Operational Stability Analysis of Blue Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes Using the Capacitance-Voltage Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Fabrication and Materials
2.2. Measurement
3. Results and Discussion
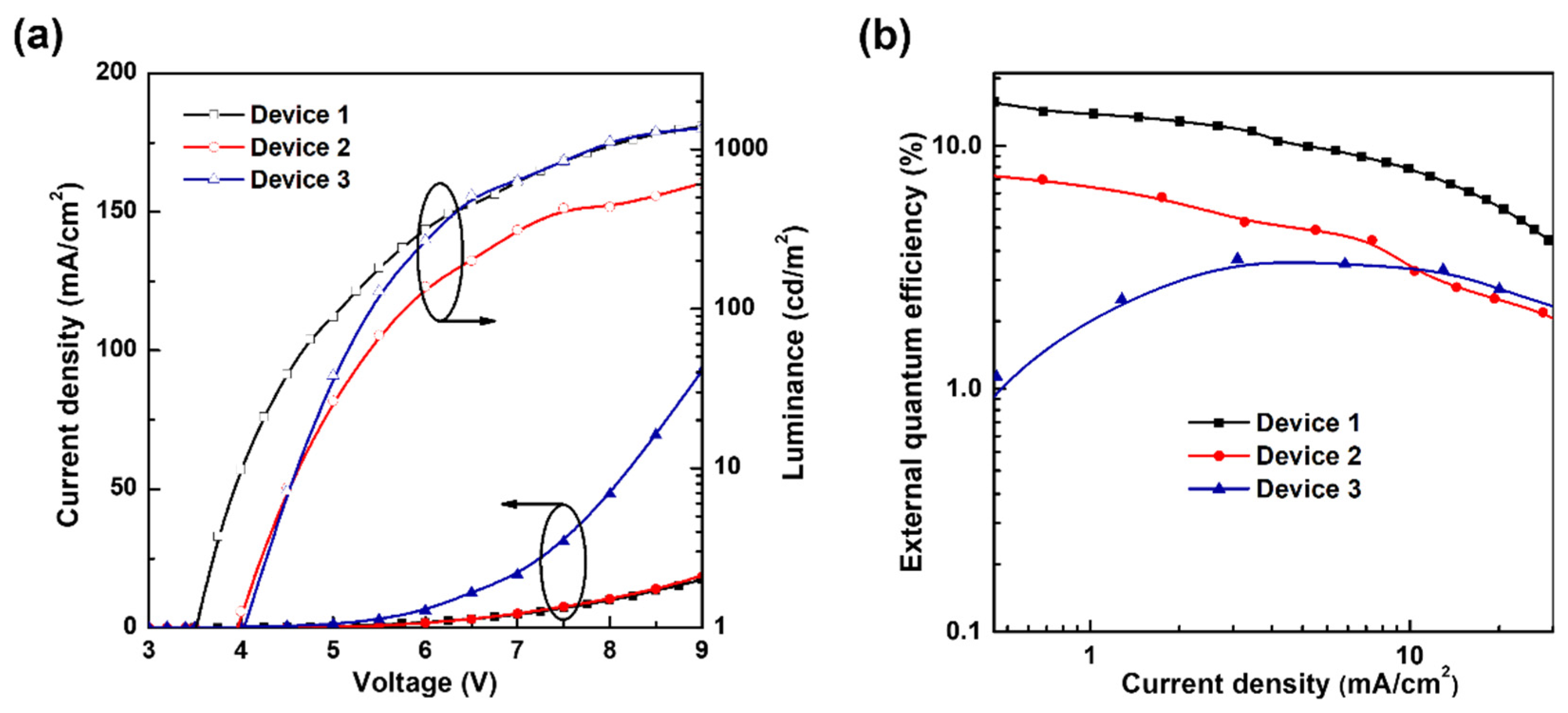
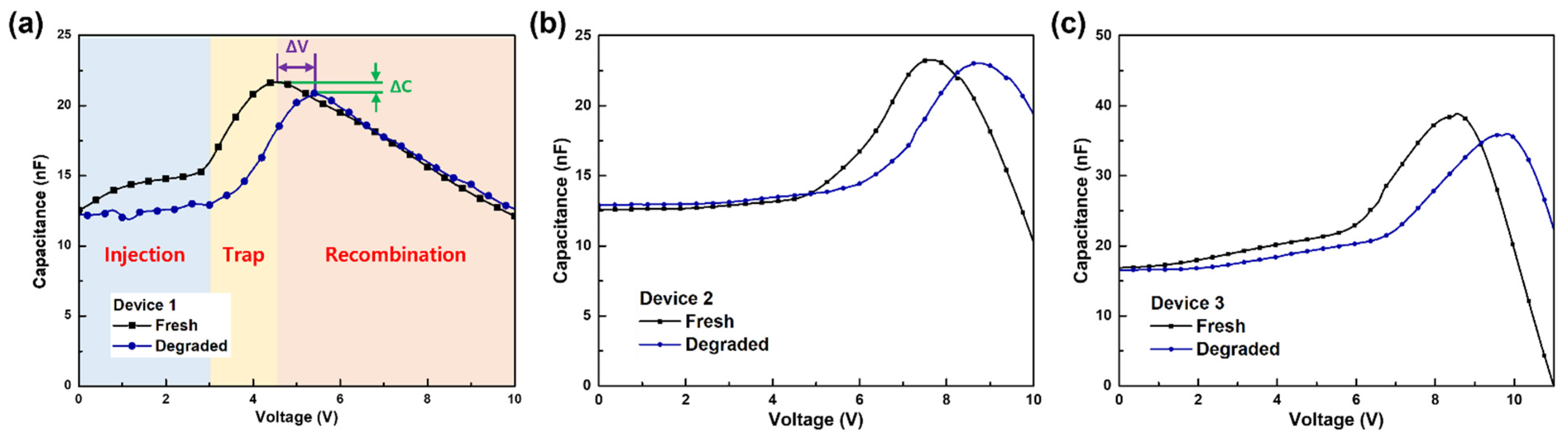
3.1. Analysis of the Degradation of Blue TADF OLEDs by Different HTL Structures
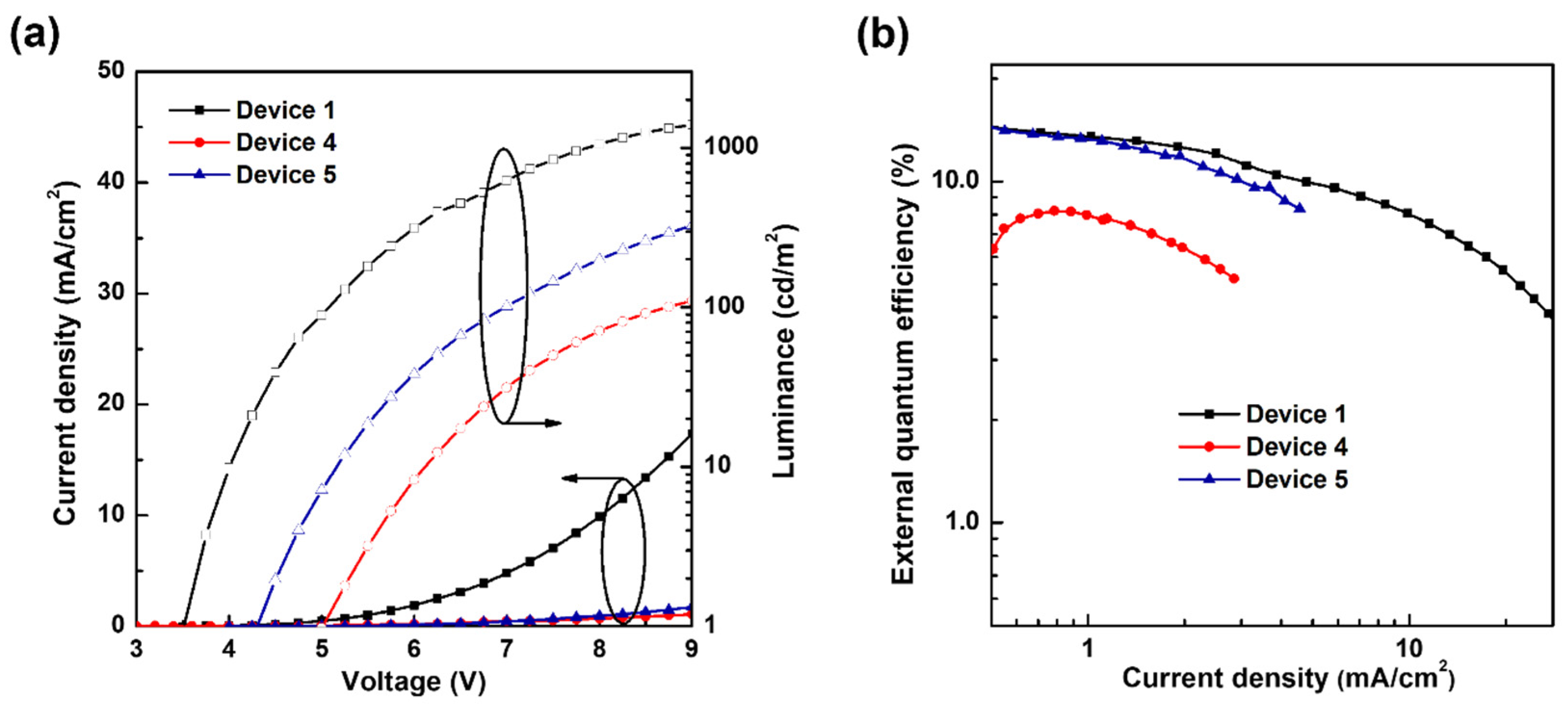
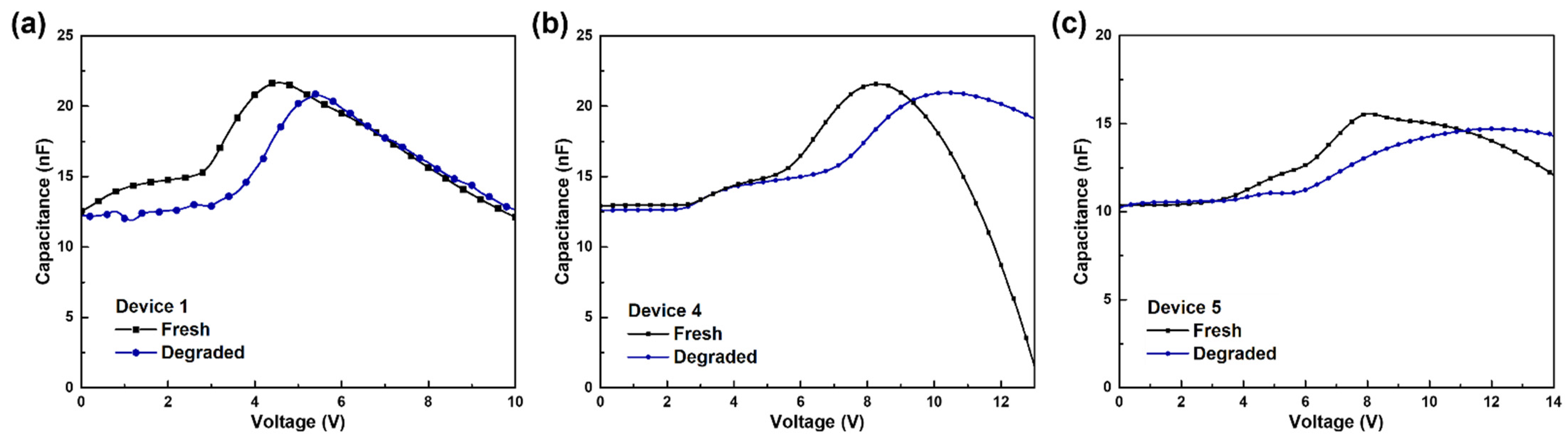
3.2. Analysis of the Degradation of Blue TADF OLEDs by Different ExBL Structures
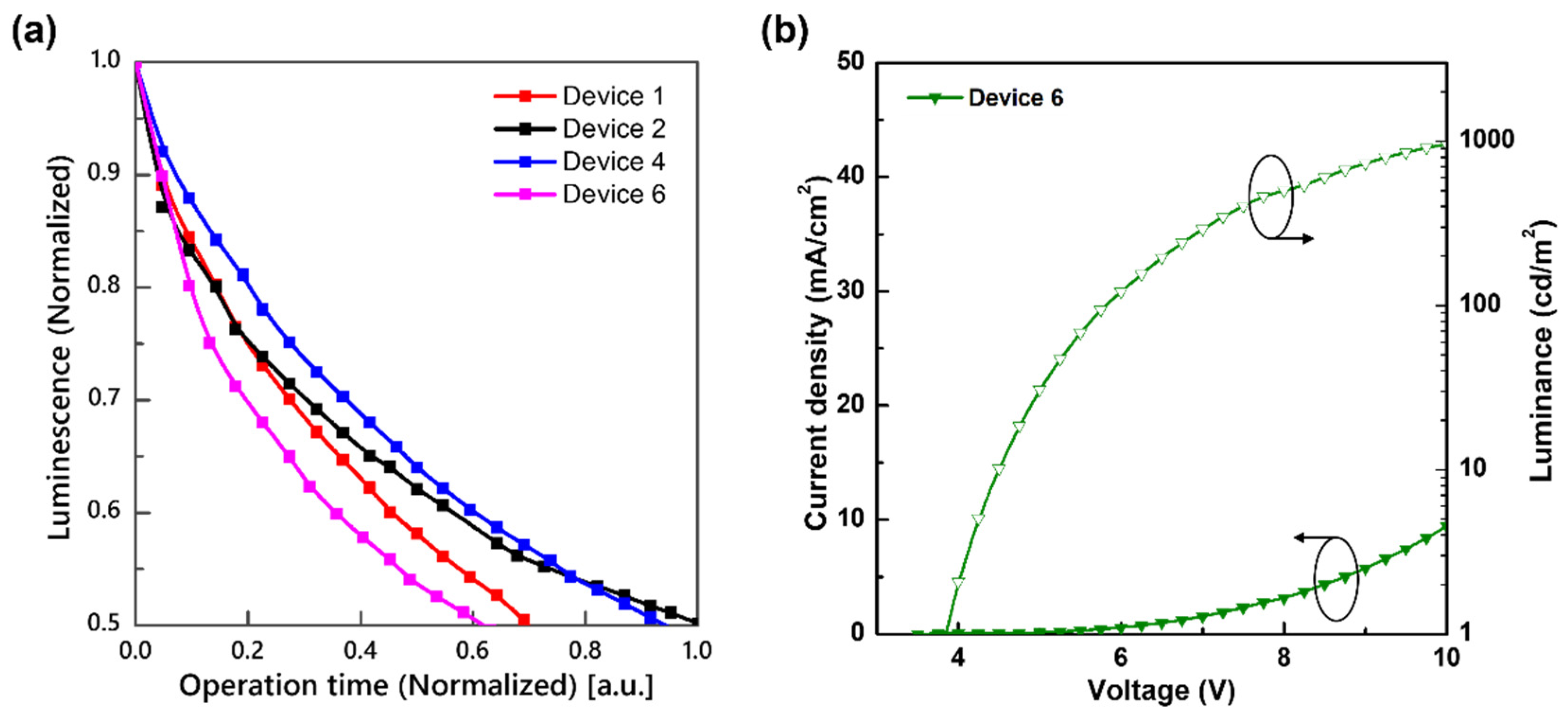
3.3. Operational Stability of Blue TADF OLEDs by Different Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Lim, H.; Cheon, H.; Woo, S.; Kwon, S.; Kim, Y.; Kim, J. Highly Efficient Deep-Blue OLEDs using a TADF Emitter with a Narrow Emission Spectrum and High Horizontal Emitting Dipole Ratio. Adv. Mater. 2020, 32, 2004083. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, J.; Lee, C.; Lee, J. Ideal Molecular Design of Blue Thermally Activated Delayed Fluorescent Emitter for High Efficiency, Small Singlet–Triplet Energy Splitting, Low Efficiency Roll-Off, and Long Lifetime. ACS Appl. Mater. Interfaces 2016, 8, 23190–23196. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Wang, K.; Zhang, M.; Zheng, C.; Sun, D.; Dai, G.; Fan, X.; Wang, D.; Liu, W.; et al. Thermally Activated Delayed Fluorescence Carbonyl Derivatives for Organic Light-Emitting Diodes with Extremely Narrow Full Width at Half-Maximum. ACS Appl. Mater. Interfaces 2019, 11, 13472–13480. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Dirican, M.; Fang, D.; Tian, Y.; Yan, C.; Xie, J.; Jia, D.; Liu, H.; Wang, J.; et al. Highly Transparent and Colorless Nanocellulose/Polyimide Substrates with Enhanced Thermal and Mechanical Properties for Flexible OLED Displays. Adv. Mater. Interfaces 2020, 7, 2000928. [Google Scholar] [CrossRef]
- Nakanotani, H.; Tsuchiya, Y.; Adachi, C. Thermally-activated Delayed Fluorescence for Light-emitting Devices. Chem. Lett. 2021, 50, 938–948. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Karunathilaka, B.; Balijapalli, U.; Senevirathne, C.; Yoshida, S.; Esaki, Y.; Goushi, K.; Matsushima, T.; Sandanayaka, A.; Adachi, C. Suppression of external quantum efficiency rolloff in organic light emitting diodes by scavenging triplet excitons. Nat. Commun. 2020, 11, 4926. [Google Scholar] [CrossRef]
- Nguyen, T.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. The Role of Reverse Intersystem Crossing Using a TADF-Type Acceptor Molecule on the Device Stability of Exciplex-Based Organic Light-Emitting Diodes. Adv. Mater. 2020, 32, 1906614. [Google Scholar] [CrossRef]
- Hall, D.; Suresh, S.; dos Santos, P.; Duda, E.; Bagnich, S.; Pershin, A.; Rajamalli, P.; Cordes, D.; Slawin, A.; Beljonne, D.; et al. Improving Processability and Efficiency of Resonant TADF Emitters: A Design Strategy. Adv. Opt. Mater. 2019, 8, 1901627. [Google Scholar] [CrossRef]
- Zhang, X.; Fuentes-Hernandez, C.; Zhang, Y.; Cooper, M.; Barlow, S.; Marder, S.; Kippelen, B. High performance blue-emitting organic light-emitting diodes from thermally activated delayed fluorescence: A guest/host ratio study. J. Appl. Phys. 2018, 124, 055501. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, D.; Duan, L. A perspective on blue TADF materials based on carbazole-benzonitrile derivatives for efficient and stable OLEDs. Appl. Phys. Lett. 2020, 116, 120503. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, M.; Zhang, Y.; Zhang, D.; Duan, L. Sterically shielded blue thermally activated delayed fluorescence emitters with improved efficiency and stability. Mater. Horiz. 2016, 3, 145–151. [Google Scholar] [CrossRef]
- Kim, J.; Park, I.; Chan, C.; Tanaka, M.; Tsuchiya, Y.; Nakanotani, H.; Adachi, C. Nanosecond-time-scale delayed fluorescence molecule for deep-blue OLEDs with small efficiency rolloff. Nat. Commun. 2020, 11, 1765. [Google Scholar] [CrossRef]
- Kumar, M.; Pereira, L. Effect of the Host on Deep-Blue Organic Light-Emitting Diodes Based on a TADF Emitter for Roll-Off Suppressing. Nanomaterials 2019, 9, 1307. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, K.; Kim, J.; Ihn, S.; Chung, Y.; Kim, J.; Lee, H.; Kim, S.; Choi, H.; Lee, J. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photonics 2021, 15, 208–215. [Google Scholar] [CrossRef]
- Wang, H.; Xie, L.; Peng, Q.; Meng, L.; Wang, Y.; Yi, Y.; Wang, P. Novel Thermally Activated Delayed Fluorescence Materials-Thioxanthone Derivatives and Their Applications for Highly Efficient OLEDs. Adv. Mater. 2014, 26, 5198–5204. [Google Scholar] [CrossRef]
- Peng, X.; Feng, H.; Zhang, J.; Liu, S.; Zhang, L.; Xie, W. Efficient trilayer phosphorescent organic light-emitting devices without electrode modification layer and its working mechanism. Nanoscale Res. Lett. 2018, 13, 310. [Google Scholar] [CrossRef]
- Kwak, K.; Cho, K.; Kim, S. Analysis of thermal degradation of organic light-emitting diodes with infrared imaging and impedance spectroscopy. Opt. Express 2013, 21, 29558. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, X.; So, F. Hole injection polymer effect on degradation of organic light-emitting diodes. Org. Electron. 2013, 14, 2518–2522. [Google Scholar] [CrossRef]
- Cao, L.; Klimes, K.; Ji, Y.; Fleetham, T.; Li, J. Efficient and stable organic light-emitting devices employing phosphorescent molecular aggregates. Nat. Photonics 2020, 15, 230–237. [Google Scholar] [CrossRef]
- Baldo, M.; Adachi, C.; Forrest, S. Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys. Rev. B 2000, 62, 10967–10977. [Google Scholar] [CrossRef]
- Halls, M.; Yoshidome, D.; Mustard, T.; Goldberg, A.; Kwak, H.; Gavartin, J. Atomic-scale simulation for the analysis, optimization and accelerated development of organic optoelectronic materials. J. Phys. Soc. Jpn. 2015, 54, 561–569. [Google Scholar]
- Tsai, M.; Lin, H.; Su, H.; Ke, T.; Wu, C.; Fang, F.; Liao, Y.; Wong, K.; Wu, C. Highly Efficient Organic Blue Electrophosphorescent Devices Based on 3,6-Bis(triphenylsilyl)carbazole as the Host Material. Adv. Mater. 2006, 18, 1216–1220. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, C.; Qin, J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.; Gan, L.; Cai, X.; Li, B.; Chen, D.; Su, S. Predicting Operational Stability for Organic Light-Emitting Diodes with Exciplex Cohosts. Adv. Sci. 2019, 6, 1802246. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Kang, S.; Kim, T. A Silane-Based Bipolar Host with High Triplet Energy for High Efficiency Deep-Blue Phosphorescent OLEDs with Improved Device Lifetime. Chem.–A Eur. J. 2020, 26, 7767–7773. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, M.; Bin, Z.; Zhang, Y.; Zhang, D.; Duan, L. Highly efficient blue thermally activated delayed fluorescent OLEDs with record-low driving voltages utilizing high triplet energy hosts with small singlet–triplet splittings. Chem. Sci. 2016, 7, 3355–3363. [Google Scholar] [CrossRef]
- Sohn, J.; Ko, D.; Lee, H.; Han, J.; Lee, S.; Lee, C. Degradation mechanism of blue thermally activated delayed fluorescent organic light-emitting diodes under electrical stress. Org. Electron. 2019, 70, 286–291. [Google Scholar] [CrossRef]
- Ihn, S.; Lee, N.; Jeon, S.; Sim, M.; Kang, H.; Jung, Y.; Huh, D.; Son, Y.; Lee, S.; Numata, M.; et al. An Alternative Host Material for Long-Lifespan Blue Organic Light-Emitting Diodes Using Thermally Activated Delayed Fluorescence. Adv. Sci. 2017, 4, 1600502. [Google Scholar] [CrossRef]
- Mohammed, S.; Taher, M.; Monzir, S.; Sofyan, A. Capacitance-voltage measurements of hetero-layer oleds treated by an electric field and thermal annealing. Int. J. Thin Film Sci. Technol. 2021, 10, 217–226. [Google Scholar]
- Zhang, C.; Xu, Z.; Wang, P.; Qin, Z.; Wageh, S.; Al-Ghamdi, A.; Zhao, S. Optical capacitance/conductance-voltage characteristics of stored charges in organic light-emitting diodes. Molecules 2020, 25, 2818. [Google Scholar] [CrossRef] [PubMed]
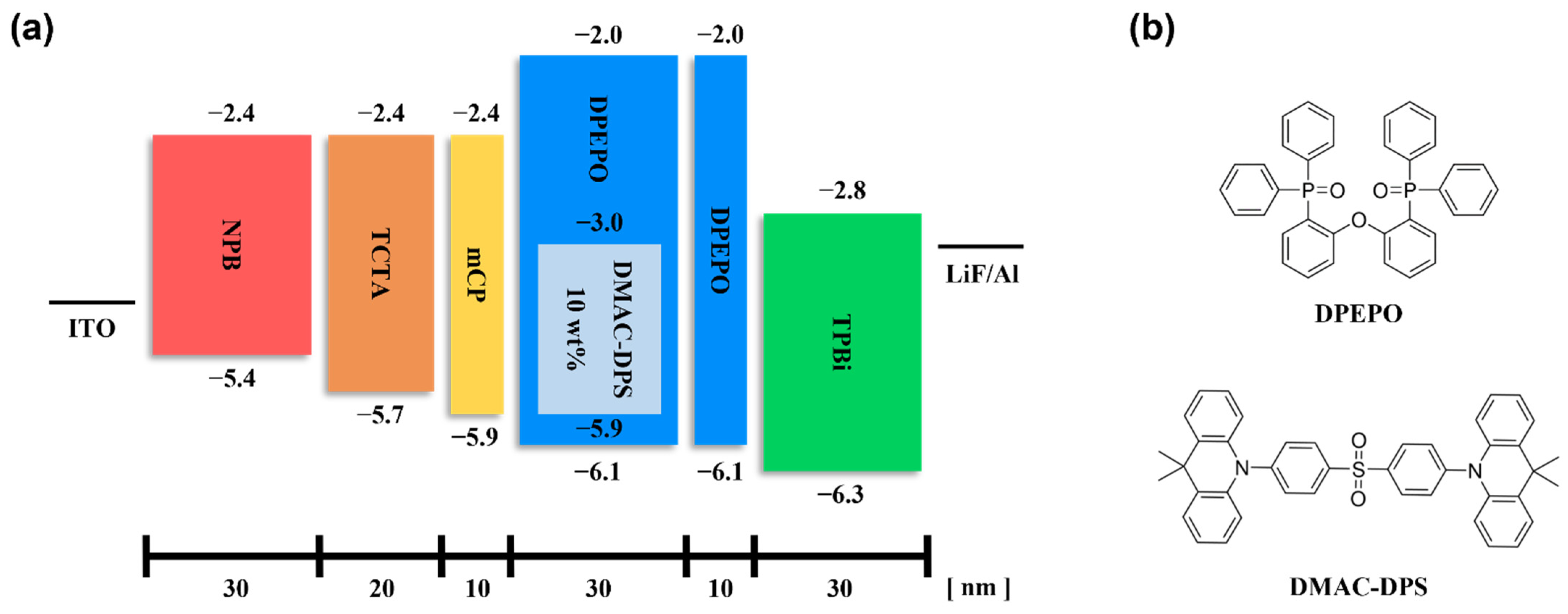
| Devices | HTL | HTL | HTL | EML | ExBL | ETL | EIL | Cathode |
|---|---|---|---|---|---|---|---|---|
| 30 nm | 20 nm | 10 nm | 30 nm | X nm | 30 nm | 2 nm | 80 nm | |
| 1 | NPB | TCTA | mCP | DPEPO: DMAC-DPS (10 wt%) | DPEPO (10) | TPBi | LiF | Al |
| 2 | TCTA | — | DPEPO (10) | |||||
| 3 | — | — | DPEPO (10) | |||||
| 4 | TCTA | mCP | — | |||||
| 5 | TCTA | mCP | DPEPO (40) |
| Turn-On Voltage (V) | Maximum External Quantum Efficiency (%) | Maximum Power Efficiency (lm/W) | |
|---|---|---|---|
| Device 1 | 3.5 | 17.7 | 17.7 |
| Device 2 | 3.9 | 7.7 | 6.1 |
| Device 3 | 4.0 | 3.4 | 2.6 |
| ΔV 1 | ΔC 2 | |
|---|---|---|
| Device 1 | 0.8 V | 3.7% |
| Device 2 | 1.1 V | 1.3% |
| Device 3 | 1.3 V | 7.4% |
| Average BDE 1 (eV) | Tg (°C) | ET (eV) | |
|---|---|---|---|
| mCP | 4.23 | 55 | 2.90 |
| TCTA | 5.24 | 151 | 2.76 |
| NPB | 5.23 | 95 | 2.29 |
| Turn-On Voltage (V) | Maximum External Quantum Efficiency (%) | Maximum Power Efficiency (lm/W) | |
|---|---|---|---|
| Device 1 | 3.5 | 17.7 | 17.7 |
| Device 4 | 5.0 | 8.2 | 4.0 |
| Device 5 | 4.2 | 14.4 | 10.0 |
| ΔV 1 | ΔC 2 | |
|---|---|---|
| Device 1 | 0.8 V | 3.7% |
| Device 4 | 2.1 V | 2.8% |
| Device 5 | 3.9 V | 5.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Park, S.-J.; Ju, B.-K. Operational Stability Analysis of Blue Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes Using the Capacitance-Voltage Method. Appl. Sci. 2022, 12, 13045. https://doi.org/10.3390/app122413045
Park J-Y, Park S-J, Ju B-K. Operational Stability Analysis of Blue Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes Using the Capacitance-Voltage Method. Applied Sciences. 2022; 12(24):13045. https://doi.org/10.3390/app122413045
Chicago/Turabian StylePark, Jun-Young, Soo-Jong Park, and Byeong-Kwon Ju. 2022. "Operational Stability Analysis of Blue Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes Using the Capacitance-Voltage Method" Applied Sciences 12, no. 24: 13045. https://doi.org/10.3390/app122413045
APA StylePark, J.-Y., Park, S.-J., & Ju, B.-K. (2022). Operational Stability Analysis of Blue Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes Using the Capacitance-Voltage Method. Applied Sciences, 12(24), 13045. https://doi.org/10.3390/app122413045






