Hamstrings on Morphological Structure Characteristics, Stress Features, and Risk of Injuries: A Narrative Review
Abstract
1. Introduction
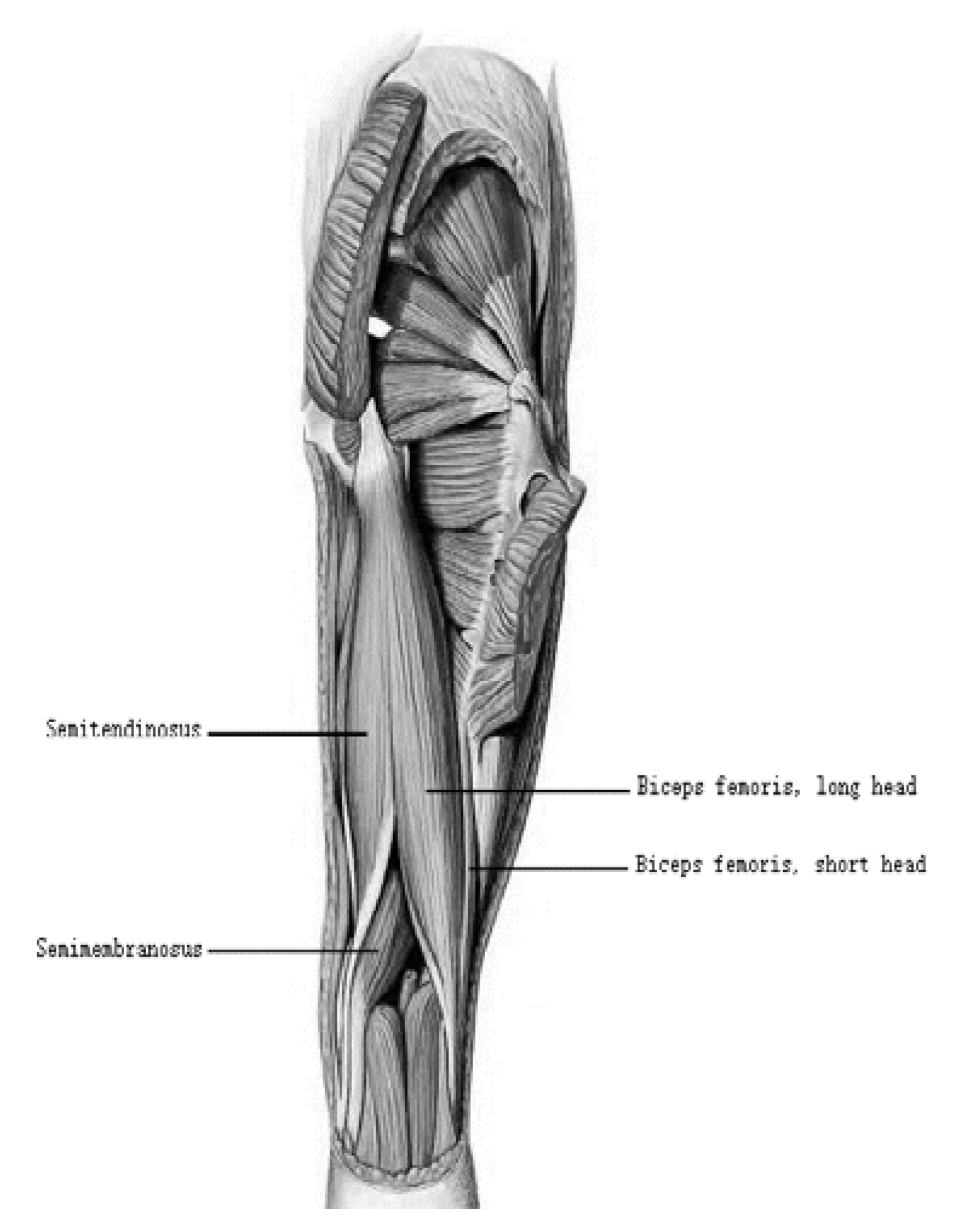
2. The Morphological Structure of Hamstrings
2.1. Morphological Diversity of the Tendons of Hamstrings
2.2. Connected Pattern Diversity of Hamstring
3. Transfer of Force among Muscles, Tendons, and Bone
3.1. The Transfer of Force between Muscle Fibers and Tendons
3.2. The Transfer of Force between Tendons and Bone
4. Possible Performance of Motor Control during Hamstrings Acute Strain
5. Differential Diagnosis
5.1. Typical Symptoms of Hamstring Strain Injuries
5.2. Grade of Hamstring Strain Injury
6. Risk Factors and Prevention for Hamstring Injury
7. Review Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekstrand, J.; Hägglund, M.; Waldén, M. Epidemiology of muscle injuries in professional football (soccer). Am. J. Sport. Med. 2011, 39, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.L.; Kerr, Z.Y.; Dompier, T.P. Epidemiology of hamstring strains in 25 NCAA sports in the 2009–2010 to 2013–2014 academic years. Am. J. Sport. Med. 2015, 43, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.N.; Sherry, M.A. Rehabilitation and return to sport after hamstring strain injury. J. Sport Health Sci. 2017, 6, 262–270. [Google Scholar] [CrossRef]
- Sherry, M. Examination and treatment of hamstring related injuries. Sport. Health 2012, 4, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Opar, D.A.; Drezner, J.; Shield, A.; Williams, M.; Webner, D.; Sennett, B.; Kapur, R.; Cohen, M.; Ulager, J.; Cafengiu, A. Acute hamstring strain injury in track-and-field athletes: A 3-year observational study at the P enn R elay C arnival. Scand. J. Med. Sci. Sport. 2014, 24, e254–e259. [Google Scholar] [CrossRef]
- Wan, X.L.; Qu, F.; Garrett, W.E.; Liu, H.; Yu, B. Relationships among hamstring muscle optimal length and hamstring flexibility and strength. J. Sport Health Sci. 2017, 6, 275–282. [Google Scholar] [CrossRef]
- Yu, B.; Li, L. Research in prevention and rehabilitation of hamstring muscle strain injury. J. Sport Health Sci. 2017, 6, 253. [Google Scholar] [CrossRef]
- Wan, X.; Qu, F.; Garrett, W.E.; Liu, H.; Yu, B. The effect of hamstring flexibility on peak hamstring muscle strain in sprinting. J. Sport Health Sci. 2017, 6, 283–289. [Google Scholar] [CrossRef]
- Wangensteen, A. Acute Hamstring Injuries: Diagnosis and Prognosis. Ph.D. Thesis. 2018. Available online: https://ostrc.no/globalas-sets/publications/phd-thesis/wangensteen_2018_phd-thesis.pdf (accessed on 2 September 2022).
- Schroeter, S.; Heiss, R.; Hammer, C.M.; Grim, C.; Engelhardt, M.; Hotfiel, T. Diagnosis of Proximal Hamstring Injuries. Sport. Orthop. Traumatol. 2022, 38, 47–57. [Google Scholar] [CrossRef]
- Jokela, A.; Stenroos, A.; Kosola, J.; Valle, X.; Lempainen, L. A systematic review of surgical intervention in the treatment of hamstring tendon ruptures: Current evidence on the impact on patient outcomes. Ann. Med. 2022, 54, 978–988. [Google Scholar] [CrossRef]
- Ekstrand, J.; Healy, J.C.; Waldén, M.; Lee, J.C.; English, B.; Hägglund, M. Hamstring muscle injuries in professional football: The correlation of MRI findings with return to play. Br. J. Sport. Med. 2012, 46, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Askling, C.M.; Tengvar, M.; Thorstensson, A. Acute hamstring injuries in Swedish elite football: A prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br. J. Sport. Med. 2013, 47, 953–959. [Google Scholar] [CrossRef]
- Kenneally-Dabrowski, C.J.; Brown, N.A.; Lai, A.K.; Perriman, D.; Spratford, W.; Serpell, B.G. Late swing or early stance? A narrative review of hamstring injury mechanisms during high-speed running. Scand. J. Med. Sci. Sport. 2019, 29, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Grange, S.; Reurink, G.; Nguyen, A.Q.; Riviera-Navarro, C.; Foschia, C.; Croisille, P.; Edouard, P. Location of Hamstring Injuries Based on Magnetic Resonance Imaging: A Systematic Review. Sport. Health 2022, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Hirata, K. Site-specific features of active muscle stiffness and proximal aponeurosis strain in biceps femoris long head. Scand J. Med. Sci. Sport. 2021, 31, 1666–1673. [Google Scholar] [CrossRef]
- Silder, A.; Thelen, D.G.; Heiderscheit, B.C. Effects of prior hamstring strain injury on strength, flexibility, and running mechanics. Clin. Biomech. 2010, 25, 681–686. [Google Scholar] [CrossRef]
- Koulouris, G.; Connell, D. Hamstring muscle complex: An imaging review. Radiographics 2005, 25, 571–586. [Google Scholar] [CrossRef]
- Chumanov, E.S.; Heiderscheit, B.C.; Thelen, D.G. Hamstring musculotendon dynamics during stance and swing phases of high-speed running. Med. Sci. Sport. Exerc. 2011, 43, 525–532. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, S.; Zhong, Y.; Fu, W.; Li, L.; Liu, Y. How joint torques affect hamstring injury risk in sprinting swing–stance transition. Med. Sci. Sport. Exerc. 2015, 47, 373. [Google Scholar] [CrossRef]
- Schache, A.G.; Brown, N.A.; Pandy, M.G. Modulation of work and power by the human lower-limb joints with increasing steady-state locomotion speed. J. Exp. Biol. 2015, 218, 2472–2481. [Google Scholar] [CrossRef]
- Nagahara, R.; Matsubayashi, T.; Matsuo, A.; Zushi, K. Alteration of swing leg work and power during human accelerated sprinting. Biol. Open 2017, 6, 633–641. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Zhu, W.; Yu, J. The late swing and early stance of sprinting are most hazardous for hamstring injuries. J. Sport Health Sci. 2017, 6, 133. [Google Scholar] [CrossRef]
- Danielsson, A.; Horvath, A.; Senorski, C.; Alentorn-Geli, E.; Garrett, W.E.; Cugat, R.; Samuelsson, K.; Hamrin Senorski, E. The mechanism of hamstring injuries—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 641. [Google Scholar] [CrossRef]
- Li, L.; Wang, D. Parallel and cross-sectional hamstring injuries in sprint running. J. Sport Health Sci. 2017, 6, 141. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy E-Book: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. JBJS 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Schweitzer, R.; Zelzer, E.; Volk, T. Connecting muscles to tendons: Tendons and musculoskeletal development in flies and vertebrates. Development 2010, 137, 2807–2817. [Google Scholar] [CrossRef]
- Zelzer, E.; Blitz, E.; Killian, M.L.; Thomopoulos, S. Tendon-to-bone attachment: From development to maturity. Birth Defects Res. C Embryo Today 2014, 102, 101–112. [Google Scholar] [CrossRef]
- Benjamin, M.; Toumi, H.; Ralphs, J.; Bydder, G.; Best, T.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef]
- Nordin, M.; Frankel, V.H. Basic Biomechanics of the Musculoskeletal System; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Rehorn, M.R.; Blemker, S.S. The effects of aponeurosis geometry on strain injury susceptibility explored with a 3D muscle model. J. Biomech. 2010, 43, 2574–2581. [Google Scholar] [CrossRef]
- van der Made, A.D.; Wieldraaijer, T.; Kerkhoffs, G.; Kleipool, R.; Engebretsen, L.; Van Dijk, C.; Golanó, P. The hamstring muscle complex. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 2115–2122. [Google Scholar] [CrossRef]
- Battermann, N.; Appell, H.-J.; Dargel, J.; Koebke, J. An anatomical study of the proximal hamstring muscle complex to elucidate muscle strains in this region. Int. J. Sport. Med. 2011, 32, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Chleboun, G.S.; France, A.R.; Crill, M.T.; Braddock, H.K.; Howell, J.N. In vivo measurement of fascicle length and pennation angle of the human biceps femoris muscle. Cells Tissues Organs 2001, 169, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Garrett Jr, W.E.; Rich, F.R.; Nikolaou, P.K.; Vogler, J. Computed tomography of hamstring muscle strains. Med. Sci. Sport. Exerc. 1989, 21, 506–514. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Marquez, J.P.; Weinberger, B.; Birman, V.; Genin, G.M. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J. Biomech. 2006, 39, 1842–1851. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Genin, G.M.; Galatz, L.M. The development and morphogenesis of the tendon-to-bone insertion—What development can teach us about healing. J. Musculoskelet. Neuronal Interact. 2010, 10, 35–45. [Google Scholar]
- Deymier, A.C.; An, Y.; Boyle, J.J.; Schwartz, A.G.; Birman, V.; Genin, G.M.; Thomopoulos, S.; Barber, A.H. Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater. 2017, 56, 25–35. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Liu, X.; Zhao, X. Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. USA 2019, 116, 10244–10249. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181. [Google Scholar]
- Knudsen, A.B.; Larsen, M.; Mackey, A.L.; Hjort, M.; Hansen, K.K.; Qvortrup, K.; Kjaer, M.; Krogsgaard, M.R. The human myotendinous junction: An ultrastructural and 3D analysis study. Scand. J. Med. Sci. Sport. 2015, 25, e116–e123. [Google Scholar] [CrossRef]
- Jakobsen, J.R.; Krogsgaard, M.R. The Myotendinous Junction-A Vulnerable Companion in Sports. A Narrative Review. Front. Physiol. 2021, 12, 635561. [Google Scholar] [CrossRef]
- Woo, S.L.Y.; Buckwalter, J.A. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, 18–20 June 1987. J. Orthop. Res. 1988, 6, 907–931. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Veeramani, R.; Karuppusamy, A. Morphometric study of pulleys of the thumb. Anat. Cell Biol. 2018, 51, 71–78. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Kalimo, H.; Jarvinen, M. Muscle injuries: Biology and treatment. Am. J. Sport. Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Takala, T.E.; Virtanen, P. Biochemical composition of muscle extracellular matrix: The effect of loading. Scand. J. Med. Sci. Sport. 2000, 10, 321–325. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Wisdom, K.M.; Delp, S.L.; Kuhl, E. Use it or lose it: Multiscale skeletal muscle adaptation to mechanical stimuli. Biomech. Model. Mechanobiol. 2015, 14, 195–215. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Williams, G.R.; Gimbel, J.A.; Favata, M.; Soslowsky, L.J. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res. 2003, 21, 413–419. [Google Scholar] [CrossRef]
- Lieber, R.L.; Ward, S.R. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am. J. Physiol. -Cell Physiol. 2013, 305, C241–C252. [Google Scholar] [CrossRef]
- Kernell, D. Muscle regionalization. Can. J. Appl. Physiol. 1998, 23, 1–22. [Google Scholar] [CrossRef]
- Schneider-Kolsky, M.E.; Hoving, J.L.; Warren, P.; Connell, D.A. A comparison between clinical assessment and magnetic resonance imaging of acute hamstring injuries. Am. J. Sport. Med. 2006, 34, 1008–1015. [Google Scholar] [CrossRef]
- Verrall, G.M.; Slavotinek, J.P.; Barnes, P.G.; Fon, G.T. Diagnostic and prognostic value of clinical findings in 83 athletes with posterior thigh injury: Comparison of clinical findings with magnetic resonance imaging documentation of hamstring muscle strain. Am. J. Sport. Med. 2003, 31, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Koulouris, G.; Connell, D. Evaluation of the hamstring muscle complex following acute injury. Skelet. Radiol. 2003, 32, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 2011, 1, 2029–2062. [Google Scholar] [PubMed]
- Hamilton, B.; Alonso, J.M.; Best, T.M. Time for a paradigm shift in the classification of muscle injuries. J. Sport Health Sci. 2017, 6, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Xia, Y.; Galatz, L.M.; Genin, G.M.; Thomopoulos, S. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect. Tissue Res. 2012, 53, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.F.; Li, L.; Chen, C.; Wu, X. Stretch Could Reduce Hamstring Injury Risk during Sprinting by Right Shifting the Length-Torque Curve. J. Strength Cond. Res. 2018, 32, 2190–2198. [Google Scholar] [CrossRef]
- Egger, A.C.; Berkowitz, M.J. Achilles tendon injuries. Curr. Rev. Musculoskelet. Med. 2017, 10, 72–80. [Google Scholar] [CrossRef]
- Kuske, B.; Hamilton, D.F.; Pattle, S.B.; Simpson, A.H. Patterns of Hamstring Muscle Tears in the General Population: A Systematic Review. PLoS ONE 2016, 11, e0152855. [Google Scholar] [CrossRef]
- Woodley, S.J.; Mercer, S.R. Hamstring muscles: Architecture and innervation. Cells Tissues Organs 2005, 179, 125–141. [Google Scholar] [CrossRef]
- Beltran, L.; Ghazikhanian, V.; Padron, M.; Beltran, J. The proximal hamstring muscle-tendon-bone unit: A review of the normal anatomy, biomechanics, and pathophysiology. Eur. J. Radiol. 2012, 81, 3772–3779. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Venkadesan, M.; Werbel, W.A.; Daoud, A.I.; D’Andrea, S.; Davis, I.S.; Mang’eni, R.O.; Pitsiladis, Y. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature 2010, 463, 531–535. [Google Scholar] [CrossRef]
- Macadam, P.; Cronin, J.B.; Uthoff, A.M.; Johnston, M.; Knicker, A.J. Role of Arm Mechanics During Sprint Running: A Review of the Literature and Practical Applications. Strength Cond. J. 2018, 40, 14–23. [Google Scholar] [CrossRef]
- Degen, R.M. Proximal Hamstring Injuries: Management of Tendinopathy and Avulsion Injuries. Curr. Rev. Musculoskelet. Med. 2019, 12, 138–146. [Google Scholar] [CrossRef]
- van Beijsterveldt, A.M.; van de Port, I.G.; Vereijken, A.J.; Backx, F.J. Risk factors for hamstring injuries in male soccer players: A systematic review of prospective studies. Scand. J. Med. Sci. Sport. 2013, 23, 253–262. [Google Scholar] [CrossRef]
- Martin, R.L.; Cibulka, M.T.; Bolgla, L.A.; Koc, T.A., Jr.; Loudon, J.K.; Manske, R.C.; Weiss, L.; Christoforetti, J.J.; Heiderscheit, B.C. Hamstring Strain Injury in Athletes. J. Orthop. Sport. Phys. Ther. 2022, 52, CPG1–CPG44. [Google Scholar] [CrossRef]
- Orchard, J.W. Hamstrings are most susceptible to injury during the early stance phase of sprinting. Br. J. Sport. Med. 2012, 46, 88–89. [Google Scholar] [CrossRef][Green Version]
- Arnold, A.S.; Thelen, D.G.; Schwartz, M.H.; Anderson, F.C.; Delp, S.L. Muscular coordination of knee motion during the terminal-swing phase of normal gait. J. Biomech. 2007, 40, 3314–3324. [Google Scholar] [CrossRef][Green Version]
- Thelen, D.G.; Chumanov, E.S.; Hoerth, D.M.; Best, T.M.; Swanson, S.C.; Li, L.; Young, M.; Heiderscheit, B.C. Hamstring muscle kinematics during treadmill sprinting. Med. Sci. Sport. Exerc. 2005, 37, 108–114. [Google Scholar] [CrossRef]
- Chu, S.K.; Rho, M.E. Hamstring injuries in the athlete: Diagnosis, treatment, and return to play. Curr. Sport. Med. Rep. 2016, 15, 184. [Google Scholar] [CrossRef]
- Askling, C.M.; Tengvar, M.; Saartok, T.; Thorstensson, A. Proximal hamstring strains of stretching type in different sports: Injury situations, clinical and magnetic resonance imaging characteristics, and return to sport. Am. J. Sport. Med. 2008, 36, 1799–1804. [Google Scholar] [CrossRef]
- Askling, C.M.; Tengvar, M.; Saartok, T.; Thorstensson, A. Acute first-time hamstring strains during slow-speed stretching: Clinical, magnetic resonance imaging, and recovery characteristics. Am. J. Sport. Med. 2007, 35, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Guex, K.; Gojanovic, B.; Millet, G.P. Influence of hip-flexion angle on hamstrings isokinetic activity in sprinters. J. Athl. Train. 2012, 47, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Tim, T.; McHugh, M. Hamstring injury rehabilitation and prevention of reinjury using lengthened state eccentric training: A new concept. Int. J. Sport. Phys. Ther. 2012, 7, 333. [Google Scholar]
- Chinn, L.; Hertel, J. Rehabilitation of ankle and foot injuries in athletes. Clin. Sport. Med. 2010, 29, 157. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.W.; Hartsell, H.D. Factors contributing to chronic ankle instability: A strength perspective. J. Athl. Train. 2002, 37, 394. [Google Scholar] [PubMed]
- Schache, A.G.; Dorn, T.W.; Blanch, P.D.; Brown, N.A.; Pandy, M.G. Mechanics of the human hamstring muscles during sprinting. Med. Sci. Sport. Exerc. 2012, 44, 647–658. [Google Scholar] [CrossRef]
- Morin, J.B.; Gimenez, P.; Edouard, P.; Arnal, P.; Jimenez-Reyes, P.; Samozino, P.; Brughelli, M.; Mendiguchia, J. Sprint Acceleration Mechanics: The Major Role of Hamstrings in Horizontal Force Production. Front. Physiol. 2015, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Croisier, J.-L. Factors associated with recurrent hamstring injuries. Sport. Med. 2004, 34, 681–695. [Google Scholar] [CrossRef]
- Kalema, R.N.; Duhig, S.J.; Williams, M.D.; Donaldson, A.; Shield, A.J. Sprinting technique and hamstring strain injuries: A concept mapping study. J. Sci. Med. Sport 2022, 25, 209–215. [Google Scholar] [CrossRef]
- Kalema, R.N.; Schache, A.G.; Williams, M.D.; Heiderscheit, B.; Siqueira Trajano, G.; Shield, A.J. Sprinting Biomechanics and Hamstring Injuries: Is There a Link? A Literature Review. Sports 2021, 9, 141. [Google Scholar] [CrossRef]
- Milanese, S.; Eston, R. Hamstring injuries and Australian Rules football: Over-reliance on Nordic hamstring exercises as a preventive measure? Open Access J. Sport. Med. 2019, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Bourne, M.N.; van Dyk, N.; Pizzari, T. Recalibrating the risk of hamstring strain injury (HSI): A 2020 systematic review and meta-analysis of risk factors for index and recurrent hamstring strain injury in sport. Br. J. Sport. Med. 2020, 54, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- van Dyk, N.; Behan, F.P.; Whiteley, R. Including the Nordic hamstring exercise in injury prevention programmes halves the rate of hamstring injuries: A systematic review and meta-analysis of 8459 athletes. Br. J. Sport. Med. 2019, 53, 1362–1370. [Google Scholar] [CrossRef]
- Mueller-Wohlfahrt, H.W.; Haensel, L.; Mithoefer, K.; Ekstrand, J.; English, B.; McNally, S.; Orchard, J.; van Dijk, C.N.; Kerkhoffs, G.M.; Schamasch, P.; et al. Terminology and classification of muscle injuries in sport: The Munich consensus statement. Br. J. Sport. Med. 2013, 47, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.L.; Hamilton, B.; Best, T.M. Palpating muscles, massaging the evidence? An editorial relating to ‘Terminology and classification of muscle injuries in sport: The Munich consensus statement’. Br. J. Sport. Med. 2012, 47, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, M.; Walden, M.; Bahr, R.; Ekstrand, J. Methods for epidemiological study of injuries to professional football players: Developing the UEFA model. Br. J. Sport. Med. 2005, 39, 340–346. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Jarvinen, M.; Kalimo, H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J. 2013, 3, 337–345. [Google Scholar] [CrossRef]
- Heiderscheit, B.C.; Sherry, M.A.; Silder, A.; Chumanov, E.S.; Thelen, D.G. Hamstring strain injuries: Recommendations for diagnosis, rehabilitation, and injury prevention. J. Orthop. Sport. Phys. Ther. 2010, 40, 67–81. [Google Scholar] [CrossRef]
- Van Wilgen, C.P.; Verhagen, E. A qualitative study on overuse injuries: The beliefs of athletes and coaches. J. Sci. Med. Sport 2012, 15, 116–121. [Google Scholar] [CrossRef]
- Brukner, P. Hamstring injuries: Prevention and treatment—An update. Br. J. Sport. Med. 2015, 49, 1241–1244. [Google Scholar] [CrossRef]
- Peetrons, P. Ultrasound of muscles. Eur. Radiol. 2002, 12, 35–43. [Google Scholar] [CrossRef]
- Sugiura, Y.; Sakuma, K.; Sakuraba, K.; Sato, Y. Prevention of Hamstring Injuries in Collegiate Sprinters. Orthop. J. Sport. Med. 2017, 5, 2325967116681524. [Google Scholar] [CrossRef]

| Tissue | Tensile Modulus | Material Properties |
|---|---|---|
| Bone | 20 GPa (20,000 MPa) | Rigidity, hardness, toughness, extensible |
| Tendon | 200 MPa | Tough, slightly elastic, flexible |
| Muscle | 1 MPa | more elastic and viscoelastic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Xi, G.; Sun, M.; Sun, Y.; Li, L. Hamstrings on Morphological Structure Characteristics, Stress Features, and Risk of Injuries: A Narrative Review. Appl. Sci. 2022, 12, 12713. https://doi.org/10.3390/app122412713
Shi Y, Xi G, Sun M, Sun Y, Li L. Hamstrings on Morphological Structure Characteristics, Stress Features, and Risk of Injuries: A Narrative Review. Applied Sciences. 2022; 12(24):12713. https://doi.org/10.3390/app122412713
Chicago/Turabian StyleShi, Yinbin, Gengsi Xi, Mengzi Sun, Yuliang Sun, and Li Li. 2022. "Hamstrings on Morphological Structure Characteristics, Stress Features, and Risk of Injuries: A Narrative Review" Applied Sciences 12, no. 24: 12713. https://doi.org/10.3390/app122412713
APA StyleShi, Y., Xi, G., Sun, M., Sun, Y., & Li, L. (2022). Hamstrings on Morphological Structure Characteristics, Stress Features, and Risk of Injuries: A Narrative Review. Applied Sciences, 12(24), 12713. https://doi.org/10.3390/app122412713









