Abstract
Taurodontism, a known morpho-anatomic variation in the shape of teeth, has already attracted substantial attention. The aim of this study is to contribute to this by discussing its direct impact on the dental practitioner via an evaluation of the prevalence of taurodontism in molars in Ashkelon, Israel. This retrospective study analyzed panoramic radiographs of 624 patients (330 males, 294 females), aged of 9–29 years—2849; first and second permanent molars were screened. Objective measurements and variables were used and analyzed using statistical SPSS version 27 (IBM, Chicago, IL, USA). Taurodontism was observed in 33.6% of the patients and was more prevalent among females (53% vs. 47%) and in maxillary molars compared to mandibular molars (57% vs. 43%). The overall prevalence of hypotaurodontism, mesotaurodontism, and hypertaurodontism was 10.8%, 0.5%, and 0.2%, respectively. No statistical difference between right and left sides was discovered, however, taurodont teeth in the upper left side exhibited the highest frequency, while the lower left side had the least. The highest occurrence of hypotaurodontism was on the upper right second molar followed by upper left first and second molars. The clinical challenge posed in endodontic, orthodontic, and restorative dentistry is discussed, and suitable alternative approaches are proposed for dental practitioners.
1. Introduction
Taurodontism is an anomaly of tooth shape that most often affects molars in deciduous as well as permanent dentition [1,2,3]. It was found in the ancient populations of Homo sapiens neanderthalensis and was assumed to be associated with thin enamel [4,5]. As the assumption that taurodontism was prehistorically advantageous for people to enable heavy masticatory habits was not proven, other external factors can be attributed to the anomaly [3,6]. It occurs in both the maxilla and mandible and is more commonly seen in molar teeth, unilaterally or bilaterally [7]. This dental anomaly is characterized by an enlarged apically displaced pulp chamber, a proportionately shortened root, and an enlarged pulp chamber in the affected dentition and apically displaced furcation areas. The bifurcation may be only a few millimeters above the apices of the roots [7]. Additionally, the teeth did not present the usual constriction at the cementoenamel junction observed in molars with a normal pulp chamber [8]. The pathogenesis of taurodontism is attributed to a disturbance in Hertwig’s epithelial root sheath (HERS), which forms the initial outline for the root’s morphology. A delay or failure in root invagination at the furcation region of the multiple roots of teeth results in the disturbance of a formation of the pulp chamber, which leads to several morphological aberrations, including taurodontism [3,9]. This vertical elongation of the pulp chamber of the teeth below the CEJ [3] may be a marker of orofacial disorders and has been reported to occur either as an isolated disorder or associated with dental anomalies and developmental syndromes [8]. Associations have been reported between taurodontism and microdontia, dens invaginatus, amelogenesis imperfecta, and dermatological diseases [10]. It has also been linked to the X-chromosome polyploidy, such as 47XXX, 48XXXX11, and 47XXY evident within the Finnish population [11,12]. An increasing trend of severity was observed with each additional X-chromosome [13,14]. In addition, Down syndrome patients have presented a greater frequency of taurodontism than in the presumed normal population [15].
Diagnosis of taurodontism has been mainly based on radiographic assessments, as the external crown morphology was within normal configurations [16]. Taurodontism was initially detected by periapical X rays and in panoramic radiographs [17,18]. Literature displays a variability of prevalence from less than 0.1% to 48% [19,20,21]. This diversity basically results from a lack of uniformity either in methodology or classification performed in different studies [22]. Taurodontism is classified according to its extent from the mildest (hypotaurodontism) through the moderate (mesotaurodontism) to the most severe manifestation (hypertaurodontism) [14]. However, different classifications of taurodontism were not always used by some of the studies that reported only on mesotaurodontism and hypertaurodontism, while ignoring hypotaurodontism and a highly affected comparison of prevalence between the population [21].
Taurodontism has an impact on the various types of treatment modalities: it complicates endodontic treatments, reduces the root canal surface area available for post anchorage, and jeopardizes orthodontic treatment due to root resorption vulnerability. Thus, the prevalence of taurodontism is important for the dental disciplines of endodontics, orthodontics, prosthodontics [3,14,21], and periodontics.
The aim of this study is to evaluate the prevalence of taurodontism amongst Israelis in one medical center using measurements from panoramic radiographs in order to discuss its implications for general dental practice treatment modalities.
2. Materials and Methods
Panoramic records of 624 patients were selected from a total of 1190 patients that attended the Department of Pediatric Dentistry at the Barzilai Hospital, Israel, between January 2015 and December 2018. Permanent first and second molars were included in this study. The age of the patients ranged from 9 to 29. The radiographic examinations were studied and measured by a single evaluator (I.H.Y.), calibrated by an experienced pediatric dentist and orthodontic specialist (U.Z. and S.E.). An intra-examiner reliability test was conducted to calibrate the consistency in the diagnosis of dental anomalies. All radiographs were taken using the same x-ray device and the same standardized method of a light table. The digital panoramic images were measured by a digital caliper using ImageJ software v.1.7.0. Repeatability was tested on 25 randomly selected radiographs examined at least three weeks after the initial examination.
2.1. Exclusion Criteria
The criterion for the exclusion of subjects was patients with a history of systemic diseases, syndromes, cleft lip and or palate, cases of ectodermal dysplasia, cleidocranial dysostosis, and Down’s syndrome. Not included in the study were also third molars, radiographically poor-quality impacted molars, fused roots, and undetectable furcation areas. Additionally excluded were root canal treated permanent molars, fractured molars, deep carious or restored molars, incomplete root formation, and molars with cemented orthodontic appliance.
2.2. Measurement System Criteria
Shifman and Chanannel [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23] developed a radiological evaluation system for taurodontism by objective measurements and variables, and their anatomical parameters are presented schematically in Figure 1. The measurements include two variables: variable 1 is defined as the height of the pulp chamber, between the lowest point of the roof and the highest point of the floor, and variable 2 is the distance between the lowest point of the roof of the pulp chamber and the apex of the longest root. They also developed a ‘taurodont index’ (TI) that is related to the height of the pulp chamber and the length of the longest root, and it is calculated as the ratio of the two variables: TI = variable 1/variable 2 × 100). Manifestation of the taurodontism [24] is expressed in order of increased severity, according to Shifman and Chanannel classification of the value of TI, hypotaurodontism (TI value 20–30), mesotaurodontism (TI value 30–40), and hypertaurodontism (TI value 40–70).
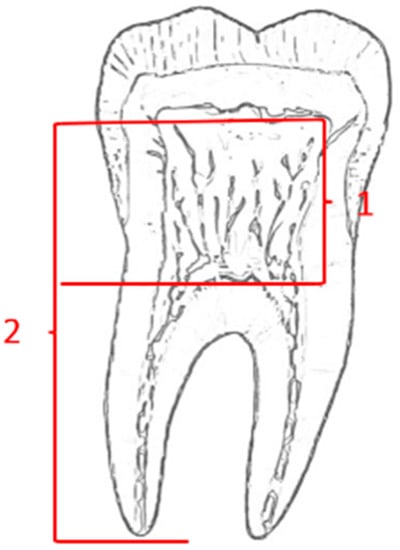
Figure 1.
Taurodontism variables by Shifman and Chanannel 1978.
2.3. Statistical Evaluation
A comparison between male and female prevalence and the location of the tooth was conducted using Chi-Square test. The significance level was determined as p < 0.05.
2.4. Ethical Approval
This article does not contain any studies with human participants or animals that were performed by any of the authors.
3. Results
The study comprised of 624 patients—330 males (52.8%) and 294 females (47.1%) with no statistical significance in an age range of 9 to 29 years. A total of 2849 permanent first and second molars were examined: 1106 lower first molars, 849 upper first molars, 655 lower second molars, and 239 upper second molars. Taurodontism was found in 329 molars (11.5% of examined teeth) among 209 patients, which consists of 33.6% of all patients—149 males (48%) and 160 females (52%) who had at least one taurodont tooth. The prevalence of taurodont molars was consistently higher in the teeth of females as demonstrated in Table 1.

Table 1.
The effect of gender on the prevalence of taurodont molars.
In general, 43% of taurodont teeth were in the mandible and 57% were in the maxilla with statistical significance (p = 0.001). Hypotaurodontism was found (Table 2) in 309 molars (10.8%), mesotaurodontism in 15 molars (0.5%), and 5 molars were found with hypertaurodontism (0.2%).

Table 2.
The distribution of taurodontism in right and left side.
Hypotaurodontism was the most common shape detected and comprised of 94.3% of the whole taurodont molars (the rest is 4.6% and 1.5% accordingly). Figure 2 describes the ratio of taurodont teeth in each location according to the quadrant of the mouth. The prevalence of taurodontism was the highest on the upper left side (p = 0.001) and the lowest on the lower left side. The distribution of hypotaurodontism in the upper left second molars was more than three times greater than in the left lower second molars (see Figure 3) and two times more prevalent in the upper right molars in comparison to lower right molars.
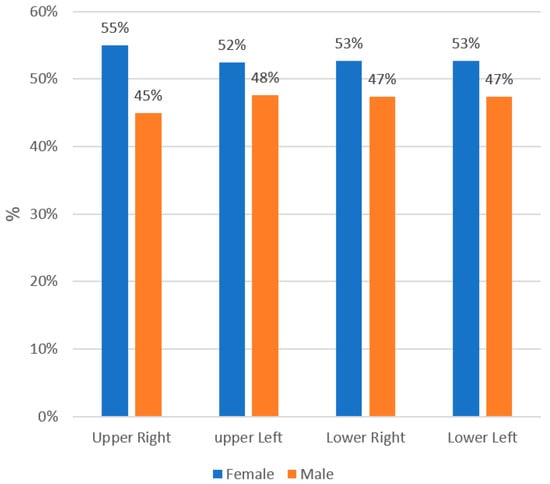
Figure 2.
The effect of gender on the prevalence of taurodont molars.
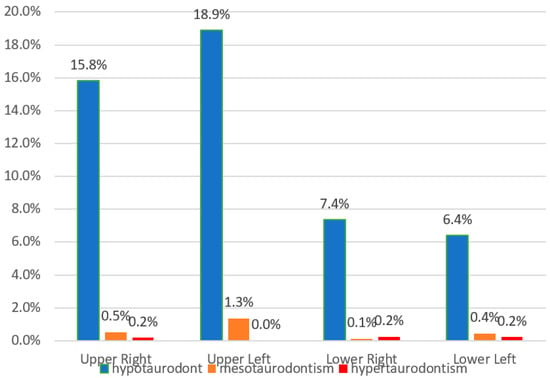
Figure 3.
The distribution of hypotaurodont, mesotaurodont, and hypertaurodont teeth in the four quadrants of the mouth.
Prevalence of hypotaurodontism (the least severe type) among every tooth is presented in Table 3. Hypotaurodontism had the highest occurrence, 23.6% in the upper right second molar, 19.4% in the upper left first molar, and 17.2% in the upper left second molar. Mesotaurodontism, which demonstrates lower occurrence compared to hypotaurodontism, occurs with the highest occurrence of 5.2% in upper left second molars.

Table 3.
The frequency of hypotaurodontism, mesotaurodontism, and hypertaurodontism in every molar.
4. Discussion
Taurodontism, which is defined as the enlargement of the pulp cavity of a molar tooth at the expense of root length [25], was investigated amongst Israelis in one medical center. The overall prevalence of taurodontism was 11.5% of all the molars examined and 33.6% of all patients. The reported prevalence of taurodont molars in the literature ranges widely. Lower prevalence of taurodontism in normal communities were reported in Germans (2%) [26], earlier (1978) in Israelis (6%) [9], moderately higher in Turks (11%) [27] and Iranians (23%) [7], with the highest reported in Brazilian (43%) [28] and Chinese (44%) [29] communities. These differences in prevalence rates reported in the literature is attributed not only to racial and ethnic differences, but also to the studies’ methodology [7]. The criteria for the diagnosis of taurodontism in some studies are not comparable, as subjective diagnosis is combined with an objective analysis of the cases presented. Moreover, the wide range in prevalence in some reports was discovered using the same Shifman and Chanannel’s methodology modified for panoramic radiographs, as performed in the current study, can be ascribed to a focus on mandibular molars, [29] as well as exclusion of third molars or inclusion of premolars [10]. The differences in the frequency of taurodontism in the Israeli population between current research and the 1978 research [9] may be due to several factors. First-possible differences may be derived from the differing methods of measurements of x-ray radiographs used. Originally, a full mouth periapical and posterior bitewing radiographs were used compared to panoramic radiographs in the current study. The age group assessed by Shifman and Chanannel (1978) was between 20–30 years of age. By contrast, this study included patients between the ages 9 and 29. Including 9 years of age and above may theoretically have led to the undervaluation of the Variable 2 (T2), which is the distance between the lowest point of the roof of the pulp chamber to the apex of the longest root, due to incomplete root development in multiple molars, which is characteristic of that cohort [14,22]. However, it should be noted that these abovementioned uncompleted roots were excluded in the current study, and all the data presented relates to completed roots only. Therefore, the age differences of these two Israeli studies are not considered a contributing or causative variable. A second factor is the inclusion of the third molars (in the 1978 research) with a very low frequency of taurodontism can lead to different understandings and findings. In general, some of the studies reporting taurodontism reported only meso- and hypertaurodontism, ignoring hypotaurodontism. [22] Finally, the variability of sample size differs. The smaller the sample size, the more unrealistic the prevalence rate. Controversy exists regarding the role of gender in the occurrence of taurodontism [7]. In the current study, taurodontism was more prevalent in females (52% vs. 48%), but no statistical significance was detected. Literature demonstrates controversy on this issue. This higher prevalence in females is somehow expected since taurodontism may be related to the X chromosome [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] and corresponds to a study carried out in China (56% in females in comparison to 44% in males) [31]. However, others have reported an equal distribution between males and females [7,25,28,32,33]. In the present study, the teeth most affected by taurodontism were the maxillary molars (57% vs. 43%). This finding is in accordance with previous studies [7,34], in which taurodont teeth were significantly more common in the maxilla than in the mandible. However, these results differ from those reported by other authors who found higher frequencies of this anomaly in lower molars [28,32]. The prevalence of taurodontism was almost equal without any statistically significant in the right and left side of the jaws (p > 0.05). Specifically, the second right maxillary molar was the most affected tooth, followed by the second left maxillary molar, the first left maxillary molar, and the first right maxillary molar. Findings coincide with the geographically universal tendency for taurodontism to increase as one proceeds distally along the molar row [25]. The exclusion of third molars can dramatically influence the overall reporting of taurodontism, for three main reasons—the low prevalence of third molars in the population, the incompletion of root formation. In addition, the third reason is biased panoramic views of the geometric distortion of tilting of mandibular third molars with respect to second molars [35]. These factors could result in either an underreporting or, in some cases, an overreporting of taurodontism [28,29]. Hypotaurodontism, the least severe type of taurodontism, was by far the most common morphology of taurodontism observed with a prevalence of 94%. The dominance of the hypotaurodontism is in accordance with and is even higher than the findings of Weckwerth’s study [28] of hypotaurodontism in 84.1% of taurodont teeth, mesotaurodontism in 10.1%, and hypertaurodontism in 5.8%.
Clinical Considerations
This highly frequent anomaly is of interest to numerous clinical, basic science, and public health fields [36]. The abnormal root canals in taurodont teeth is both in terms of shape and the number of encounters with complete filling of the root canal system [16]. The complex anatomy of the roots poses a challenge for a complete filling of the root canal system [7] (Figure 4). Thus, vital pulpotomy may be a suitable alternative to pulpectomy in severe cases of hypertaurodont teeth [5], a solution to be seriously considered. In the deciduous dentition, when pulp has a large extent, pulpotomy itself is not easy to perform. The extraction of a taurodont tooth is usually complicated because of a shift in the furcation area down to the apical third with the roots turning shorter and thinner. An apically placed furcation in the alveolar bone is the apparent preferred periodontal approach [7]. However, this specific different morphology may well affect the gingival line, relative to the neighboring normal teeth. Such an irregular gingival line may create difficulties in maintaining oral hygiene and cause gingival inflammation and a risk for periodontal breakdown, which may be a challenge to solve. The taurodont teeth occupy a smaller space in the alveolar bone, which may affect their stability compared to a normal tooth following their reduced ability to withstand normal occlusal wear forces [14,36]. This raises a question regarding their ability to maintain a functional passive eruption as compensation [37]. Stability should be considered when such a tooth is used as an abutment for either prosthetic or orthodontic purposes [7,16]. During orthodontic treatment, special attention must be given to the orthodontic force application due to the reduced alveolar bone support and risk factors for induced inflammatory root resorption of the shortened roots [38]. An alternative treatment of clear aligners is a viable option over the fixed appliances based on their effectiveness in shortening treatment duration [39] combined with superior periodontal health [40].
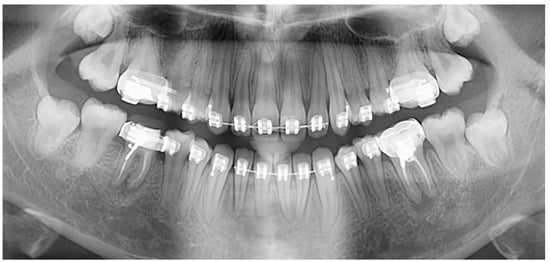
Figure 4.
Clinical challenges that taurodont teeth posed for the general practitioner.
The enlarged pulp chamber increases the chance of pulp exposure due to decay or during tooth preparation. The apically positioned canal orifices and the complexity of root canal anatomy and accessibility eliminates their feasibility as a source for core retention through a post [41]. As mentioned, a deeply located furcation in the bone is considered a protective mechanism against periodontal problems, [23] but the absence of the cervical constriction poses some difficulties for preparing a tooth for a crown. The first problem is the amount of tooth material that needs to be removed in these robust and square teeth, in an attempt to avoid an over-contoured crown in the gingival interface, which might block the imperative embrasure space and cause inflammation and home oral hygiene. A second aspect is the relative long crown and short root substance in a taurodont tooth, implicating a variation in the attachment apparatus with a possible decrease in alveolar stability, which jeopardizes the tooth’s prognosis. A third and separate consideration is the enlarged pulp chamber, prone to be exposed during an extensive tooth preparation [41]. A root canal, followed by a core build up and the preparation amount demanded, may cause a problem in the cervical part in an attempt to place a crown on sound tooth material [42,43]. An important matter to be discussed is whether a post is needed for the core build up, when on the one hand there is a large pulp chamber, but on the other, there are limited retention abilities of the shortened roots necessary to supply retention forces for the core build up (Figure 3).
Preparing a compromised root in length and form for a post is definitely in question, but due to the improved abilities today to adhesively cement composite core material to the dentinal walls, this post becomes redundant [44,45,46,47].
The relatively large sample size and the strict exclusion criteria conducted were among the strengths of this study and increased the reliability of our findings. The gold standard of assessing root length is a periapical radiograph. However, the assessment of taurodontism according Shifman and Chanannel’s methodology, which is modified for panoramic radiographs, corresponds to the American Dental Association philosophy of limiting radiation exposure in younger patients. Generally, a two-dimensional (2D) radiograph has a limitation when diagnosing dental anomalies and angulation errors, leading to distortions such as elongation or foreshortening of the image. This can ultimately skew linear measurements in 2D radiography and affect the visualization of important structures that may impact measurement accuracy [14,21].
5. Conclusions
Taurodontism is a morpho-anatomical change in the shape of a tooth, which involves enlargement of the body of the tooth and shortening of the roots. This highly prevalent phenomenon, predominant more in upper dentition that occurs more in second molars relative to first molars, requires meticulous attention both for the clinical and radiological diagnosis. Clinicians need to be more aware of the various and different dental treatment modalities that can be chosen and implemented according to the diagnosis.
Author Contributions
Conceptualization, investigation, methodology, writing—original draft, and writing-review and editing, S.E.; investigation, methodology, resources, and writing—original draft preparation I.H.Y., formal Analysis, O.C.; investigation and writing—original draft, A.S.; conceptualization, methodology, and writing—review and editing, U.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The research is exempt from IRB approval to ICH-GCP.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dipali, S.; Vikram, G.; Janardan, G.; Devendra, E. Prevalence of taurodontism among the patients visiting a dental teaching hospital in Pune, India. J. Indian Assoc. Public Health Dent. 2015, 13, 4. [Google Scholar]
- Daito, M.; Hieda, T. Taurodont teeth in primary dentition. Jpn. J. Pedodont. 1971, 9, 94–106. [Google Scholar]
- Zilberman, U.; Skinner, M.; Smith, P. Tooth components of mandibular deciduous molars of Homo sapiens and Homo sapiens neanderthalensis: A radiographic study. Am. J. Phys. Anthrop. 1992, 87, 255–262. [Google Scholar] [CrossRef]
- Zilberman, U.; Smith, P. A comparison of tooth structure in Neanderthals and early Homo sapiens: A radiographic study. J. Anat. 1992, 180, 387–393. [Google Scholar]
- Jafarzadeh, H.; Azarpazhooh, A.; Mayhall, J.T. Taurodontism: A review of the condition and endodontic treatment challenges. Int. Endod. J. 2008, 41, 375–388. [Google Scholar] [CrossRef]
- Benazzi, S.; Nguyen, H.; Kullmer, O.; Hublin, J. Exploring the biomechanics of taurodontism. J. Anat. 2015, 226, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Jamshidi, D.; Tofangchiha, M.; Jafari, N.; Mohammadpour, M.; Nouri, B.; Hosseinzadehe, K. Prevalence of Taurodont Molars in a Selected Iranian Adult Population. Iran Endod. J. 2017, 12, 282–287. [Google Scholar] [PubMed]
- Cichon, J.; Pack, R. Taurodontism: Review of literature and report of case. J. Am. Dent. Assoc. 1985, 111, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Shifman, A.; Chanannel, I. Prevalence of taurodontism found in radiographic dental examination of 1200 young adult Israeli patients. Community Dent. Oral Epidemiol. 1978, 6, 200–203. [Google Scholar] [CrossRef]
- Haskova, J.; Gill, D.; Figueiredo, J.; Tredwin, C.; Naini, F. Taurodontism—A Review. Dent. Update 2009, 36, 235–236, 239–240, 243. [Google Scholar] [CrossRef] [PubMed]
- Alvesalo, L.; Varrela, J. Taurodontism and the presence of an extra Y chromosome: Study of 47, XYY males and analytical review. Hum. Biol. 1991, 63, 31–38. [Google Scholar] [PubMed]
- Varrela, J.; Alvesalo, L. Taurodontism in 47, XXY males: An effect of the extra X chromosome on root development. J. Dent. Res. 1988, 67, 501–502. [Google Scholar] [CrossRef]
- Varrela, J.; Alvesalo, L. Taurodontism in females with extra X chromosomes. J. Craniofac. Genet. Dev. Biol. 1989, 9, 129–133. [Google Scholar]
- MacDonald, D. Taurodontism. Oral Radiol. 2020, 36, 129–132. [Google Scholar] [CrossRef]
- Jaspers, M. Taurodontism in the Down syndrome. Oral Surg. Oral Med. Oral Pathol. 1981, 51, 632–636. [Google Scholar] [CrossRef]
- Dineshshankar, J.; Sivakumar, M.; Balasubramanium, A.; Kesavan, G.; Karthikeyan, M.; Prasad, V. Taurodontism. J. Pharm. Bioallied. Sci. 2014, 6 (Suppl. 1), S13–S15. [Google Scholar] [CrossRef]
- Langland, O.E.; Langlais, R.P.; McDavid, W.D.; DelBalso, A.M. Panoramic Radiography, 2nd ed.; Lea and Febiger: Philadelphia, PA, USA, 1989; pp. 52–57. [Google Scholar]
- Tulensalo, T.; Ranta, R.; Kataja, M. Reliability in estimating taurodontism of permanent molars from orthopantomograms. Community Dent. Oral Epidemiol. 1989, 17, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, H.; Duarte, C.; Al Baloushi, W. Prevalence of Dental Anomalies in Patients from a Teaching Dental Hospital in the UAE. Int. J. Orofac. Res. 2018, 3, 32–36. [Google Scholar]
- Sarr, M.; Toure, B.; Kane, A.; Fall, F.; Wone, M. Taurodontism and the pyramidal tooth at the level of the molar. Prevalence in the Senegalese population 15 to 19 years of age. Odontostomatol. Trop. 2000, 23, 31–34. [Google Scholar] [PubMed]
- MacDonald-Jankowski, D. Multiple dental developmental anomalies. Dentomaxillofac. Radiol. 1991, 20, 166–168. [Google Scholar] [CrossRef]
- Samji, Z.H. Investigating the Prevalence of Taurodontism in an Adolescent Population Using Dental Panoramic Radiographs. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Faculty of Graduate and Postdoctoral Studies (Craniofacial Science), Vancouver, The University of British Columbia, 2021. Available online: https://open.library.ubc.ca/soa/cIRcle/collections/ubctheses/24/items/1.0400885 (accessed on 1 July 2021).
- Shifman, A.; Buchner, A. Taurodontism. Report of sixteen cases in Israel. Oral Surg. Oral Med. Oral Pathol. 1976, 41, 400–405. [Google Scholar] [CrossRef]
- Shaw, J.C. Taurodont teeth in South African races. J. Anat. 1928, 62, 476–498. [Google Scholar]
- Constant, D.; Grine, F.E.A. Review of taurodontism with new data on indigenous southern African populations. Arch. Oral Biol. 2001, 46, 1021–1029. [Google Scholar] [CrossRef]
- Bürklein, S.; Breuer, D.; Schäfer, E. Prevalence of taurodont and pyramidal molars in a German population. J. Endod. 2011, 37, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Bilge, N.; Yeşiltepe, S.; Törenek Ağırman, K.; Çağlayan, F.; Bilge, O. Investigation of prevalence of dental anomalies by using digital panoramic radiographs. Folia Morphol. 2018, 77, 323–328. [Google Scholar] [CrossRef]
- Weckwerth, G.M.; Santos, C.F.; Brozoski, D.T.; Centurion, B.S.; Pagin, O.; Lauris, J.R.P.; Carvalho, I.M.M.; Neves, L.T. Taurodontism, Root Dilaceration, and Tooth Transposition: A Radiographic Study of a Population with Nonsyndromic Cleft Lip and/o Palate. Cleft Palate Craniofac. J. 2016, 53, 404–412. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D. Taurodontism. In Japanese Society for Oral and Maxillofacial Radiology; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar]
- Varrela, J.; Alvesalo, L.; Mayhall, J. Taurodontism in 45,X females. J. Dent. Res. 1990, 69, 494–495. [Google Scholar] [CrossRef]
- MacDonald-Jankowski, D.S.; Li, T.T. Taurodontism in a young adult Chinese population. Dentomaxillofac. Radiol. 1993, 22, 140–144. [Google Scholar] [CrossRef]
- Gupta, S.; Saxena, P. Prevalence of taurodontism and its association with various oral conditions in an Indian population. Oral Health Prev. Dent. 2013, 11, 155–160. [Google Scholar]
- Ruprecht, A.; Batniji, S.; El-Neweihi, E. The incidence of taurodontism in dental patients. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 743–747. [Google Scholar] [CrossRef]
- Bronoosh, P.; Haghnegahdar, A.; Dehbozorgi, M. Prevalence of Taurodontism in Premolars and Molars in the South of Iran. J. Dent. Res. Dent. Clin. Dent. Prospect. 2012, 6, 21–24. [Google Scholar]
- Lupi, S.M.; Galinetto, P.; Cislaghi, M.; Rodriguez y Baena, A.; Scribante, A.; Rodriguez y Baena, R. Geometric distortion of panoramic reconstruction in third molar tilting assessments: A comprehensive evaluation. Dentomaxillofac. Radiol. 2018, 47, 20170467. [Google Scholar] [CrossRef]
- Yassin, S. Prevalence and distribution of selected dental anomalies among Saudi children in Abha, Saudi Arabia. J. Clin. Exp. Dent. 2016, 8, e485–e490. [Google Scholar] [CrossRef]
- Ainamo, A.; Ainamo, J. The dentition is intended to last a lifetime. Int. Dent. J. 1984, 34, 87–92. [Google Scholar]
- Kjar, I. Morphological characteristics of dentitions developing excessive root resorption during orthodontic treatment. Eur. J. Orthod. 1995, 16, 25–34. [Google Scholar] [CrossRef]
- Ke, Y.; Zhu, Y.; Zhu, M. A comparison of treatment effectiveness between clear aligner and fixed appliance therapies. BMC Oral Health 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Levrini, L.; Mangano, A.; Montanari, P.; Margherini, S.; Caprioglio, A.; Abbate, G. Periodontal health status in patients treated with the Invisalign® system and fixed orthodontic appliances: A 3 months clinical and microbiological evaluation. Eur. J. Dent. 2015, 9, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Tsesis, I.; Shifman, A.; Kaufman, A. Taurodontism: An endodontic challenge. Report of a case. J. Endod. 2003, 29, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature, Part II (Evaluation of fatigue behavior, interfaces, and in vivo studies). Quintessence Int. 2008, 39, 1197–1212. [Google Scholar]
- Zarow, M.; Ramírez-Sebastià, A.; Paolone, G.; de Ribot Porta, J.; Mora, J.; Espona, J.; Durán-Sindreu, F.; Roig, M. A new classification system for the restoration of root filled teeth. Int. Endod. J. 2018, 51, 318–334. [Google Scholar] [CrossRef] [Green Version]
- Slutzky-Goldberg, I.; Slutzky, H.; Gorfil, C.; Smidt, A. Restoration of Endodontically Treated Teeth Review and Treatment Recommendations. Int. J. Dent. 2009, 2009, 150251. [Google Scholar] [CrossRef] [Green Version]
- Magne, P.; Carvalho, A.O.; Bruzi, G.; Anderson, R.E.; Maia, H.P.; Giannini, M. Influence of no-ferrule and no-post buildup design on the fatigue resistance of endodontically treated molars restored with resin nanoceramic CAD/CAM crowns. Oper. Dent. 2014, 39, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Gresnigt, M.M.M.; Özcan, M.; van den Houten, M.L.A.; Schipper, L.; Cune, M.S. Fracture strength, failure type and Weibull characteristics of lithium disilicate and multiphase resin composite endocrowns under axial and lateral forces. Dent. Mater. 2016, 32, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Dartora, N.R.; Moris, I.C.M.; Poole, S.F.; Bacchi, A.; Sousa-Neto, M.D.; Silva-Sousa, Y.T.; Gomes, E.A. Mechanical behavior of endocrowns fabricated with different CAD-CAM ceramic systems. J. Prosthet. Dent. 2021, 125, 117–125. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).