Oral Immune-Related Adverse Events Associated with PD-1 Inhibitor Treatment: A Case Series
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Oral irEAs
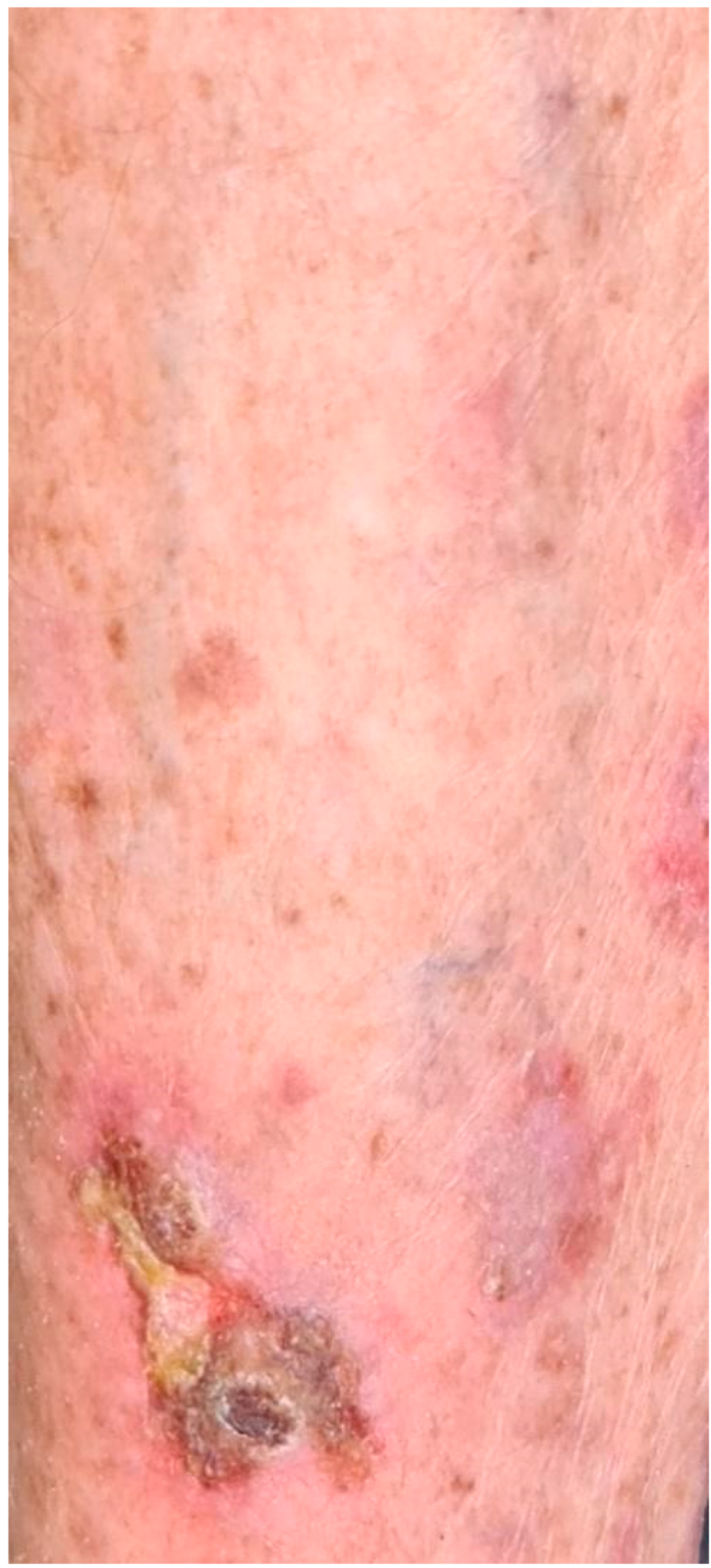
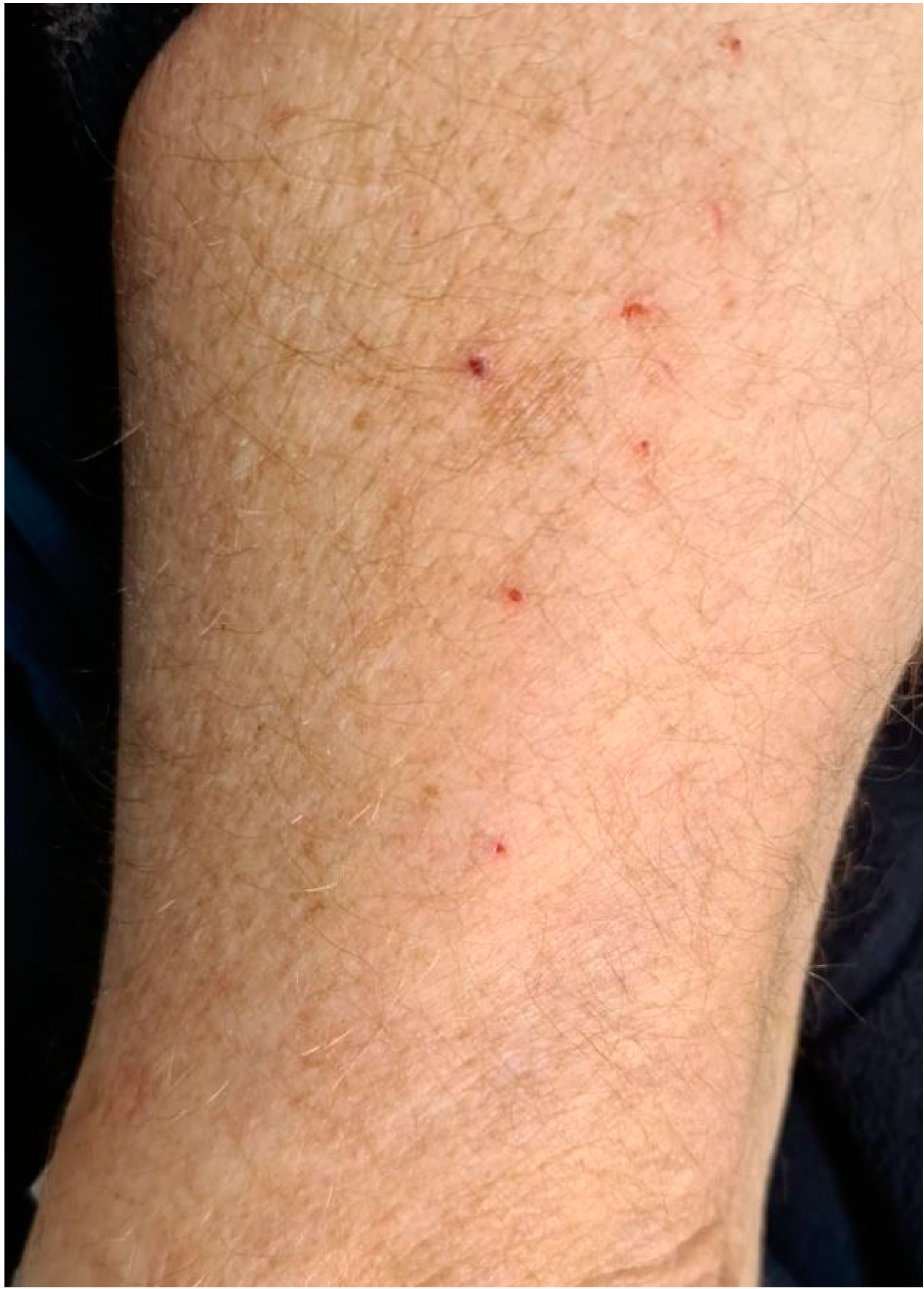
3.2. Cutaneous irAEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23 (Suppl. S8), viii6–viii9. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Botticelli, A.; Cecere, F.; Cognetti, F.; Giusti, R.; Gelibter, A.; Lugini, A.; Nelli, F.; Nuti, M.; Santini, D.; et al. L’immunoterapia nel tumore del polmone non a piccole cellule: Ritorno al futuro [Immunotherapy in non-small cell lung cancer patients: Back to the future]. Recenti Prog. Med. 2019, 110, 587–593. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An anti-CTLA-4 antibody for metastatic melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety profiles of anti-CTLA4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Yang, Q. Risk of dermatologic and mucosal adverse events associated with PD-1/PD-L1 inhibitors in cancer patients: A meta-analysis of randomized controlled trials. Medicine 2019, 98, e15731. [Google Scholar] [CrossRef]
- Dika, E.; Lambertini, M.; Gouveia, B.; Mussi, M.; Marcelli, E.; Campione, E.; Gurioli, C.; Melotti, B.; Alessandrini, A.; Ribero, S. Oral Manifestations in Melanoma Patients Treated with Target or Immunomodulatory Therapies. J. Clin. Med. 2021, 10, 1283. [Google Scholar] [CrossRef]
- Sibaud, V.; Eid, C.; Belum, V.R.; Combemale, P.; Barres, B.; Lamant, L.; Mourey, L.; Gomez-Roca, C.; Estilo, C.L.; Motzer, R.; et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e464–e469. [Google Scholar] [CrossRef] [PubMed]
- Fazer, C.; Price, K.A. Management of immune-related dermatitis and mucositis associated with pembrolizumab in metastatic human papillomavirus-associated squamous cell carcinoma of the oropharynx. JCO Oncol. Pract. 2020, 16 (Suppl. S2), 20s–24s. [Google Scholar] [CrossRef] [PubMed]
- Acero Brand, F.Z.; Suter, N.; Adam, J.P.; Faulques, B.; Maietta, A.; Soulières, D.; Blais, N. Severe immune mucositis and esophagitis in metastatic squamous carcinoma of the larynx associated with pembrolizumab. J. Immunother. Cancer 2018, 6, 22. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19 (Suppl. S1), 31–39. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.M.; Baer, A.N.; Lipson, E.J.; Allen, C.; Hinrichs, C.; Rajan, A.; Pelayo, E.; Beach, M.; Gulley, J.L.; Madan, R.A.; et al. Sicca Syndrome Associated with Immune Checkpoint Inhibitor Therapy. Oncologist 2019, 24, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Palaia, G.; Grassotti, B.; Tenore, G.; Ciolfi, C.; Podda, G.; Impellizzeri, A.; Mohsen, A.; Galluccio, G.; Romeo, U. Effects of laser photobiomodulation in the management of oral lichen planus: A literature review. Clin. Ther. 2021, 172, 467–483. [Google Scholar] [CrossRef]
- Zumelzu, C.; Alexandre, M.; Le Roux, C.; Weber, P.; Guyot, A.; Levy, A.; Aucouturier, F.; Mignot-Grootenboer, S.; Caux, F.; Maubec, E.; et al. Mucous membrane pemphigoid, bullous pemphigoid, and anti-programmed death-1/programmed death-ligand 1: A case report of an elderly woman with mucous membrane pemphigoid developing after pembrolizumab therapy for metastatic melanoma and review of the literature. Front. Med. 2018, 5, 268. [Google Scholar]
- Dascorresponding, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Klein, B.A.; Shazib, M.A.; Villa, A.; de Abreu Alves, F.; Vacharotayangul, P.; Sonis, S.; Fedele, S.; Treister, N.S. Immune checkpoint inhibitors in cancer therapy: Review of orofacial adverse events and role of the oral healthcare provider. Front. Oral Health 2022, 3, 968157. [Google Scholar] [CrossRef]
- Riudavets, M.; Barba, A.; Maroto, P.; Sullivan, I.G.; Anguera, G.; Paez, D.; del Carpio, L.; Callejo, A.; Gonzalez Blanco, C.; Garcia Planellas, E.; et al. Correlation between immune-related adverse events (irAEs) and efficacy in patients with solid tumors treated with immune-checkpoints inhibitors (ICIs). J. Clin. Oncol. 2018, 36 (Suppl. S15), 3064. [Google Scholar] [CrossRef]
- Tattersall, I.W.; Leventhal, J.S. Cutaneous Toxicities of Immune Checkpoint Inhibitors: The Role of the Dermatologist. Yale J. Biol. Med. 2020, 93, 123–132. [Google Scholar] [PubMed]
- Del Vecchio, A.; Tenore, G.; Luzi, M.C.; Palaia, G.; Mohsen, A.; Pergolini, D.; Romeo, U. Laser Photobiomodulation (PBM)-A Possible New Frontier for the Treatment of Oral Cancer: A Review of In Vitro and In Vivo Studies. Healthcare 2021, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; D’Alessandro, L.; Pergolini, D.; Carletti, R.; Di Gioia, C.R.T.; Romeo, U. In vivo clinical and histological thermal effect of a 445 nm diode laser on oral soft tissues during a biopsy. J. Oral Sci. 2021, 63, 280–282. [Google Scholar] [CrossRef]
- Palaia, G.; Renzi, F.; Pergolini, D.; Del Vecchio, A.; Visca, P.; Tenore, G.; Romeo, U. Histological Ex Vivo Evaluation of the Suitability of a 976 nm Diode Laser in Oral Soft Tissue Biopsies. Int. J. Dent. 2021, 2021, 6658268. [Google Scholar] [CrossRef]
- Pergolini, D.; Del Vecchio, A.; Mohsen, M.; Tenore, G. Inter-incisor ossifying fibroma removal by diode laser. Dent. Cadmos 2020, 88, 266–268. [Google Scholar] [CrossRef]
- Klein, B.A.; Alves, F.A.; de Santana Rodrigues Velho, J.; Vacharotayangul, P.; Hanna, G.J.; LeBoeuf, N.R.; Shazib, M.A.; Villa, A.; Woo, S.B.; Sroussi, H.; et al. Oral manifestations of immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Oral Dis. 2022, 28, 9–22. [Google Scholar] [CrossRef]




| Patient | County’ | Sex | Cancer Diagnosis | Therapy Anti PD-1 | Dosage | Smoke | Radiotherapy | Chemotherapy | Pathological History |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | K urothelial | pembrolizumab | 200 mg flat dose q21 | Yes | No | No | Systemic lupus erythematosus |
| 2 | 67 | M | K parotid | pembrolizumab | 200 mg flat dose q21 | Former smoker | 10 sessions | No | Hypertension |
| 3 | 76 | M | Pulmonary ADK | pembrolizumab | 200 mg flat dose q21 | No | No | No | Renal failure, aortic stenosis |
| 4 | 68 | M | K squamous skin | cemiplimab | 350 mg dropped dose q 21 | No | 2 sessions | No | Rheumatoid arthritis |
| 5 | 36 | M | Melanoma | nivolumab | 240 mg q 14 | No | No | No | Nothing to detect |
| 6 | 81 | M | K squamous cell oral cavity | pembrolizumab | 200 mg flat dose q21 | Former smoker | No | No | Hypertension; diabetes II; dyslipidemia |
| 7 | 68 | F | K squamous cell oral cavity | pembrolizumab | 200 mg flat dose q21 | No | No | No | Diabetes II; hypertension; chronic HBV; diverticular pathology |
| 8 | 70 | M | K urothelial | pembrolizumab | 200 mg flat dose q21 | Yes | No | Cisplatin-gemcitabine | Nothing to detect |
| 9 | 63 | M | Lung adenocarcinoma | pembrolizumab | 200 mg flat dose q21 | No | No | No | Atrial fibrillation |
| 10 | 69 | M | Pulmonary adenocarcinoma | nivolumab | 240 mg q 14 | Former smoker | No | Cisplatin alimta | Hypertension; dyslipidemia; partial thyroidectomy; coronary stent |
| 11 | 66 | F | Lung adenocarcinoma | pembrolizumab | 200 mg flat dose q21 | No | No | Cisplatin pemetrexed | Nothing to detect |
| 12 | 75 | M | Pulmonary adenocarcinoma | pembrolizumab | 200 mg flat dose q21 | No | No | No | Nothing to detect |
| 13 | 71 | M | Pulmonary adenocarcinoma | pembrolizumab | 200 mg flat dose q21 | No | 10 sessions | No | Hypertension |
| Patient | Clinical Description | Type of Lesion | Anatomical Site | Therapy | Anti-PD-1 Dose for Oral irAEs |
|---|---|---|---|---|---|
| 1 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect | |
| 2 | Erythematous area | Epithelial atrophy without dysplasia | Palate | Aminogam® mouthwash | 1st dose |
| 3 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect | |
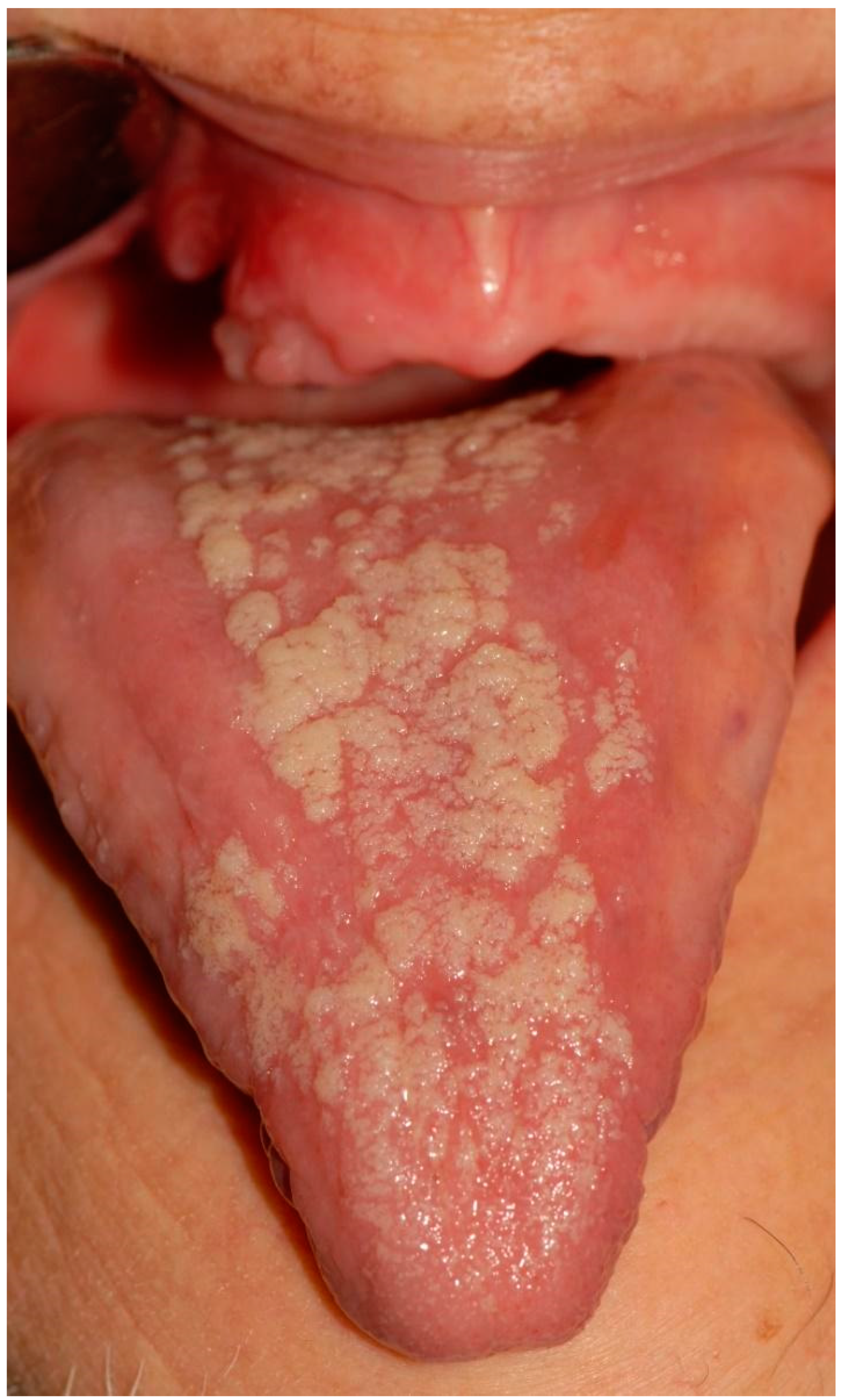
| 4 | Removable whitish plates Erythematous area Xerostomia | Candidiasis (pseudomembranous candidiasis, median rhomboid glossitis) | Dorsal tongue Oral mucous membranes | Nystatin Mucosamin® mouthwash | 3rd dose 9th dose |
| 5 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect | |
| 6 | Non-removable whitish lesions | Hyperkeratosis | Palate Cheek mucosa | Aminogam® mouthwash | 4th dose |
| 7 | Non-removable white lesions | Hyperkeratosis | Dorsal tongue Palate | Aminogam® mouthwash | 3rd dose |
| 8 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 9 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect | |
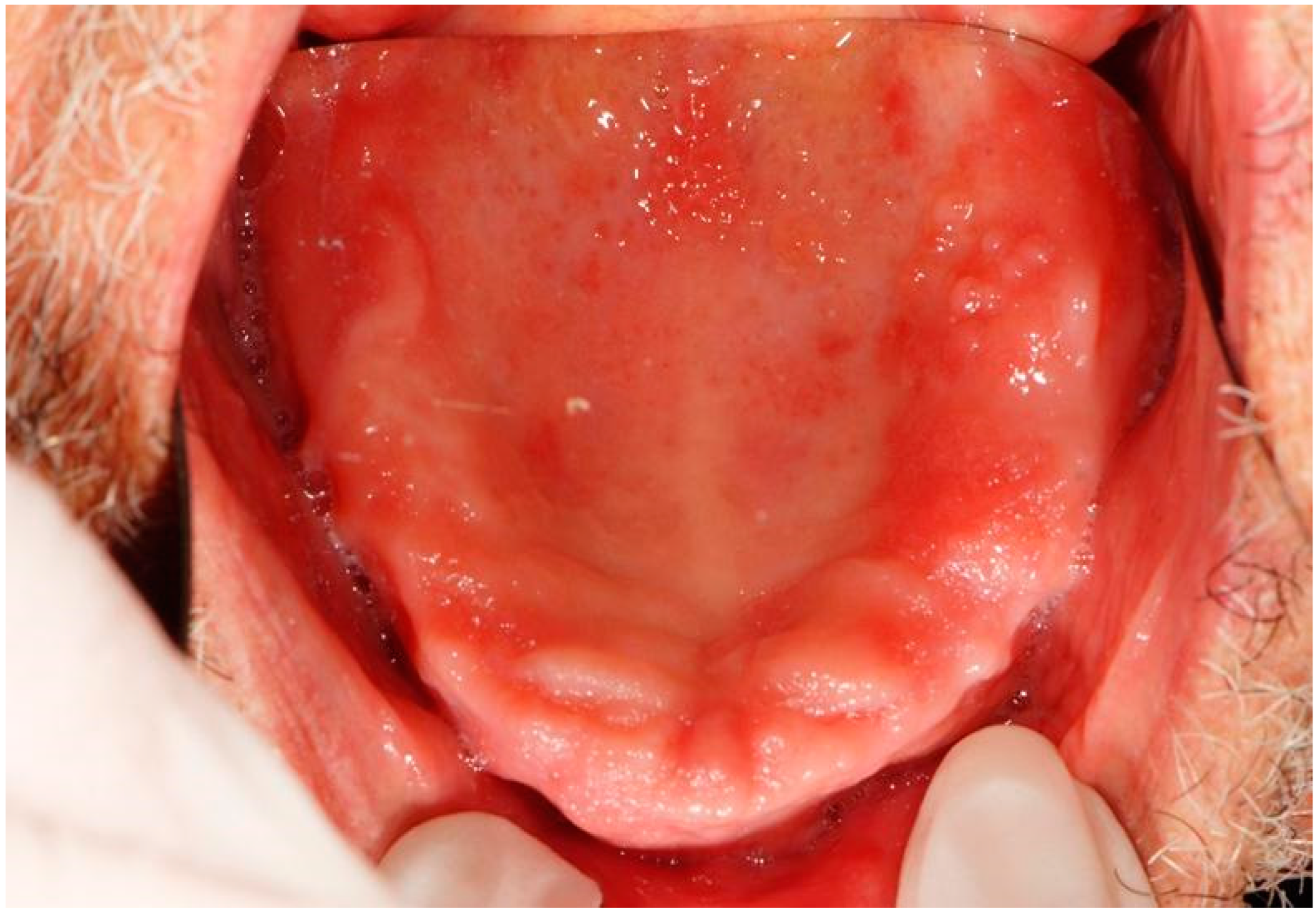
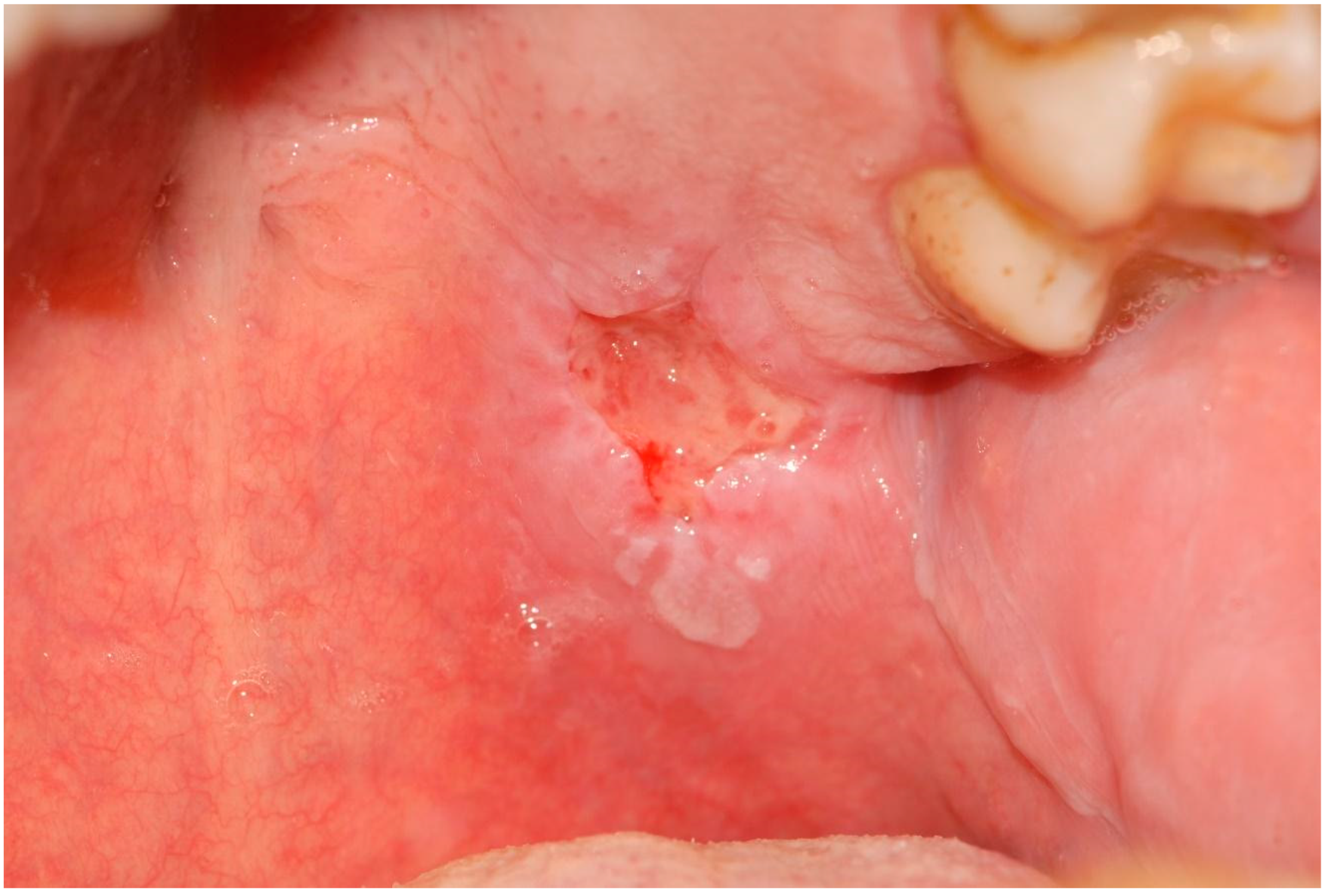
| 10 | Erythematous and ulcerative areas | Epithelial atrophy without dysplasia Ulcer | Palate | 2nd dose | |
| 11 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 12 | Non-removable whitish lesions | Epithelial atrophy and hyperkeratosis without dysplasia | Cheek Dorsal tongue | Aminogam® mouthwash | 4th dose |
| 13 | Erythematous area Ulcer Removable whitish plates | Epithelial atrophy without dysplasia Ulcer Candidiasis | Palate Tuberosity Dorsal tongue Alveolar process | Nystatin | 2nd dose 4th dose |
| Patient | Lesion | Body Area | Treatment | Dermatological Anti-PD-1 Dose |
|---|---|---|---|---|
| 1 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 2 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 3 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
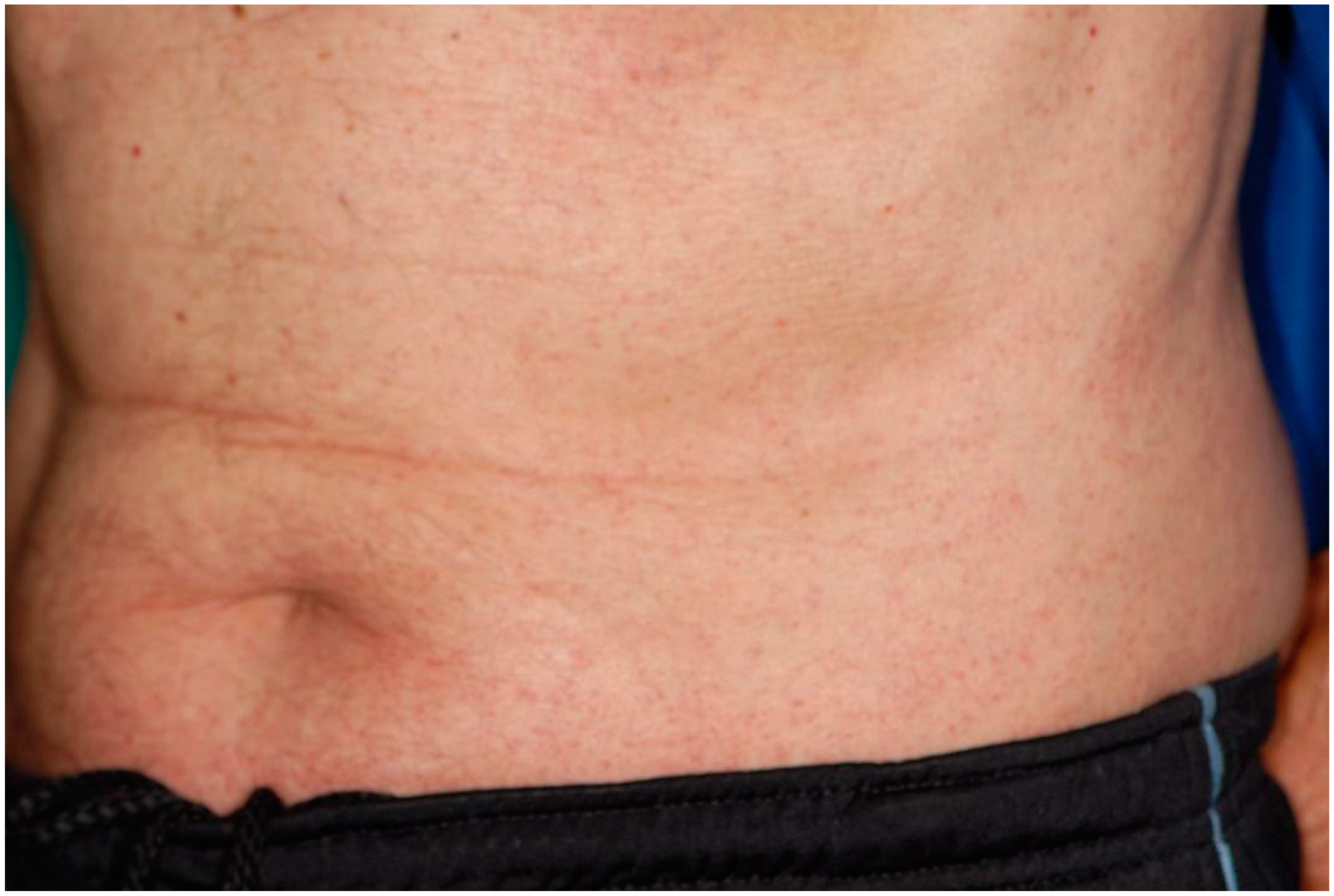
| 4 | ERYTHEMA | BACK | TOPICAL STEROID | 3rd dose |
| 5 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 6 | ERYTHEMA | LEGS | TOPICAL STEROID | 3rd dose |
| 7 | ERYTHEMA | ABDOMEN | TOPICAL STEROID | 3rd dose |
| 8 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 9 | Nothing to detect | Nothing to detect | Nothing to detect | Nothing to detect |
| 10 | ERYTHEMA | ARMS, LEGS, ABDOMEN | TOPICAL STEROID | 3rd dose |
| 11 | ERYTHEMA | FACE, CHEST | TOPICAL STEROID | 3rd dose |
| 12 | ERYTHEMA | ABDOMEN | TOPICAL STEROID | 1st dose |
| 13 | ERYTHEMA | ABDOMEN | TOPICAL STEROID | 2nd dose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergolini, D.; Botticelli, A.; Fascetti, R.; Rocchetti, F.; Cirillo, A.; Tenore, G.; Palaia, G.; Polimeni, A.; Romeo, U. Oral Immune-Related Adverse Events Associated with PD-1 Inhibitor Treatment: A Case Series. Appl. Sci. 2022, 12, 12994. https://doi.org/10.3390/app122412994
Pergolini D, Botticelli A, Fascetti R, Rocchetti F, Cirillo A, Tenore G, Palaia G, Polimeni A, Romeo U. Oral Immune-Related Adverse Events Associated with PD-1 Inhibitor Treatment: A Case Series. Applied Sciences. 2022; 12(24):12994. https://doi.org/10.3390/app122412994
Chicago/Turabian StylePergolini, Daniele, Andrea Botticelli, Roberta Fascetti, Federica Rocchetti, Alessio Cirillo, Gianluca Tenore, Gaspare Palaia, Antonella Polimeni, and Umberto Romeo. 2022. "Oral Immune-Related Adverse Events Associated with PD-1 Inhibitor Treatment: A Case Series" Applied Sciences 12, no. 24: 12994. https://doi.org/10.3390/app122412994
APA StylePergolini, D., Botticelli, A., Fascetti, R., Rocchetti, F., Cirillo, A., Tenore, G., Palaia, G., Polimeni, A., & Romeo, U. (2022). Oral Immune-Related Adverse Events Associated with PD-1 Inhibitor Treatment: A Case Series. Applied Sciences, 12(24), 12994. https://doi.org/10.3390/app122412994












