Abstract
Organic/inorganic halide perovskite materials have attracted substantial attention in solar cells, and they have achieved significant improvements in recent years. In perovskite solar cells (PSCs), the engineering of interfacial properties between multilayers is an important determinant of performance and stability. Here, we designed a bilayer structure of hole transporting layer by inserting poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA), which can improve the interfacial properties between the perovskite and the nickel oxide (NiO) hole transport layer in p-i-n planar PSCs. We observed that the hole transporting PTAA/NiO bilayer leads to higher performance by optimizing the energy level and accelerating the extraction of charges from the interface. The power conversion efficiency of the PSC was improved from 17.29% to 19.05% when the PTAA/NiO bilayer was introduced instead of the NiO monolayer. Ultimately, we confirmed that this interface engineering provides thermal and air stability of PSCs.
1. Introduction
Hybrid organic/inorganic halide perovskite (ABX3, A = methyl ammonium, formamidinium, or metallic Cs+; B = Pb2+, Sn2+; X = I−, Br−, Cl−) materials have attracted substantial attention for use as next-generation solar cells [1,2]. Perovskite materials have many advantages, such as an excellent absorption coefficient, tunable bandgap, low exciton binding energies, high carrier mobility, long charge diffusion length, ambipolar charge transport, etc. [3,4,5,6,7,8,9]. Based on these characteristics, organic-inorganic halide perovskite solar cells (PSCs) have attracted attention, as their power conversion efficiency (PCE) has increased remarkably from 3.8% in 2009 to 25.7% in recent years [10]. Despite these advantages, many efforts are needed to achieve high efficiency and high stability in PSCs due to the loss of the incomplete interface between the perovskite and the interfacial layer [11]. In particular, PSC performance is highly dependent on the interfacial charge transport layer (CTL) as well as the quality of the perovskite active layer. By strategically selecting the CTL of the PSC, it is possible to control the high-quality perovskite active layer and inhibit carrier recombination in the device, thereby promoting carrier injection into the electrode [12,13,14]. This charge transport layer is an important factor in the performance and stability of PSCs [15,16,17].
Planar PSCs can be divided into two well-known categories, which include n-i-p and p-i-n structures, both of which are beneficial for high-performance solar cells [18,19]. Recently, many studies have examined p-i-n devices due to their advantages, such as low hysteresis effect, low process temperature, and potential tandem solar cells [20]. A material commonly used as a hole transporting layer (HTL) in the p-i-n structure is poly(3,4-ethylenedioxythiophene)polystyrene-sulfonate (PEDOT:PSS), which has a high work function, simple process, optical transparency and high conductivity [21]. However, PEDOT:PSS-based p-i-n PSCs exhibit low open circuit voltage (VOC) and insufficient stability due to their acidic and hygroscopic properties as well as a large energy barrier between PEDOT:PSS and perovskite [22,23,24]. Therefore, researchers are exploring various materials that could replace PEDOT:PSS. Recently, nickel oxide (NiO) has been widely used as a promising candidate for HTL in p-i-n structures because of its high light transmittance through a large band gap (3.6–4.0 eV), deep valence band compatible with perovskite layer, and thermal and chemical stability [25,26,27]. However, there are still some obstacles to achieving the high performance and high stability of NiO-based PSCs: low intrinsic conductivity, energy level at NiO/perovskite interface, and deprotonation reactions of under-coordinated Ni ions [28]. Therefore, this represents one of the technological challenges in improving charge transport and minimizing charge recombination at the interface of perovskite/HTL.
Recently, there have been many studies to strategically improve the performance and stability of PSCs through surface engineering, such as improving charge extraction by reducing recombination by controlling interface defects and traps, energy alignment, and improving the quality of perovskite films [29,30,31,32]. For surface engineering, various materials such as organic materials, self-assembled monolayer (SAM), and passivation materials have been reported. It has been reported that hole extraction is improved by tailoring the energy level of the HTL through addition of F6TCNNQ and F2HCNQ molecules and insertion of polymers such as insulating polystyrene (PS) and poly(methyl methacrylate) (PMMA), thereby suppressing energy loss and improving VOC [33,34,35]. In addition, by using a salt such as alkali halide, defects and traps present at the CTL/perovskite interface of PSCs were passivated, and interfacial recombination was relieved, resulting in a VOC of 1.15 V [36]. Additionally, surface treatment of SAM dipole molecules such as 2PACz and MeO-2PACz have been reported [37,38]. The effect of interfacial engineering to eliminate the causes of non-radiative recombination losses by minimizing these interfacial defects, incomplete energy level alignment, and interfacial reaction losses is an important part in achieving efficient and stable PSCs. In particular, the interfacial engineering approach with the addition of PTAA polymer has proven effective in improving the energy level alignment and crystallinity of perovskite and HTL [39]. However, the effects of interfacial engineering have not been sufficiently discussed, and they remain to be understood. Moreover, although an approach of previous studies inserted a PTAA between the perovskite and the HTL, there have been no investigations into the thermal and air stability of PSCs with PTAA.
In this study, we demonstrated highly thermal and air stable PSCs by replacing NiO with a PTAA/NiO bilayer that acts as an HTL. The PTAA/NiO bilayer showed a higher work function and better charge carrier mobility than the NiO film. It also inhibited interfacial recombination and accelerated charge transfer at the HTL interface, thereby increasing the VOC and current density (JSC) of PSCs simultaneously. The optimized PTAA/NiO bilayer-based PSC showed a PCE of 19.05%, which was improved over that of the NiO monolayer-based PSC with a PCE of 17.29%. In addition, the encapsulated PTAA/NiO bilayer-based PSCs showed excellent long-term stability in an environment with 85% RH and 85 °C temperature.
2. Materials and Methods
2.1. Materials
NiO (NCT-7) was purchased from 1-Material. Lead (ΙΙ) iodide (PbI2, 99%), cesium iodide (CsI, 99.99%), and lead bromide (PbBr2, 99%) were obtained from Tokyo Chemical Industry. Formamidinium iodide (FAI, 98%) and methylammonium bromide (MABr, >99%) were obtained from Great Cell Solar. N,N-dimethylformamide (DMF, 99.8%), dimethyl sulfoxide (DMSO, 99.7%), and PTAA (PTAA, 99%) were all purchased from Sigma-Aldrich, and C60 was obtained from Nano-C.
2.2. Fabrication of PSCs
For PSC fabrication, a 2.5 × 2.5 cm2 indium tin oxide (ITO, AMG-Tech, Atlanta, GA, USA) glass was pretreated with ultraviolet (UV)-ozone for 30 min (Figure 1). The NiO was spin-casted (at 5000 rpm for 30 s) on clean ITO substrates as an HTL and then annealed at 350 °C for 30 min in the air. When the PTAA/NiO layer was employed, a PTAA solution was prepared by dissolving 1 mg PTAA in 1 mL toluene. The PTAA solutions were spin-coated on the NiO substrates at 3000 rpm for 30 s and heated at 100 °C for 10 min in a glove box. The perovskite solution of Cs0.175FA0.750MA0.075Pb(I0.880Br0.120)3 was prepared by mixing 1.45M CsI, FAI, PbI2, PbBr2, and MABr in a 4:1 ratio solution of DMF and DMSO. Next, the perovskite solution (200 µL) was spin-coated onto the HTL/ITO/glass substrate at 500 rpm for 5 s and 4000 rpm for 45 s [40]. At 30 s before the end of spin-coating, chlorobenzene or anisole (0.4 mL) was dripped onto the substrate, and the sample was subsequently heated at 100 °C for 30 min in a glove box under N2 atmosphere. Then, the electron transport material C60 of 35 nm and SnO2 of 3 nm were deposited by using a thermal evaporator at a high vacuum of 8 × 10−6 Torr. Finally, an Ag electrode of 120 nm was deposited by using a thermal evaporator. The effective device area, which was defined by a shadow mask, was 1.12 cm2.
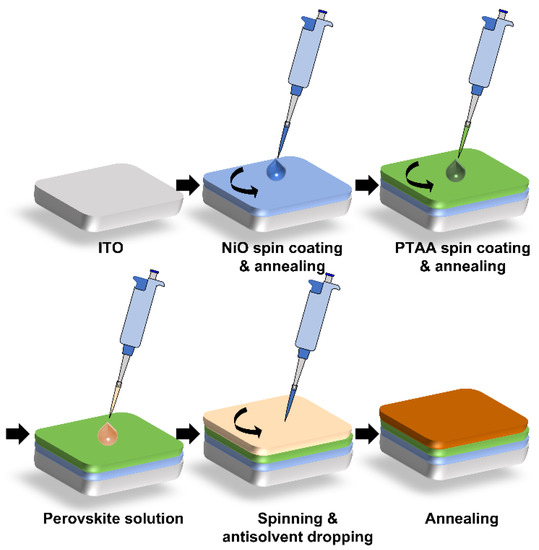
Figure 1.
Fabrication of perovskite/PTAA/NiO films.
2.3. Characterization and Measurement
Steady-state photoluminescence (PL) was obtained from a spectrofluorophotometer (SHIMADZU, Kyoto, Japan, RF-6000) using an Xe lamp (power intensity < 100 mW cm−2, wavelength of 532 nm) as an excitation source. The valence band maximum (VBM) values of the films were determined by photoelectron spectroscopy (UPS) (K-Alpha+, Thermo Fisher Scientific, Waltham, MA, USA) using an He I (21.22 eV) excitation source. The conduction band minimum (CBM) value was calculated by adding the optical band gap (Eg) derived from a UV-Vis spectrophotometer (Lambda 750, PerkinElmer, Waltham, MA, USA). The device performance of solar cells was measured by a Keithley 4200 source meter using an Oriel solar simulator (Newport, Irvine, CA, USA, class AAA) under AM 1.5 G (100 mW cm−2) irradiation. Transient photovoltage (TPV) and transient photocurrent (TPC) measurements were conducted using a multifunctional semiconductor-parameter test system (T-4000; McScience, Suwon, Republic of Korea) [40].
3. Results
3.1. Performance of NiO and the PTAA/NiO-Based PSCs
To investigate the performance and stability of the perovskite device using the PTAA/NiO bilayer HTL, we fabricated a p-i-n device with the Ag/SnO2/C60/perovskite/PTAA/NiO/ITO structure shown in Figure 2. Specifically, Ag, SnO2, C60, perovskite, PTAA, NiO, and ITO were used as back metal, electron transport layer, Cs0.175FA0.750MA0.075Pb(I0.880Br0.120)3 based photoactive layer, bilayer HTL, and front transparent electrode, respectively.
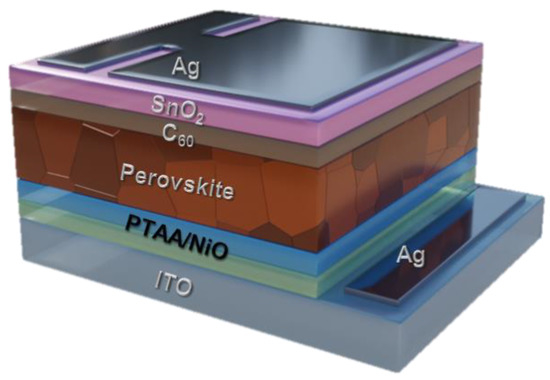
Figure 2.
Structural layout of PTAA/NiO-based PSCs.
Based on this structure, the outputs of PSCs and various electrical and optical performances were analyzed. The current–voltage (J–V) curves and external quantum efficiency (EQE) of the best NiO and PTAA/NiO bilayer-based PSCs are shown in Figure 3. As presented in Figure 3a, the PTAA/NiO bilayer-based PSC significantly improved PCE from 17.29% to 19.05%, and VOC, JSC and FF all increased from 1.10 V to 1.15 V, from 20.93 mA cm−2 to 21.69 mA cm−2, and from 74.73% to 76.47% %, respectively, as listed in Table 1. As shown in Figure 3b, the optimized PTAA/NiO bilayer exhibited higher EQE values compared to NiO devices, which supports that devices with PTAA/NiO bilayer promote efficient charge extraction and higher Voc [40]. The device performance improvement was consistent with the J–V and EQE spectra of NiO and PTAA/NiO bilayer-based PSCs. The JSC calculated by integrating the EQE curves (20.30 mA cm−2 for NiO-based and 21.08 mA cm−2 for PTAA/NiO) showed a small deviation (3%) from the JSC obtained from the J–V curves, thus indicating that the measurements are reliable.
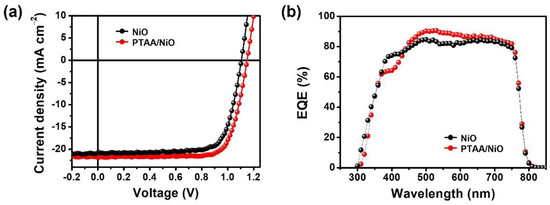
Figure 3.
(a) J–V curves of the NiO and PTAA/NiO PSCs with active area of 1.12 cm2. (b) EQE spectra of NiO and PTAA/NiO based PSCs.

Table 1.
Device performance of the NiO monolayer and PTAA/NiO bilayer-based PSCs under AM 1.5 G illumination at 100 mW cm−2.
Figure 4 summarizes the changes in all output parameters: PCE, fill factor (FF), open circuit voltage (VOC), and short circuit current (JSC) using corresponding representative J–V curves for devices manufactured with different HTLs. The PSCs that had the PTAA layer inserted performed significantly better than the device without the PTAA layer in all parameters. The PTAA/NiO bilayer-based PSCs showed higher output performances than NiO-based PSCs.
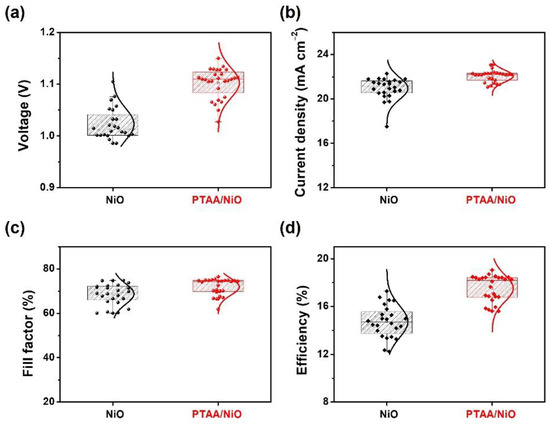
Figure 4.
Statistical comparison of photovoltaic parameters with optimization condition of NiO and PTAA/NiO; (a) VOC, (b) JSC, (c) FF, and (d) PCE.
3.2. Properties of the NiO and PTAA/NiO Films
To further investigate the mechanism behind this output improvement, the detailed optical and electrical properties of the NiO and PTAA/NiO films, surface energy, UV–Vis, energy band level diagram, and PL studies were examined. The energy levels of the individual components and the alignment of the energy levels of device structures were studied using UPS. As shown in Figure 5a, the work functions (WFs) for NiO and PTAA through the UPS were determined to be 4.6 eV and 4.8 eV, respectively [41]. The optical properties of NiO, PTAA/NiO, and PTAA HTL films were investigated by UV-Vis spectroscopy. The UV-Vis spectra data are shown in Figure 5b, and the bandgaps calculated from the Tauc plot method are shown in Figure 5c,d [42]. The obtained band gaps of NiO and PTAA of HTL were 3.7 eV and 4.0 eV, respectively. The energy level alignments of ITO as the bottom electrode, NiO and PTAA as HTL, perovskite as the photoactive layer, and C60 as the electron transport layer are shown in Figure 5e, and the perovskite was based on the triple cation composition Cs0.175FA0.750MA0.075Pb(I0.880Br0.120)3. The obtained valence bands of NiO and PTAA film were 0.6 eV and 0.6 eV, respectively, which means that that of PTAA was closer to the perovskite film than that of NiO. From the UPS results, it was concluded that HTL with bilayers of PTAA/NiO has a more suitable energy level alignment and can better perform the charge extraction role in perovskite active layer.
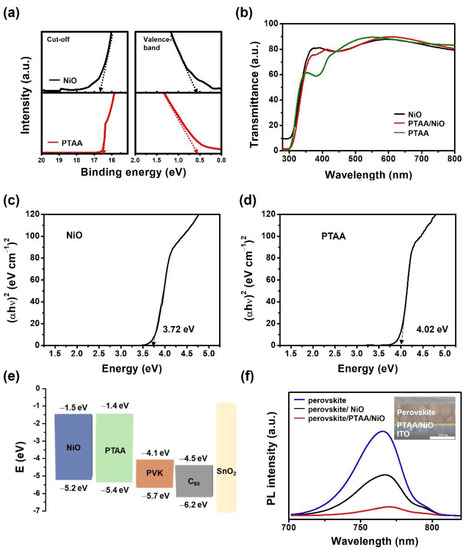
Figure 5.
(a) UPS spectra for NiO and PTAA films. (b) UV–Vis transmittance spectra of various HTL films; NiO, PTAA, PTAA/NiO (bilayer) on ITO substrate. Tauc plot of (c) NiO film and (d) PTAA film. (e) Energy level diagram of the whole layer PSC device. (f) Steady-state PL spectra of perovskite, NiO/perovskite, and perovskite /PTAA/NiO films. Inset: the cross-view SEM image of perovskite/PTAA/NiO film.
To further investigate the effect of PTAA/NiO film on the performance of the PSCs, the steady-state PL spectra of perovskite and HTL with perovskite film were measured and are shown in Figure 5f. We prepared PL spectra of perovskite films based on HTL films such as perovskite/glass, perovskite/NiO/glass, and perovskite/PTAA/NiO/glass. In the PL peak emission, the perovskite film showed a strong emission peak at about 765 nm. When the NiO as HTL was introduced under the perovskite film, the PL peak intensity decreased dramatically. Further, when PTAA was introduced on NiO to form a PTAA/NiO bilayer, the PL peak intensity was further reduced. Through the inserted cross-section SEM image, it was confirmed that the thickness of perovskite/PTAA/NiO was approximately 30 nm, and that of perovskite was 500 nm. Unfortunately, PTAA was too thin to be distinguished. However, PTAA/NiO bilayer HTL showed more efficient PL quenching than the NiO film obtained from steady-state PL. The finding of a lower PL intensity in the PL results means that carrier extraction and transport is most efficient when the PTAA/NiO bilayer is used as HTL, and that the energy level alignment of HTL between electrode and perovskite is substantially improved and the carrier recombination is reduced [43,44]. These results are consistent with the higher Jsc and FF values of PTAA/NiO bilayer PSCs in their J–V characteristics.
Differences in the wettability of the substrates can modulate the nucleation and growth processes of perovskite crystals, thus affecting the quality of the perovskite films. As shown in Figure 6, the contact angles of the two substrates with water were 55.9° and 85.6° for NiO and PTAA/NiO, respectively, and the measured contact angles for the PTAA/NiO surface were significantly increased compared to those of the NiO surface. As is well known, large contact angle values such as for the PTAA film indicate that the surface properties are hydrophobic. These HTL surface properties affect the crystal formation of perovskite, and in particular, hydrophobic surface properties can form large crystals of perovskite film [39]. Therefore, the surface properties of HTL not only affect the film quality of the perovskite, but also contribute to charge transport and extraction at the interface with the perovskite. Figure 6 shows the SEM top view images (c and d) of the perovskite films on NiO layers without and with the PTAA layer. The perovskite on the PTAA/NiO bilayer showed a much larger particle size (~550 nm), thus indicating improved crystallization even with the hydrophobic properties of the substrate. The use of a hydrophobic substrate, which is known to be beneficial for crystallization of the perovskite layer, suppresses non-uniform nucleation of the perovskite and improves the grain boundary expansion rate of the perovskite to form a high-quality perovskite film [45]. These properties enhance the charge extraction ability of the perovskite and PSCs and contribute to the low series resistance of the interface to form an excellent FF.
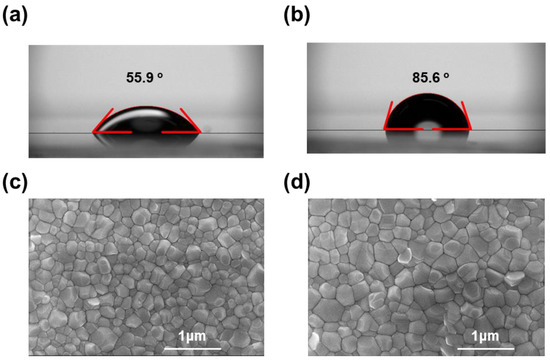
Figure 6.
Contact angle images of water droplets on the surface of (a) NiO film, (b) PTAA/NiO film. Top-view SEM images of (c) perovskite/NiO film, (d) perovskite/ PTAA/NiO film.
3.3. Charge Carrier Dynamic Mechanism of NiO and the PTAA/NiO-Based Device
The enhanced properties of PSC devices with the bilayer of HTL can be further validated through optical and electrical analyses such as TPV, TPC, trap density (Ntrap), and charge mobility measurements, as shown in Figure 7. As shown in Figure 7a,b, the charge carrier transport, lifetime and extraction ability of the device were obtained by TPV and TPC. As can be seen in Figure 7a, the decay times of the photovoltage (0.6 ms) of the device have a lower decay time than the PTAA/NiO bilayer (1.1 ms) device. The better electronic quality and lower defect concentration in the PTAA/NiO-based device are consistent with the higher Voc of that device [46]. Meanwhile, the TPC decay times were 0.37 μs and 0.48 μs in NiO and PTAA/NiO bilayer PSCs, respectively, thus indicating better charge transport in the PTAA/NiO bilayer devices, as shown in Figure 7b. To evaluate the two types of HTL in detail, the Ntrap values of NiO and PTAA/NiO based hole-only devices were measured through space-charge-limited current measurement in the following ways. Dark J–V curves for hole-only devices with Au/PTAA/Perovskite /NiO/ITO and Au/PTAA/Perovskite/PTAA/NiO/ITO are shown in Figure 7c. The Ntrap can be expressed using the following equation [47]:
and can be estimated as the trap-filled limiting voltage (VTFL), which is the starting voltage between the ohmic region and the trap-filled region. The VTFL values obtained from the J–V curves of the different perovskite devices for NiO and PTAA/NiO were 0.86 V and 0.54 V, respectively. In addition, the Ntrap values of the perovskite film containing NiO and PTAA/NiO obtained through this were 1.34 × 1016 cm−3 and 8.48 × 1015 cm−3, respectively. The reduced VTFL and Ntrap values in the bilayer perovskite show that the PTAA interlayer has the effect of reducing Ntrap and recombination sites as well as improving overall device performance.
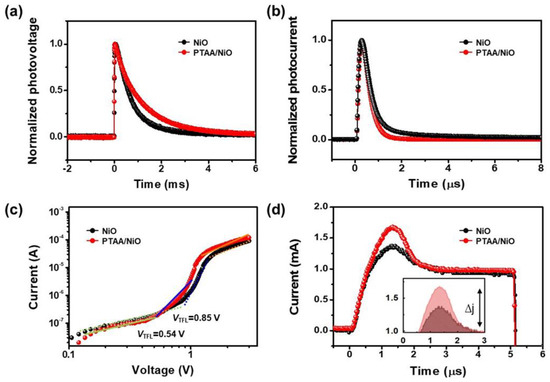
Figure 7.
(a) Normalized TPV curves and (b) TPC curves of NiO and PTAA/NiO bilayer-based PSCs. (c) Dark J–V curves of NiO and PTAA/NiO bilayer-based hole-only devices. (d) Photo-CELIV measurement of PSCs (the inset indicates the extracted charges).
In addition, a more-detailed investigation of the charge mobility and recombination process of working PSCs was performed using photo-generated charge extraction by linearly increasing voltage (photo-CELIV) method, as shown in Figure 7d. The charge carrier mobility of PSCs with and without a PTAA layer in the HTL were calculated using the equations of current density at maximum peak (Δj) and the time at maximum current density of 3.7 × 10−3 and 1.8 × 10−3 cm2 V−1 s−1, respectively. In addition, the inset image in Figure 7d shows the extractable charge values, suggesting that the PTAA/NiO bilayer device has a larger extractable charge. This shows that it is possible to inhibit the recombination of charges.
3.4. Thermal and Air Stability of NiO and the PTAA/NiO-Based PSCs
Interface engineering of PSCs has proven successful in suppressing device degradation and extending device lifetime under various environmental conditions. The PSC stability test results are shown in Figure 8, and the PCE was normalized to the initial values obtained after device preparation. The output stability of the unencapsulated PTAA/NiO bilayer device in an air atmosphere at room temperature and 30 ± 10% relative humidity maintained 74% of the initial PCE value even after 30 days, as shown in Figure 8a, whereas the NiO-based device only exhibited 50% of the initial efficiency after the same conditions were maintained for the same time. Finally, as encapsulated, PTAA/NiO bilayer-based PSC was compared to NiO-based PSC under extreme conditions (85% RH/85 °C temperature), and the results are shown in Figure 8b. The NiO-based PSCs retained 84% of the initial PCE for 10 days, whereas the PTAA/NiO bilayer PSCs retained 98% of the initial PCE, thus indicating better stability.
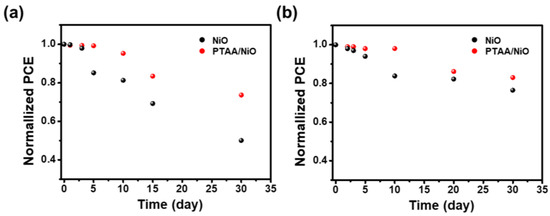
Figure 8.
Stability of normalized PCE of NiO and PTAA/NiO bilayer devices (a) under air atmosphere at room temperature (unencapsulated); (b) under 85% RH/85 °C temperature environment (encapsulated).
4. Conclusions
In this work, we used PTAA/NiO bilayers as the HTL in PSCs with p-i-n structures and showed significant improvement in device PCE compared to devices with an HTL of NiO. When the PTAA/NiO bilayer was used as the HTL, the PCE increased from 17.29% to 19.05%. Because PTAA has a lower work function compared to NiO, the energy band alignment of PTAA/NiO bilayer-based PSCs is better than that of NiO monolayer-based PSCs when PTAA is present between the perovskite and NiO. Moreover, the analysis of the charge transport behavior showed that the device using the PTAA/NiO bilayer HTL had high performance due to its improved carrier transport, carrier extraction and reduced recombination. It can, therefore, be suggested that the PTAA/NiO bilayer as an HTL controlled by interface engineering is a promising pathway to further improving the stability of PSCs.
Author Contributions
Y.-S.L. and S.-N.K. equally contributed to this work. Y.-S.L.: Writing—original draft, Methodology and Data Curation. S.-N.K.: Writing-review and editing, Formal Analysis, and Visualization. S.-I.N.: Conceptualization. D.K.: Conceptualization, Supervision. S.-W.K.: Supervision, Writing—review and editing, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by SKKU Excellence in Research Award Research Fund (S-2021-2596-000), Sungkyunkwan University, 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-J.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photon. 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Sum, T.C.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef]
- Song, T.-B.; Chen, Q.; Zhou, H.; Jiang, C.; Wang, H.-H.; Yang, Y.; Liu, Y.; You, J.; Yang, Y. Perovskite solar cells: Film formation and properties. J. Mater. Chem. A 2015, 3, 9032–9050. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- NREL. Best Research-Cell Efficiencies; National Renewable Energy Laboratory: Golden, CO, USA, 2022. [Google Scholar]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sheri, M.; Page, Z.A.; Emrick, T.; Saeki, A.; Liu, Y.; Russell, T.P. Understanding Hole Extraction of Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 56068–56075. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A.; et al. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Seybold, M.; Agresti, A.; Pescetelli, S.; Matteocci, F.; Haider, M.I.; Birkhold, S.T.; Hu, H.; Giridharagopal, R.; Sultan, M.; et al. Perovskite-Polymer Blends Influencing Microstructures, Nonradiative Recombination Pathways, and Photovoltaic Performance of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 42542–42551. [Google Scholar] [CrossRef]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Fujikawa, N.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; et al. CH3NH3SnxPb(1–x)I3 Perovskite Solar Cells Covering up to 1060 nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, Z.; Zhou, Y.; Liu, H.; Shen, W. Highly efficient and stable perovskite solar cells via bilateral passivation layers. J. Mater. Chem. A 2019, 7, 21730–21739. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, N.-G. On the Current–Voltage Hysteresis in Perovskite Solar Cells: Dependence on Perovskite Composition and Methods to Remove Hysteresis. Adv. Mater. 2019, 31, 1805214. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Han, W.; Ren, G.; Liu, J.; Li, Z.; Bao, H.; Liu, C.; Guo, W. Recent Progress of Inverted Perovskite Solar Cells with a Modified PEDOT:PSS Hole Transport Layer. ACS Appl. Mater. Interfaces 2020, 12, 49297–49322. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jiang, F.; Qin, F.; Meng, W.; Jiang, Y.; Xiong, S.; Tong, J.; Li, Z.; Liu, Y.; Zhou, Y. Nonreduction-Active Hole-Transporting Layers Enhancing Open-Circuit Voltage and Efficiency of Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 33899–33906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chueh, C.-C.; Eslamian, M.; Jen, A.K.Y. Modulation of PEDOT:PSS pH for Efficient Inverted Perovskite Solar Cells with Reduced Potential Loss and Enhanced Stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Peng, J.; Wang, W.; Xia, Z.; Yuan, J.; Lu, J.; Huang, X.; Ma, W.; Song, H.; Chen, W.; et al. Sequential Deposition of CH3NH3PbI3 on Planar NiO Film for Efficient Planar Perovskite Solar Cells. ACS Photonics 2014, 1, 547–553. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, H.; Xiao, S.; Xue, Q.; Zhang, T.; Zhu, Z.; Li, Q.; Hu, C.; Yang, Y.; Hu, Z.; et al. Effects of a Molecular Monolayer Modification of NiO Nanocrystal Layer Surfaces on Perovskite Crystallization and Interface Contact toward Faster Hole Extraction and Higher Photovoltaic Performance. Adv. Funct. Mater. 2016, 26, 2950–2958. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Zuo, Z.; Zhang, M.; Zhao, Z.; Shen, Y.; Zhou, H.; Chen, Q.; Yang, Y.; Wang, M. Hole Selective NiO Contact for Efficient Perovskite Solar Cells with Carbon Electrode. Nano Lett. 2015, 15, 2402–2408. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Duan, X.; Rao, L.; Gong, C.; Fan, B.; Xing, Z.; Meng, X.; Xie, B.; Hu, X. Printable and Homogeneous NiOx Hole Transport Layers Prepared by a Polymer-Network Gel Method for Large-Area and Flexible Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2106495. [Google Scholar] [CrossRef]
- Ozturk, T.; Sarilmaz, A.; Akin, S.; Dursun, H.; Ozel, F.; Akman, E. Quinary Nanocrystal-Based Passivation Strategy for High Efficiency and Stable Perovskite Photovoltaics. Solar RRL 2022, 6, 2100737. [Google Scholar] [CrossRef]
- Ono, L.K.; Qi, Y. Surface and Interface Aspects of Organometal Halide Perovskite Materials and Solar Cells. J. Phys. Chem. Lett. 2016, 7, 4764–4794. [Google Scholar] [CrossRef]
- Correa Baena, J.P.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Srimath Kandada, A.R.; Zakeeruddin, S.M.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Akman, E.; Karapinar, H.S. Electrochemically stable, cost-effective and facile produced selenium@activated carbon composite counter electrodes for dye-sensitized solar cells. Solar Energy 2022, 234, 368–376. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Wang, L.; Wu, Y.; Tu, B.; Yu, B.; Liu, F.; Tam, H.-W.; Wang, G.; Djurišić, A.B.; et al. Molecule-Doped Nickel Oxide: Verified Charge Transfer and Planar Inverted Mixed Cation Perovskite Solar Cell. Adv. Mater. 2018, 30, 1800515. [Google Scholar] [CrossRef]
- Ru, P.; Bi, E.; Zhang, Y.; Wang, Y.; Kong, W.; Sha, Y.; Tang, W.; Zhang, P.; Wu, Y.; Chen, W.; et al. High Electron Affinity Enables Fast Hole Extraction for Efficient Flexible Inverted Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1903487. [Google Scholar] [CrossRef]
- Lian, X.; Chen, J.; Shan, S.; Wu, G.; Chen, H. Polymer Modification on the NiOx Hole Transport Layer Boosts Open-Circuit Voltage to 1.19 V for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 46340–46347. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, Y.; Chen, G.; Wu, Y.; Tu, B.; Liu, F.-Z.; Huang, L.; Ng, A.M.C.; Djurišić, A.B.; He, Z. Alkali Chlorides for the Suppression of the Interfacial Recombination in Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803872. [Google Scholar] [CrossRef]
- Phung, N.; Verheijen, M.; Todinova, A.; Datta, K.; Verhage, M.; Al-Ashouri, A.; Köbler, H.; Li, X.; Abate, A.; Albrecht, S.; et al. Enhanced Self-Assembled Monolayer Surface Coverage by ALD NiO in p-i-n Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 2166–2176. [Google Scholar] [CrossRef]
- Levine, I.; Al-Ashouri, A.; Musiienko, A.; Hempel, H.; Magomedov, A.; Drevilkauskaite, A.; Getautis, V.; Menzel, D.; Hinrichs, K.; Unold, T.; et al. Charge transfer rates and electron trapping at buried interfaces of perovskite solar cells. Joule 2021, 5, 2915–2933. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, R.; Zhang, Z.; Xiong, J.; He, Z.; Fan, B.; Dai, Z.; Yang, B.; Xue, X.; Cai, P.; et al. Achieving efficient inverted planar perovskite solar cells with nondoped PTAA as a hole transport layer. Org. Electron. 2019, 71, 106–112. [Google Scholar] [CrossRef]
- Niu, T.; Zhu, W.; Zhang, Y.; Xue, Q.; Jiao, X.; Wang, Z.; Xie, Y.-M.; Li, P.; Chen, R.; Huang, F.; et al. D-A-π-A-D-type Dopant-free Hole Transport Material for Low-Cost, Efficient, and Stable Perovskite Solar Cells. Joule 2021, 5, 249–269. [Google Scholar] [CrossRef]
- Choi, M.-J.; Lee, Y.-S.; Cho, I.H.; Kim, S.S.; Kim, D.-H.; Kwon, S.-N.; Na, S.-I. Functional additives for high-performance inverted planar perovskite solar cells with exceeding 20% efficiency: Selective complexation of organic cations in precursors. Nano Energy 2020, 71, 104639. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Sol. B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kwon, S.-N.; Yu, J.-H.; Na, S.-I. A systematic approach to ZnO nanoparticle-assisted electron transport bilayer for high efficiency and stable perovskite solar cells. J. Alloy. Compd. 2019, 801, 277–284. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Liu, X.; Wang, S.; Yan, Z.; Chen, L.; Li, G.; Zhang, X.; Sun, W.; Lan, Z. High-effective SnO2-based perovskite solar cells by multifunctional molecular additive engineering. J. Alloy. Compd. 2021, 886, 161352. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, H.; Liu, C.; Arain, Z.; Ding, Y.; Ma, S.; Liu, X.; Hayat, T.; Alsaedi, A.; Dai, S. Bi-functional additive engineering for high-performance perovskite solar cells with reduced trap density. J. Mater. Chem. A 2019, 7, 6450–6458. [Google Scholar] [CrossRef]
- Bube, R.H. Trap Density Determination by Space-Charge-Limited Currents. J. Appl. Phys. 1962, 33, 1733–1737. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).