during the stimulation studies on human subjects, Hohenfellner performed bladder neck incisions to reduce this unwanted effect and promote voiding. Second, electrical stimulation of the urethral sphincter: the unwanted effect of electrical field spread during stimulation was inferred from the palpation of strong contractions of the anus and perineum. Third, dyssynergia caused by anesthesia: this anesthesia effect was recently demonstrated in swine with intact spinal cords [18].
Reasonable electrode design and stimulation methods were used in this study. The electrode was similar to the clinically successful 1 cm disk electrodes implanted on the bladder wall by Magasi and Simon [14]. Implantation locations on the ventral side of the bladder above the trigone area are recommended sites [14,15,17]. Established stimulation methods were used, including long pulse durations, high stimulating frequencies, and current-response tests [13,14,15]. Finally, the use of two stimulators with simultaneous pulses is known to produce strong electric fields; this is important for bladder nerve activation, but also increases the spread of the electrical field [15]. With the identified limitations of bladder responses to stimulation encountered here, however, improved stimulation methods must be considered. For example, identifying effective stimulation sites on the bladder wall prior to permanent implantation should be conducted; placing electrodes closer together could reduce the spread of the electrical field, and tests with larger surface area electrodes to stimulate the broad plexus of nerves innervating the bladder could be helpful [23].
A positive finding of this study was that the helical-wire hook electrodes remained secure on the bladder wall during the four weeks (Figure 4A). The difficult implanting of the electrode in the bladder wall using a 14-gauge needle with a risk of bladder wall perforation in the first minipig led to the method of implantation using imbrication in the last two animals (Figure 3). Imbrication was effective because electrode migration did not occur. This is an improvement compared to previous reports of electrode implants on the bladder wall having migration problems [16,17]. The current electrode should be considered for use in future DBWS studies, and it may have wider application in the neuroprosthetics field [24]. It is composed of the same materials as used in the Peterson electrode, which is commercially available for direct diaphragm stimulation in SCI patients with respiratory insufficiency [18,25]. Our electrode, however, has many more strands of wire than the Peterson electrode, which gives it several positive attributes. The extra strands give it sufficient rigidity to hold a helix for self-securing, imbrication, and to resist kinking. The stimulating tip is resistant to corrosion due to its large surface area. The design of the electrode lead is straight wire, which reduces encapsulation and the risk of chronic dislodgement and migration (Figure 3B).
6. Conclusions
Our 28-day study has confirmed the utility of the new electrode design, preventing migration from the bladder wall and making it suitable for long-term electrode implants. However, in a setting of an incomplete sacral spinal cord lesion in a minipig, DBWS required anesthesia to avoid pain and to allow for the use of high stimulating currents (60 mA), which induced only moderate vesical wall contractions and no voiding, as well as strong skeletal muscle contractions. Future DBWS studies should include a complete sacral lesion, and alternative animal species and implantation sites, to induce adequate detrusor contraction and efficient voiding with minimal side effects.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app12031149/s1, Experimental Model.
Author Contributions
Conceptualization, J.W. and H.M.; methodology, J.W., I.H., B.T., A.A.C., R.B., D.N., O.G., M.P. and H.M.; validation, J.W., R.B. and H.M.; formal analysis, J.W., R.B. and H.M.; investigation, J.W., I.H., B.T., A.A.C., R.B., S.L., A.R., D.N., O.G., M.P. and H.M.; resources, J.W., I.H., O.G. and H.M.; data curation, J.W., R.B. and H.M.; writing—original draft preparation, J.W., R.B., A.D.C. and H.M.; writing—review and editing, J.W., R.B. and H.M.; supervision, J.W., R.B. and H.M.; project administration, J.W., R.B. and H.M.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Neuro-Urology Society, Grant for DBWS, 2020.
Institutional Review Board Statement
The study was approved by the Ethics Committee for Research on Animals, of the Banat University of Agricultural Sciences and Veterinary Medicine, Timisoara, Romania (Ethics Committee Approval No. 73/30 January 2019), and by the Romanian Veterinarian Authority (Approval No. 5/5 February 2020).
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Adrian Zeltner from Ellegaard Göttingen Minipigs for his excellent support. We would like to express our appreciation to the team of the Horia Cernescu Research Unit of the Banat University of Agriculture Science and Veterinary Medicine, Timisoara, Romania, for their excellent care of the minipigs. We would like to thank Esra Keller, from the Spinal Cord Injury and Tissue Regeneration Center Salzburg, and the Institute for Molecular Regenerative Medicine, Paracelsus Medical University, Salzburg, Austria, for her advice on the use of her minipig urodynamic model in this project, support and advice about the study, and review of the manuscript. Finally, we thank Ervin Kocjancic, Farshad Moghaddam, and Achim Herms, Head of the Neuro-Urology Unit at the Department of Urology of the Medical University Innsbruck, Austria, for discussions about the protocol and results, and for technical assistance and editorial comments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bladder Management for Adults with Spinal Cord Injury: A Clinical Practice Guideline for Health-Care Providers. Todd Linsenmeyer, MD, Chairman; Consortium for Spinal Cord Medicine. Available online: https://pva-cdnendpoint.azureedge.net/prod/libraries/media/pva/library/publications/cpgbladdermanageme_1ac7b4.pdf (accessed on 14 February 2021).
- Hayes, K.C.; Bassett-Spiers, K.; Das, R.; Ethans, K.D.; Kagan, C.; Kramer, J.L.; Linsenmeyer, T.; Moore, K.N.; Razvi, H.; Reid, G.; et al. Research priorities for urological care following spinal cord injury: Recommendations of an expert panel. Can. J. Urol. 2007, 14, 3416–3423. [Google Scholar]
- Munce, S.E.P.; Fehlings, M.G.; Straus, S.E.; Nugaeva, N.; Jang, E.; Webster, F.; Jaglal, S.B. Views of people with traumatic spinal cord injury about the components of self-management programs and program delivery: A Canadian pilot study. BMC Neurol. 2014, 14, 209. [Google Scholar] [CrossRef]
- Jaglal, S.B.; Munce, S.E.P.; Guilcher, S.J.; Couris, C.M.; Fung, K.; Craven, B.C.; Verrier, M. Health system factors associated with rehospitalizations after traumatic spinal cord injury: A population-based study. Spinal Cord 2009, 47, 604–609. [Google Scholar] [CrossRef]
- Brindley, S.; Polkey, E.; Rushton, M.; Cardozo, L. Sacral anterior root stimulators for bladder control in paraplegia: The first 50 cases. Neurol. Neurosurg. Psychiatr. 1986, 49, 1102–1114. [Google Scholar] [CrossRef]
- Walter, J.; Wheeler, J.S.; Bresler, L.; Sayers, J.S.; Singh, S. Neuroprosthetics for SCI bladder Management: The Argument for Direct Bladder Stimulation. Int. J. Phys. Med. Rehabil. 2014, 2, 5. [Google Scholar] [CrossRef]
- Burghele, T.; Ichim, V.; Demetresco, M. Electrical stimulation of the medullated bladder: Experimental study. J. Urol. Med. Chir. 1958, 64, 317–329. [Google Scholar]
- Susset, J.G.; Boctor, Z.N. Implantable electrical vesical stimulator: Clinical experience. J. Urol. 1967, 98, 673–678. [Google Scholar] [CrossRef]
- Hald, T.; Meier, W.; Khalili, A. Clinical experience with a radio-linked bladder stimulator. J. Urol. 1967, 97, 73–78. [Google Scholar] [CrossRef]
- Schoenberg, H.W.; Young, D.G.; Murphy, J.J. Electrical stimulation of the urinary bladder. Surg. Forum 1962, 13, 509–510. [Google Scholar] [PubMed]
- Habib, H.N. Neural trigger points for evacuation of neurogenic bladder by electro-stimulation. Surg. Forum 1963, 14, 489–491. [Google Scholar]
- Merrill, D.C. Clinical experience with the Mentor bladder stimulator III. Patients with urinary vesical hypotonia. J. Urol. 1975, 113, 335–337. [Google Scholar] [CrossRef]
- Jonas, U.; Hohenfellner, R. Later results of bladder stimulation in 11 patients: Follow-up to 4 years. J. Urol. 1978, 120, 565–568. [Google Scholar] [CrossRef]
- Magasi, P.; Simon, Z. Electrical stimulation of the bladder and gravidity. Urol. Int. 1986, 41, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Wheeler, J.S.; Cai, W.; King, W.W.; Wurster, R.D. Evaluation of a suture electrode for direct bladder stimulation in a lower motor neuron lesioned animal model. IEEE Trans. Rehabil. Eng. 1999, 7, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Wheeler, J.S.; Cai, W.; Scarpine, V.; Wurster, R.D. Direct bladder stimulation with suture electrodes promotes voiding in a spinal animal model: A technical Report. J. Rehab. Res. Dev. 1997, 34, 72–81. [Google Scholar]
- Bresler, L.; Walter, J.S.; Jahoda, A.; Wheeler, J.S.; Turk, T.; Wurster, R.D. Effective methods of pelvic plexus nerve and bladder stimulation in anesthetized animal model. J. Rehab. Res. Dev. 2008, 45, 627–638. [Google Scholar] [CrossRef]
- Walter, J.S.; Allen, J.C.; Sayers, S.; Singh, S.; Cera, L.; Thomas, D.; Wheeler, J.S. Evaluation of bipolar Permaloc® electrodes for direct bladder stimulation. Bentham Open Rehab. J. 2012, 5, 14–21. [Google Scholar] [CrossRef]
- Foditsch, E.E.; Roider, K.; Patras, I.; Hutu, I.; Bauer, S.; Janetschek, G.; Zimmermann, R. Structural Changes of the Urinary Bladder After Chronic Complete Spinal Cord Injury in Minipigs. Int. Neurourol. J. 2017, 21, 12–19. [Google Scholar] [CrossRef]
- Wyndaele, J.; Vo THi, H.; Pham, B. The use of one-channel water cystometry in patients with a spinal cord lesion: Practicalities, clinical value and limitations for the diagnosis of neurogenic bladder dysfunction. Spinal Cord 2009, 47, 526–530. [Google Scholar] [CrossRef]
- Walter, J.S.; Thomas, D.; Sayers, S.; Perez-Tamayo, R.A.; Crish, A.; Singh, S. Respiratory responses to stimulation of abdominal and upper-thorax intercostal muscles using multiple Permaloc® electrodes. J. Rehab. Res. Dev. 2015, 52, 85–96. [Google Scholar] [CrossRef]
- De Jonge, M.C.; Kornelis, J.A.; van den Berg, J.W. Electrically induced evacuation of the neurogenic bladder. Acute and long-term experiments (2 years) with paraplegic dogs. Acta Neurol. Scand. 1966, 42, 155–173. [Google Scholar] [CrossRef]
- Van Balken, M.; Vergunst, H.; Bemelmans, B.L. The use of electrical devices for the treatment of bladder dysfunction: A review of methods. J. Urol. 2004, 172, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, H.; Fischer, J. Sacral anterior root stimulation: Prerequisites and indications. J. Neurourol. Urodyn. 1993, 12, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Posluszny, J.; Dieter, R.; Dieter, R.S.; Sayers, S.; Kiratipath, I.; Staunton, C.; Thomas, D.; Rabbat, M.; Singh, S. Stimulation of Abdominal and Upper Thoracic Muscles with Surface Electrodes for Respiration and Cough: Acute Studies in Adult Canines. J. Spinal Cord Med. 2018, 41, 326–336. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).