How the Rigid and Deformable Image Registration Approaches Affect the Absorbed Dose Estimation Using Images Collected before and after Transarterial Radioembolization with 90Y Resin Microspheres in a Clinical Setting
Abstract
1. Introduction
2. Materials and Methods
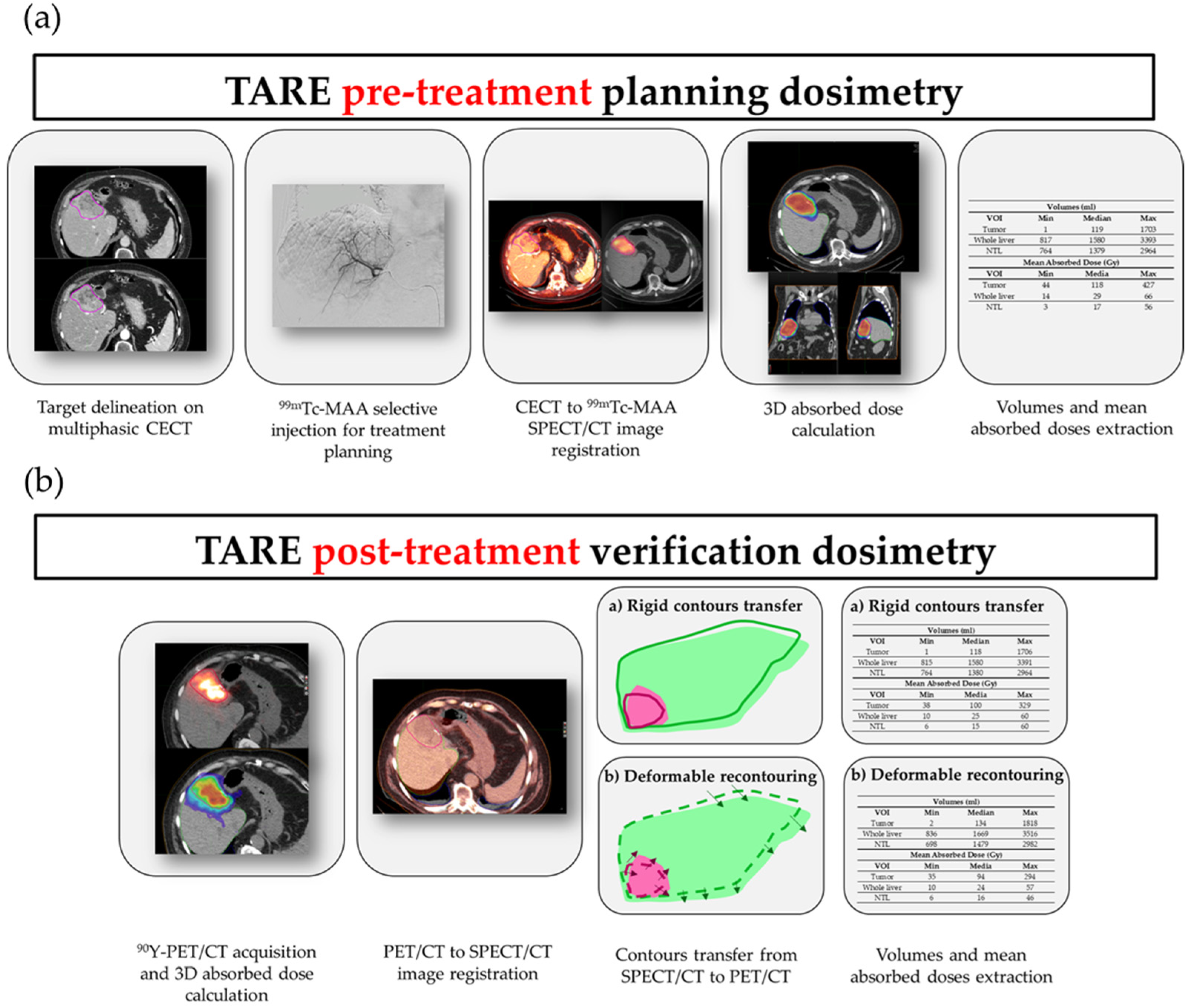
2.1. Planning and Verification Dosimetry Workflows
2.1.1. Patient Eligibility for TARE Treatment
2.1.2. Pre-Treatment Images and Angiographic Procedure
2.1.3. SPECT/CT Imaging
2.1.4. Pre-Treatment Dosimetry and Activity Determination
2.1.5. Post-Treatment Residual Activity Assessment
2.1.6. PET/CT Imaging
2.2. Voxel-Based Dosimetry Tools
2.3. Patients
2.4. Volumes and Mean Absorbed Dose Comparisons and Statistical Analysis
2.5. Survival Analysis
3. Results
3.1. Patient Cohort
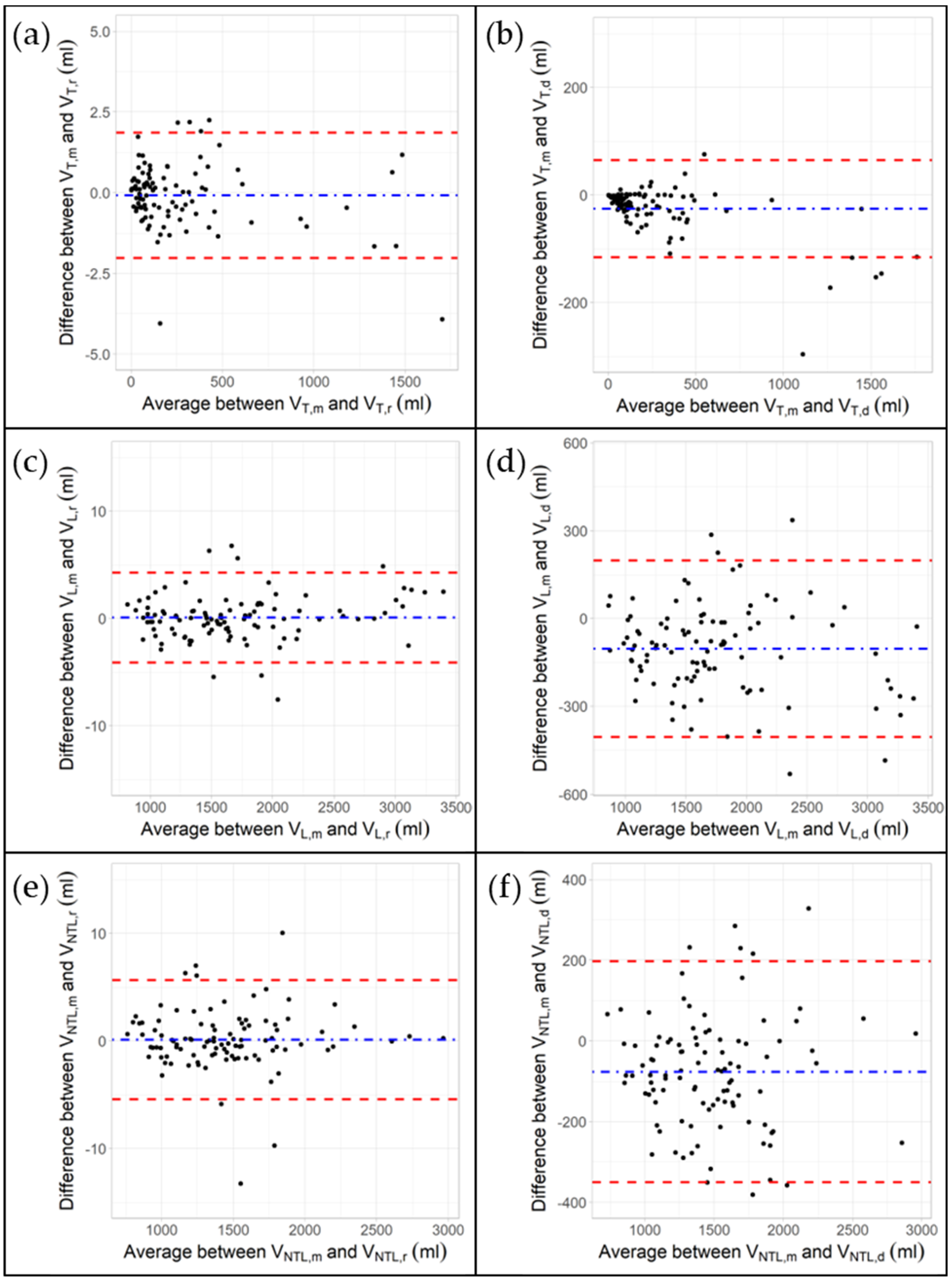
3.2. Impact of Rigid and Deformable Image Registration Algorithms on VOI Volumes
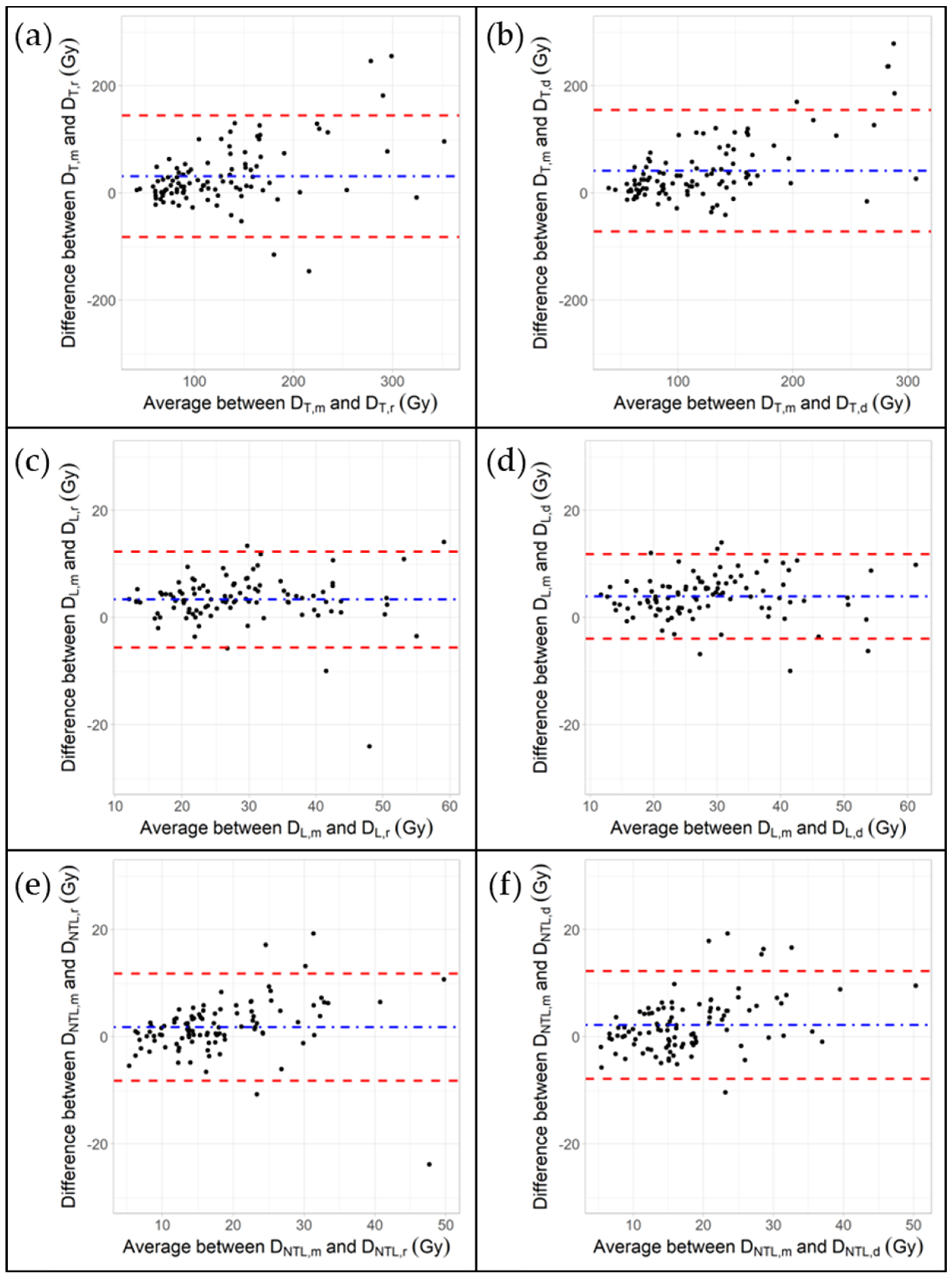
3.3. Impact of Rigid and Deformable Image Registration Algorithms on Mean Absorbed Doses
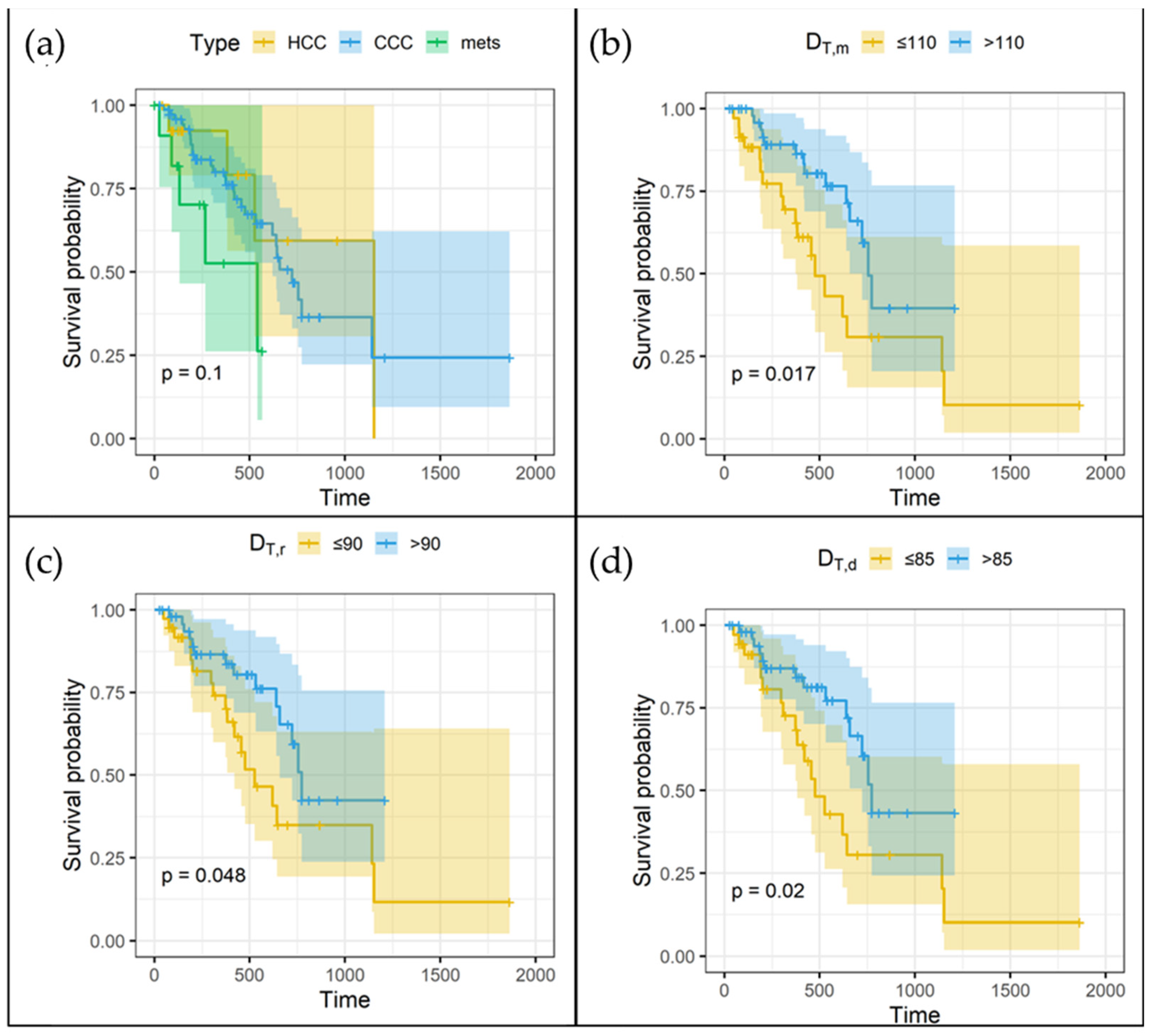
3.4. Overall Survival and Mean Absorbed Doses
4. Discussion
4.1. Bland–Altman Plots
4.2. Concordance Correlation Coefficients
4.3. Dose–Survival Relationship
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonné, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lam, M.; Chiesa, C.; Konijnenberg, M.; Cremonesi, M.; Flamen, P.; Gnesin, S.; Bodei, L.; Kracmerova, T.; Luster, M.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Padia, S.A.; Lam, M.; Bell, J.; Chiesa, C.; Fowers, K.; Hamilton, B.; Herman, J.; Kappadath, S.C.; Leung, T.; et al. Clinical and dosimetric considerations for Y90: Recommendations from an international multidisciplinary working group. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, M.; Chiesa, C.; Strigari, L.; Ferrari, M.; Botta, F.; Guerriero, F.; De Cicco, C.; Bonomo, G.; Orsi, F.; Bodei, L.; et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front. Oncol. 2014, 4, 210. [Google Scholar] [CrossRef]
- Braat, A.J.A.T.; Prince, J.F.; van Rooij, R.; Bruijnen, R.C.G.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: A cohort study. Eur. Radiol. 2018, 28, 920–928. [Google Scholar] [CrossRef]
- Della Gala, G.; Bardiès, M.; Tipping, J.; Strigari, L. Overview of commercial treatment planning systems for targeted radionuclide therapy. Phys. Med. 2021, 92, 52–61. [Google Scholar] [CrossRef]
- Nodari, G.; Popoff, R.; Riedinger, J.M.; Lopez, O.; Pellegrinelli, J.; Dygai-Cochet, I.; Tabouret-Viaud, C.; Presles, B.; Chevallier, O.; Gehin, S.; et al. Impact of contouring methods on pre-treatment and post-treatment dosimetry for the prediction of tumor control and survival in HCC patients treated with selective internal radiation therapy. EJNMMI Res. 2021, 11, 24. [Google Scholar] [CrossRef]
- Meyers, N.; Jadoul, A.; Bernard, C.; Delwaide, J.; Lamproye, A.; Detry, O.; Honoré, P.; Gerard, L.; Hustinx, R. Inter-observer variability of (90)Y PET/CT dosimetry in hepatocellular carcinoma after glass microspheres transarterial radioembolization. EJNMMI Phys. 2020, 7, 29. [Google Scholar] [CrossRef]
- Kyme, A.Z.; Fulton, R.R. Motion estimation and correction in SPECT, PET and CT. Phys. Med. Biol. 2021, 66, 18TR02. [Google Scholar] [CrossRef]
- Morán, V.; Prieto, E.; Sancho, L.; Rodríguez-Fraile, M.; Soria, L.; Zubiria, A.; Martí-Climent, J.M. Impact of the dosimetry approach on the resulting (90)Y radioembolization planned absorbed doses based on (99m)Tc-MAA SPECT-CT: Is there agreement between dosimetry methods? EJNMMI Phys. 2020, 7, 72. [Google Scholar] [CrossRef]
- Pasciak, A.S.; Erwin, W.D. Effect of voxel size and computation method on Tc-99m MAA SPECT/CT-based dose estimation for Y-90 microsphere therapy. IEEE Trans. Med. Imaging 2009, 28, 1754–1758. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Pimpinella, M.; Capogni, M.; De Coste, V.; Filippi, L.; Spezi, E.; Patterson, N.; Mariotti, F.; Ferrari, P.; Chiaramida, P.; et al. Phantom validation of quantitative Y-90 PET/CT-based dosimetry in liver radioembolization. EJNMMI Res. 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Knešaurek, K. Comparison of posttherapy (90)Y positron emission tomography/computed tomography dosimetry methods in liver therapy with (90)Y microspheres. World J. Nucl. Med. 2020, 19, 359–365. [Google Scholar] [CrossRef]
- Hermann, A.L.; Dieudonné, A.; Ronot, M.; Sanchez, M.; Pereira, H.; Chatellier, G.; Garin, E.; Castera, L.; Lebtahi, R.; Vilgrain, V. Relationship of Tumor Radiation-absorbed Dose to Survival and Response in Hepatocellular Carcinoma Treated with Transarterial Radioembolization with (90)Y in the SARAH Study. Radiology 2020, 296, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Richetta, E.; Pasquino, M.; Poli, M.; Cutaia, C.; Valero, C.; Tabone, M.; Paradisi, B.P.; Pacilio, M.; Pellerito, R.E.; Stasi, M. PET-CT post therapy dosimetry in radioembolization with resin (90)Y microspheres: Comparison with pre-treatment SPECT-CT (99m)Tc-MAA results. Phys. Med. 2019, 64, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kafrouni, M.; Allimant, C.; Fourcade, M.; Vauclin, S.; Guiu, B.; Mariano-Goulart, D.; Ben Bouallègue, F. Analysis of differences between (99m)Tc-MAA SPECT- and (90)Y-microsphere PET-based dosimetry for hepatocellular carcinoma selective internal radiation therapy. EJNMMI Res. 2019, 9, 62. [Google Scholar] [CrossRef]
- Jadoul, A.; Bernard, C.; Lovinfosse, P.; Gérard, L.; Lilet, H.; Cornet, O.; Hustinx, R. Comparative dosimetry between (99m)Tc-MAA SPECT/CT and (90)Y PET/CT in primary and metastatic liver tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 828–837. [Google Scholar] [CrossRef]
- d’Abadie, P.; Walrand, S.; Hesse, M.; Amini, N.; Lhommel, R.; Sawadogo, K.; Jamar, F. Accurate non-tumoral 99mTc-MAA absorbed dose prediction to plan optimized activities in liver radioembolization using resin microspheres. Phys. Med. 2021, 89, 250–257. [Google Scholar] [CrossRef]
- Gramenzi, A.; Golfieri, R.; Mosconi, C.; Cappelli, A.; Granito, A.; Cucchetti, A.; Marinelli, S.; Pettinato, C.; Erroi, V.; Fiumana, S.; et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: A cohort study with propensity score analysis. Liver Int. 2015, 35, 1036–1047. [Google Scholar] [CrossRef]
- Kim, S.P.; Cohalan, C.; Kopek, N.; Enger, S.A. A guide to (90)Y radioembolization and its dosimetry. Phys. Med. 2019, 68, 132–145. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Haslerud, T.; Reichmann, K.; Meyer, C.; Habibi, E.; Fimmers, R.; Muckle, M.; Sabet, A.; Biersack, H.J.; Ezziddin, S. Residual activity after radioembolization of liver tumours with 90Y resin microspheres. A safe calculation method. Nuklearmedizin 2014, 53, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gehealthcare.com/en-sg/-/jssmedia/widen/2018/01/25/0204/gehealthcarecom/migrated/2018/03/22/0155/ocuments-us-global-products-pet-ct-whitepaper-q-clear-ge-healthcare-white-paper_qclear_pdf.pdf?rev=-1 (accessed on 30 November 2022).
- Rowley, L.M.; Bradley, K.M.; Boardman, P.; Hallam, A.; McGowan, D.R. Optimization of Image Reconstruction for (90)Y Selective Internal Radiotherapy on a Lutetium Yttrium Orthosilicate PET/CT System Using a Bayesian Penalized Likelihood Reconstruction Algorithm. J. Nucl. Med. 2017, 58, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ma, H.; Esquinas, P.L.; Uribe, C.F.; Tolhurst, S.; Bénard, F.; Liu, D.; Rahmim, A.; Celler, A. Impact of image reconstruction method on dose distributions derived from(90)Y PET images: Phantom and liver radioembolization patient studies. Phys. Med. Biol. 2020, 65, 215022. [Google Scholar] [CrossRef]
- Calusi, S.; Labanca, G.; Zani, M.; Casati, M.; Marrazzo, L.; Noferini, L.; Talamonti, C.; Fusi, F.; Desideri, I.; Bonomo, P.; et al. A multiparametric method to assess the MIM deformable image registration algorithm. J. Appl. Clin. Med. Phys. 2019, 20, 75–82. [Google Scholar] [CrossRef]
- Brosch, J.; Gosewisch, A.; Kaiser, L.; Seidensticker, M.; Ricke, J.; Zellmer, J.; Bartenstein, P.; Ziegler, S.; Ilhan, H.; Todica, A.; et al. 3D image-based dosimetry for Yttrium-90 radioembolization of hepatocellular carcinoma: Impact of imaging method on absorbed dose estimates. Phys. Med. 2020, 80, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Rolland, Y.; Edeline, J. (90)Y-Loaded Microsphere SIRT of HCC Patients with Portal Vein Thrombosis: High Clinical Impact of 99mTc-MAA SPECT/CT-Based Dosimetry. Semin. Nucl. Med. 2019, 49, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Allimant, C.; Kafrouni, M.; Delicque, J.; Ilonca, D.; Cassinotto, C.; Assenat, E.; Ursic-Bedoya, J.; Pageaux, G.P.; Mariano-Goulart, D.; Aho, S.; et al. Tumor Targeting and Three-Dimensional Voxel-Based Dosimetry to Predict Tumor Response, Toxicity, and Survival after Yttrium-90 Resin Microsphere Radioembolization in Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 1662–1670.e4. [Google Scholar] [CrossRef]
- Kao, Y.H.; Steinberg, J.D.; Tay, Y.S.; Lim, G.K.; Yan, J.; Townsend, D.W.; Budgeon, C.A.; Boucek, J.A.; Francis, R.J.; Cheo, T.S.; et al. Post-radioembolization yttrium-90 PET/CT—Part 2: Dose-response and tumor predictive dosimetry for resin microspheres. EJNMMI Res. 2013, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Sciuto, R.; Rea, S.; Carpanese, L.; Pizzi, G.; Soriani, A.; Iaccarino, G.; Benassi, M.; Ettorre, G.M.; Maini, C.L. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: Radiobiologic considerations. J. Nucl. Med. 2010, 51, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Guiu, B.; Edeline, J.; Rolland, Y.; Palard, X. Trans-arterial Radioembolization Dosimetry in 2022. CardioVasc. Interv. Radiol. 2022, 45, 1608–1621. [Google Scholar] [CrossRef]
- Walrand, S.; Hesse, M.; Chiesa, C.; Lhommel, R.; Jamar, F. The low hepatic toxicity per Gray of 90Y glass microspheres is linked to their transport in the arterial tree favoring a nonuniform trapping as observed in posttherapy PET imaging. J. Nucl. Med. 2014, 55, 135–140. [Google Scholar] [CrossRef][Green Version]
- van Roekel, C.; Bastiaannet, R.; Smits, M.L.J.; Bruijnen, R.C.; Braat, A.; de Jong, H.; Elias, S.G.; Lam, M. Dose-Effect Relationships of (166)Ho Radioembolization in Colorectal Cancer. J. Nucl. Med. 2021, 62, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.L.J.; Dassen, M.G.; Prince, J.F.; Braat, A.; Beijst, C.; Bruijnen, R.C.G.; de Jong, H.; Lam, M. The superior predictive value of (166)Ho-scout compared with (99m)Tc-macroaggregated albumin prior to (166)Ho-microspheres radioembolization in patients with liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Mahvash, A.; Abdelsalam, M.; Kaseb, A.O.; Kappadath, S.C. Planning dosimetry for (90) Y radioembolization with glass microspheres: Evaluating the fidelity of (99m) Tc-MAA and partition model predictions. Med. Phys. 2020, 47, 5333–5342. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Della Gala, G.; Paolani, G.; Zagni, F.; Strolin, S.; Civollani, S.; Calderoni, L.; Cappelli, A.; Mosconi, C.; Lodi Rizzini, E.; et al. A novel tool for motion-related dose inaccuracies reduction in (99m)Tc-MAA SPECT/CT images for SIRT planning. Phys. Med. 2022, 98, 98–112. [Google Scholar] [CrossRef]
- van der Vos, C.S.; Koopman, D.; Rijnsdorp, S.; Arends, A.J.; Boellaard, R.; van Dalen, J.A.; Lubberink, M.; Willemsen, A.T.M.; Visser, E.P. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 4–16. [Google Scholar] [CrossRef]
- Sanders, J.C.; Ritt, P.; Kuwert, T.; Vija, A.H.; Maier, A.K. Fully Automated Data-Driven Respiratory Signal Extraction from SPECT Images Using Laplacian Eigenmaps. IEEE Trans. Med. Imaging 2016, 35, 2425–2435. [Google Scholar] [CrossRef]
- Robert, A.; Rit, S.; Baudier, T.; Jomier, J.; Sarrut, D. Data-Driven Respiration-Gated SPECT for Liver Radioembolization. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 778–787. [Google Scholar] [CrossRef]

| VOI | Min Volume (mL) | Median Volume (mL) | Max Volume (mL) |
|---|---|---|---|
| Treated tumor | 1.3 | 118.5 | 1702.9 |
| Whole liver | 816.8 | 1580.2 | 3393.3 |
| NTL | 764.2 | 1379.3 | 2964.1 |
| Var.1 | Var.2 | ICC § | Pearson § | CCCo (95% CI) |
|---|---|---|---|---|
| DT,r | DT,d. | 0.875 | 0.908 | 0.875 (0.826; 0.911) |
| DT,m | DT,r | 0.590 | 0.707 | 0.605 (0.491; 0.699) |
| DT,m | DT,d | 0.486 | 0.721 | 0.524 (0.418; 0.617) |
| VT,r | VT,d | 0.990 | 0.995 | 0.990 (0.986; 0.993) |
| VT,m | VT,r | 1.000 | 1.000 | 1.000 (1.000; 1.000) |
| VT,m | VT,d | 0.990 | 0.995 | 0.990 (0.986; 0.992) |
| DNTL,r | DNTL,d. | 0.869 | 0.876 | 0.790 (0.713; 0.846) |
| DNTL,m | DNTL,r | 0.820 | 0.843 | 0.821 (0.748; 0.873) |
| DNTL,m | DNTL,d | 0.787 | 0.842 | 0.789 (0.713; 0.846) |
| VNTL,r | VNTL,d | 0.927 | 0.940 | 0.927 (0.894; 0.950) |
| VNTL,m | VNTL,r | 0.996 | 0.996 | 0.996 (0.994; 0.997) |
| VNTL,m | VNTL,d | 0.929 | 0.944 | 0.929 (0.897; 0.951) |
| DL,r | DL,d. | 0.930 | 0.931 | 0.929 (0.897; 0.952) |
| DL,m | DL,r | 0.860 | 0.907 | 0.862 (0.807; 0.903) |
| DL,m | DL,d | 0.859 | 0.925 | 0.863 (0.810; 0.902) |
| VL,r | VL,d | 0.959 | 0.971 | 0.959 (0.941; 0.972) |
| VL,m | VL,r | 0.998 | 0.998 | 0.998 (0.997; 0.999) |
| VL,m | VL,d | 0.957 | 0.971 | 0.957 (0.938–0.971) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Gala, G.; Santoro, M.; Paolani, G.; Strolin, S.; Cappelli, A.; Mosconi, C.; Lodi Rizzini, E.; Strigari, L. How the Rigid and Deformable Image Registration Approaches Affect the Absorbed Dose Estimation Using Images Collected before and after Transarterial Radioembolization with 90Y Resin Microspheres in a Clinical Setting. Appl. Sci. 2022, 12, 12767. https://doi.org/10.3390/app122412767
Della Gala G, Santoro M, Paolani G, Strolin S, Cappelli A, Mosconi C, Lodi Rizzini E, Strigari L. How the Rigid and Deformable Image Registration Approaches Affect the Absorbed Dose Estimation Using Images Collected before and after Transarterial Radioembolization with 90Y Resin Microspheres in a Clinical Setting. Applied Sciences. 2022; 12(24):12767. https://doi.org/10.3390/app122412767
Chicago/Turabian StyleDella Gala, Giuseppe, Miriam Santoro, Giulia Paolani, Silvia Strolin, Alberta Cappelli, Cristina Mosconi, Elisa Lodi Rizzini, and Lidia Strigari. 2022. "How the Rigid and Deformable Image Registration Approaches Affect the Absorbed Dose Estimation Using Images Collected before and after Transarterial Radioembolization with 90Y Resin Microspheres in a Clinical Setting" Applied Sciences 12, no. 24: 12767. https://doi.org/10.3390/app122412767
APA StyleDella Gala, G., Santoro, M., Paolani, G., Strolin, S., Cappelli, A., Mosconi, C., Lodi Rizzini, E., & Strigari, L. (2022). How the Rigid and Deformable Image Registration Approaches Affect the Absorbed Dose Estimation Using Images Collected before and after Transarterial Radioembolization with 90Y Resin Microspheres in a Clinical Setting. Applied Sciences, 12(24), 12767. https://doi.org/10.3390/app122412767








