3.1. Phytochemical Composition and Ascorbic Acid Content
Table 4 summarises the general phytochemical composition of the five noni juice brands, as measured using benchtop spectrophotometric methods. The Fijian noni juice showed both the highest TPC (1761 mg GAE L
−1) and antioxidant capacity (817 mg TE L
−1). The range of TPCs found here (1106–1761 mg GAE L
−1) were slightly higher than the TPC of commercial noni juice reported by Bramorski et al. [
14] (919 mg GAE L
−1), but a little lower than the 2100 mg GAE L
−1 reported by Yang et al. [
13] in freshly produced noni juice from Guam. The same authors found that TPC values reduced significantly with storage [
13], thus appearing to explain why the TPC values found in the present commercial samples were lower than those typically found in fresh juice. However, it is important to note that the ripeness of the noni fruits used to manufacture the juice can also influence their TPC and antioxidant capacity [
28].
There was a positive correlation between the TPC and antioxidant capacity; however, this correlation was not significant (r
3 = 0.81;
p > 0.05). However, previous research has reported a correlation between TPC and DPPH antioxidant capacity in this matrix [
13].
In the crude crystals, the highest TPC was seen in DHN, although there was no significant variation between the different brands. The crystals from CIN also showed a very high FRAP (6389 mg TE 100 g−1), although this brand had the lowest FRAP for the neat juice. In contrast to the results for the juice, the TPC and FRAP of the crude crystal extracts were not strongly correlated (r4 = 0.18).
There was a lower level of variation in the ascorbic acid content of the different noni juice brands (
Table 4). The FN and TON noni juice brands showed the highest ascorbic acid contents (20.1 and 19.7 mg L
−1, respectively), while DHN had the lowest ascorbic acid (12.2 mg L
−1). The ascorbic acid content was only reported on the label of one of the noni juice bottles (TON—16.67 mg L
−1), which was slightly lower than the experimentally determined value.
The U.S. Department of Agriculture (USDA) FoodData central has reported a vitamin C content of 30 mg/100 mL in the “Pure Noni” brand of Noni juice sourced from Florida [
29], which was significantly higher than the values quantified in this study. In a review, the ascorbic acid content in Noni fruit on a wet weight basis was reported to be in the range of 53.2–76.2 mg/100 g (approximately 5–8 mg L
−1) [
30]. Previous research has indicated that illumination during storage often affects the chemical properties and components of noni juice, including vitamin C. Although minimal exposure to light would have occurred during shipping or storage at the laboratory, the storage conditions post-manufacture are unknown for these samples.
3.2. AChE Inhibitory Activity
As shown in
Table 5, all of the noni juice brands showed relatively low activity against AChE, ranging from 9.6% inhibition for DHN to 30.2% inhibition for CIN. This indicates that, in contrast the results found by Jeyabalan et al. [
24], commercial samples of noni juice are unlikely to provide significant AChE inhibitory activity, particularly not at the level required to alleviate neurological conditions such as Alzeimer’s disease or dementia.
Given the low inhibitory values, dose–response experiments were not conducted on the samples.
3.3. Quantitative Phenolic Profiling by LC-MS
Targeted LC-MS profiling of the noni juice samples revealed the presence of 21 phenolic compounds putatively identified across all five noni juice brands. Most of these compounds (14) were common to all samples, while several were only found in one or two samples (e.g., neochlorogenic acid, cyanidin 3-glucoside, sinapic acid, ellagic acid, kaempferol).
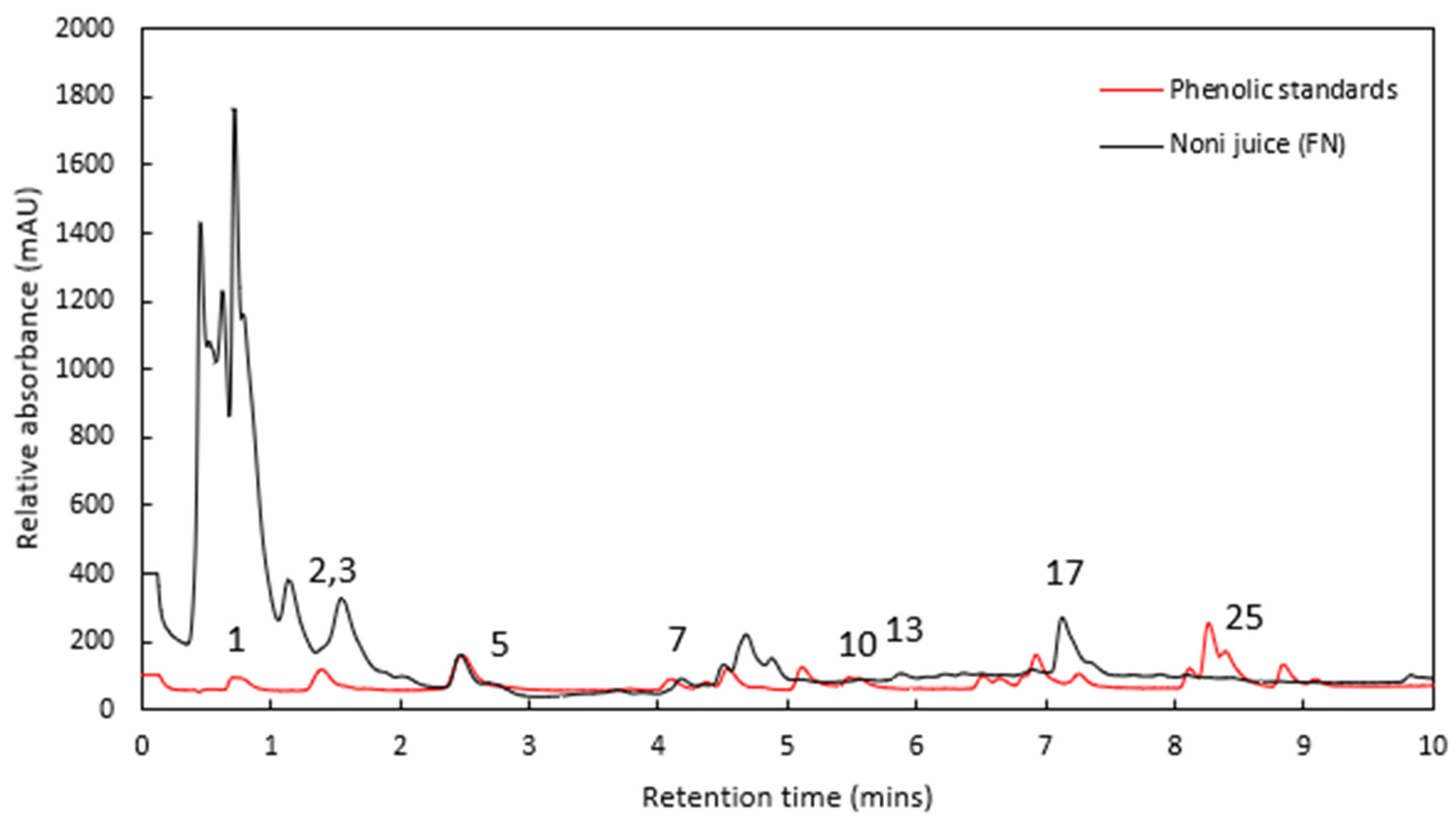
Figure 2 shows the chromatogram traces for one of the noni juice samples overlaid with the combined standards solution, at a wavelength of 254 nm.
The phenolic profiles varied markedly between the various brands. The most abundant compound in three of the brands (TON, DHN and LHN) was rutin (6.8–41.2 mg L
−1). In contrast, the CIN brand showed virtually no rutin (0.09 mg L
−1). Similar differences were observed between brands for most of the detected phenolic compounds (
Table 6). This agrees with the results of Yan et al. [
17], who observed a high level of variation in seven phenolic constituents between eight brands of noni juice produced in China, Taiwan and Fiji. Similarly, Kim et al. [
31] observed that the processing methods had a significant impact on the total phenolic content and bioactivity of noni juice samples. Consequently, it is likely that this high level of variation in composition between brands can be attributed to the different starting materials and processes used in their manufacture.
All six of the phenolic compounds reported by Yan et al. [
17] were also detected in this work (gentisic acid, caffeic acid,
p-coumaric acid, ferulic acid, sinapic acid and quercetin). Additional compounds reported here include five hydroxybenzoic acids (gallic, protocatechuic, 4-hydroxybenzoic, salicylic and vanillic acid), two hydroxycinnamic acids (chlorogenic and neochlorogenic acid), one dimerised polyphenol (ellagic acid) and five flavonoids (luteolin, rutin, quercetin-3-glucoside and kaempferol).
Five anthocyanins were also detected in the noni juice samples—cyanidin, cyanidin-3-glucoside, malvidin-3-glucoside, pelargonidin and delphinidin. Although there have been limited studies reporting individual anthocyanins from
M. citrifolia fruit, [
32] did detect a moderate concentration of total anthocyanins (16 mg/100 g) in this species. Furthermore, many brands of noni juice are reported to include grape and blueberry juice, in addition to noni fruit juice [
14]. Glycosides of all four anthocyanin types detected here (cyanidin, malvidin, pelargonidin and delphinidin) have been reported from grapes or blueberries [
33,
34]. Consequently, this may be the source of some of these anthocyanins, rather than the noni fruit itself.
3.4. Assessment of Antioxidant Activity of the Major Phenolic Compounds
Previous preliminary work has isolated the most antioxidant-active fractions of noni juice and found that these included phenol and flavonoid compounds [
35]. However, the exact identity of the most antioxidant-active compounds has not been described to date. Consequently, in order to identify the major phenolic compounds contributing to the high total phenolic content and antioxidant activity of the noni juice, the TPC and FRAP assays were performed on pure standards of the most abundant phenolic compounds identified in this study—rutin, gentisic acid and 4-hydroxybenzoic acid. These were chosen due to their high concentrations (i.e., >3 mg L
−1) across one or more noni juice brands.
These results (
Table 7) indicated that gentisic acid showed the highest total phenolic content and antioxidant activity out of the three major phenolic acids. This compound showed a total phenolic content approximately 10% higher than gallic acid and an antioxidant activity over 7 times higher than Trolox, a synthetic derivative of vitamin E. This agreed with previous research, which found that gentisic acid had the second-highest antioxidant activity out of a number of phenolic compounds tested using the DPPH assay [
36].
Interestingly, 4-hydroxybenzoic acid showed no antioxidant activity using the FRAP assay. This concurs with theoretical modelling that suggests that 4-hydroxybenzoic acid would have the lowest antioxidant activity out of a number of 4-hydroxybenzoic acid derivatives [
37].
Although the possibility of interactions or matrix effects was not considered in this study, gentisic acid showed the highest antioxidant activity, followed by rutin. Interestingly, the LHN sample contained only slightly less gentisic acid than rutin; this sample also showed the highest antioxidant capacity (
Table 4). In contrast, the rutin content of the DHN brand of noni juice was over 60 times higher than the gentisic acid concentration in this sample; it contained the second-lowest antioxidant capacity. Further investigations would be required to determine any possible matrix effects on the antioxidant activity.
3.5. Cytotoxic Activity
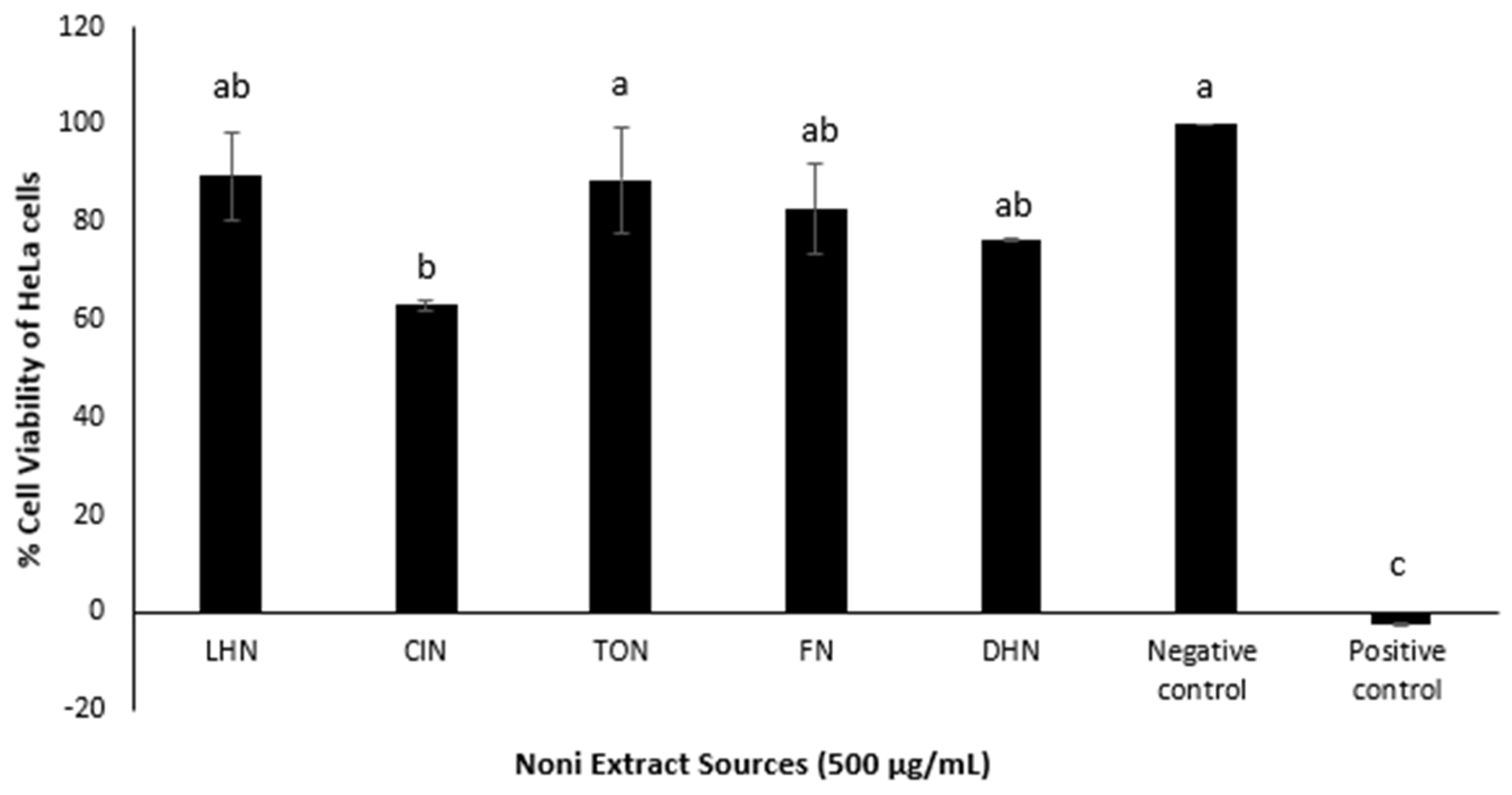
The various Noni juice crystals showed significantly different (
p < 0.05) activity against the HeLa cells (
Figure 3). The CIN extracts showed the highest potency (cell viability of 63 ± 1%), significantly different to the negative control, whereas the FN, LHN and DHN showed lower potency (76–90% cell viability). Aside from CIN, the effects of all extracts on the HeLa cells were not significantly different from that of the negative control; hence deeming them to have no or low cytotoxicity. It is possible that longer cell exposure times (>48 h) to the Noni juice extracts would have resulted in a greater reduction in cell viability. Nevertheless, these results suggest that commercial samples of noni juice do not appear to have very high cytotoxic activity against the tested cell lines.
Previously, Gupta et al. [
38] reported that Noni juice and cisplatin either alone or in combination were able to induce apoptosis in HeLa cells through the mitochondrial pathway. Their findings also reported that cisplatin showed higher cell potency compared to Noni juice, comparative to the results of this study. However, the combination of Noni juice and cisplatin showed additive effects, suggesting that it can be used as a chemo adjuvant in the treatment of cervical cancer [
38,
39]. Another study suggested that in vitro, a ‘concentrated component’ in Noni juice and not the pure Noni juice may firstly stimulate the immune system to ‘possibly’ assist the body fight the cancer and then kill a small percentage (0–36%) of cancer depending on the type [
2]. This may explain the low potency of the Noni juice extracts (0–30%) (
Figure 3) obtained in this study. Further fractionation and isolation of Noni juice is required to identify the ‘component’ responsible for the cytotoxic activity.
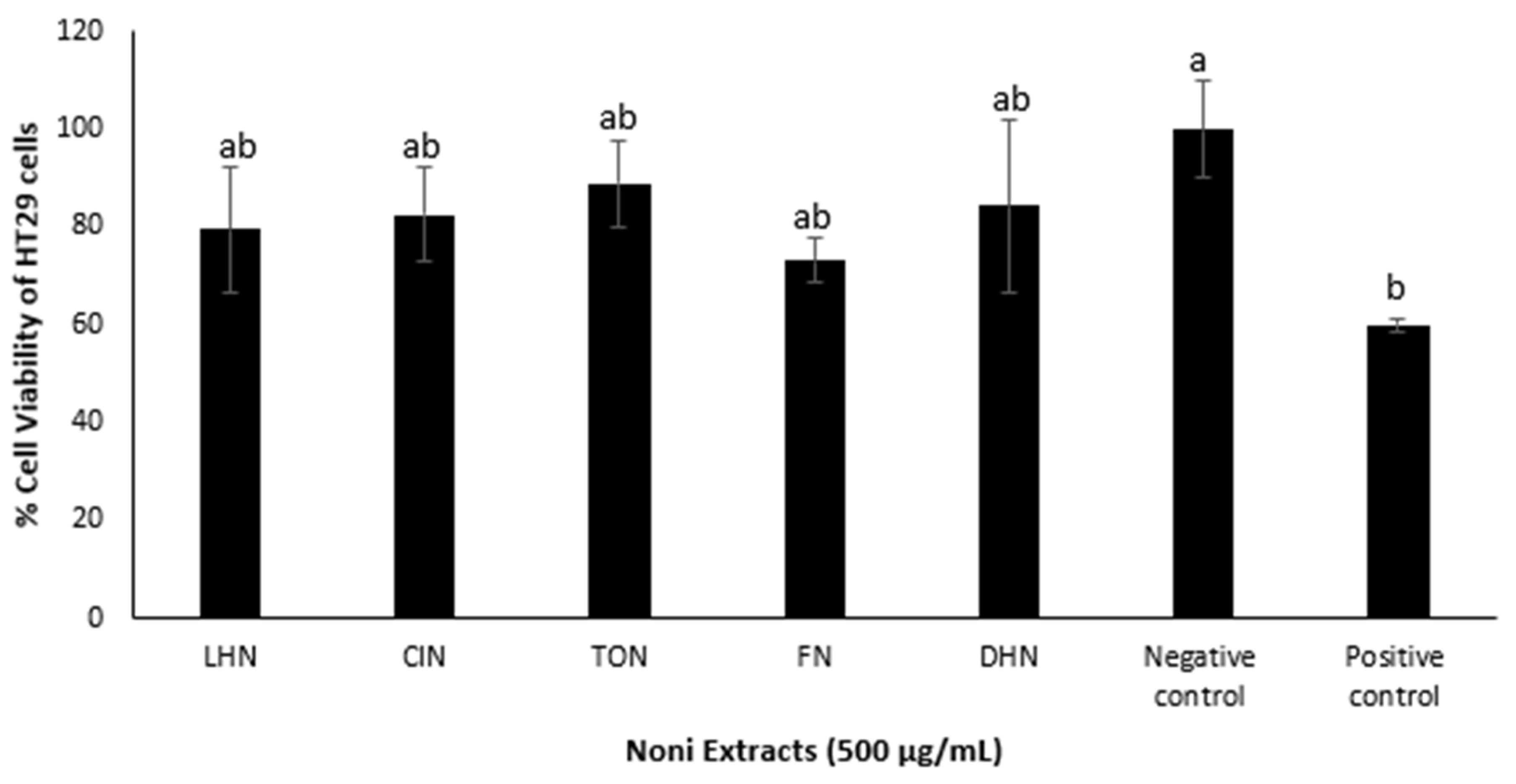
To further investigate the cytotoxic activity, colon cancer cell lines (HT29 cells) were treated with the Noni juice extracts, with the findings shown in
Figure 4. Cell viability decreased to 70–89% upon treatment with the Noni juice extracts, which was lower than the negative control (100% cell viability). This indicated that Noni extracts to some degree induced a cytotoxic effect on HT29 cell lines. There were no significant differences found between the various sources of Noni juice extracts, hence a comparison of their cytotoxicity is inconclusive.
In general, the HeLa cell line was more susceptible to the TON, CIN and DHN, whereas FN and LHN were found to be more potent against HT29. Furthermore, although some correlation between the phytochemistry and cytotoxic activity of the five sources of Noni juice was noted in this study, comparison in terms of TPC and cytotoxic activity against the HT29 cell line was inconclusive.