Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro
Abstract
1. Introduction
2. Materials and Methods
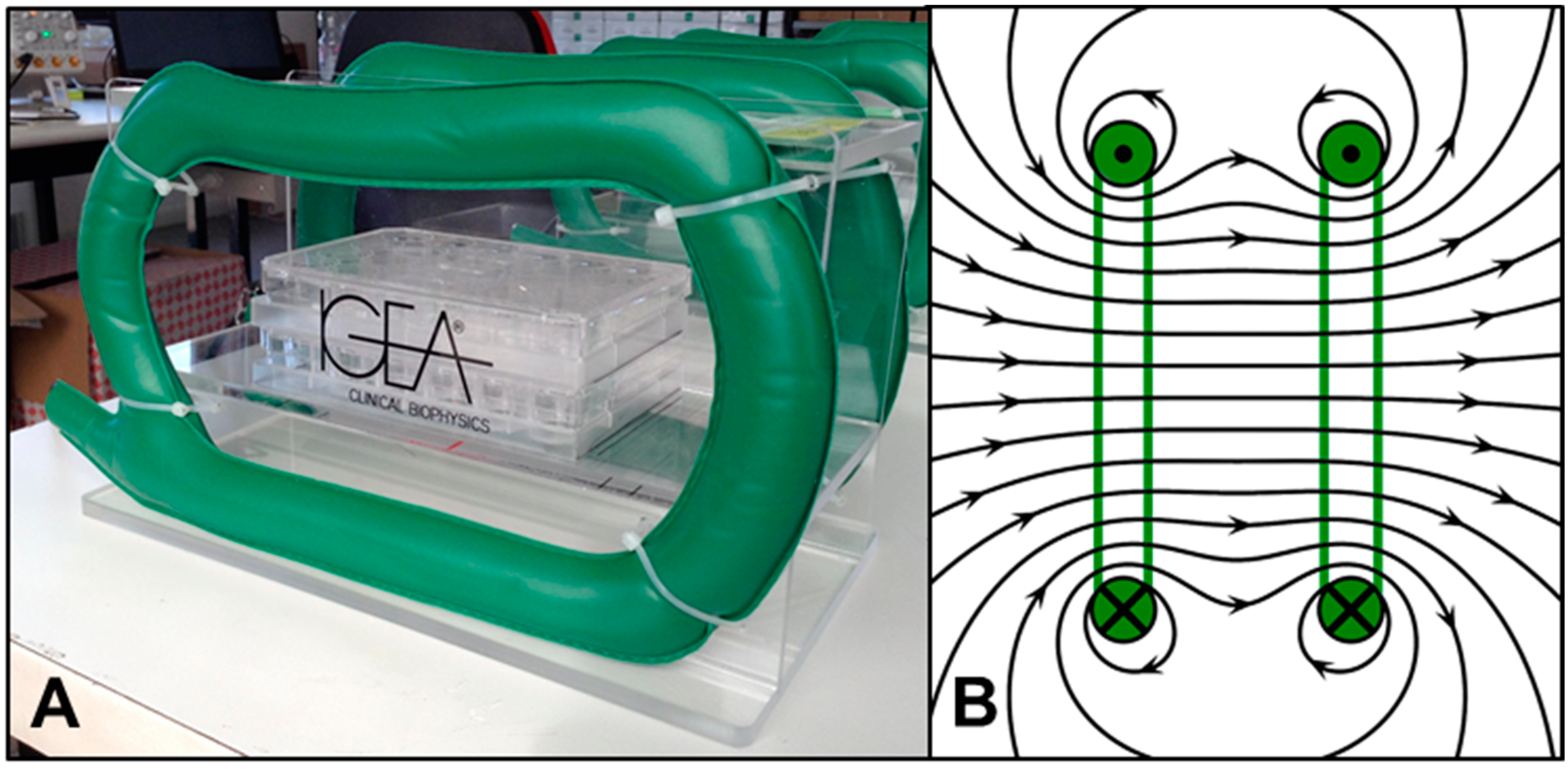
2.1. PEMF System
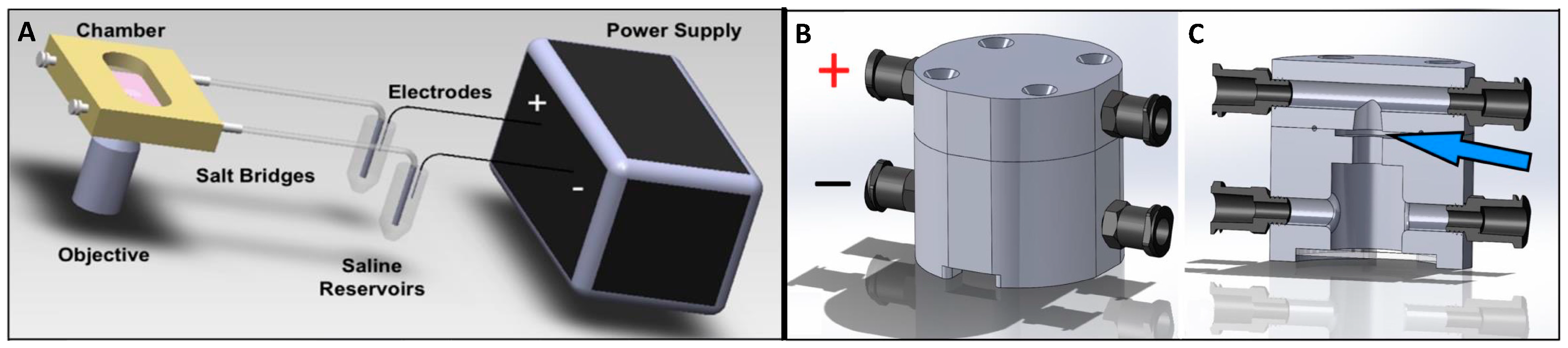
2.2. Galvanotaxis Chamber for 2D Culture
2.3. Galvanotaxis Chamber for 3D Culture
2.4. Bovine Synovium and Cartilage Explant Harvest
2.5. Bovine FLS and Chondrocyte Isolation
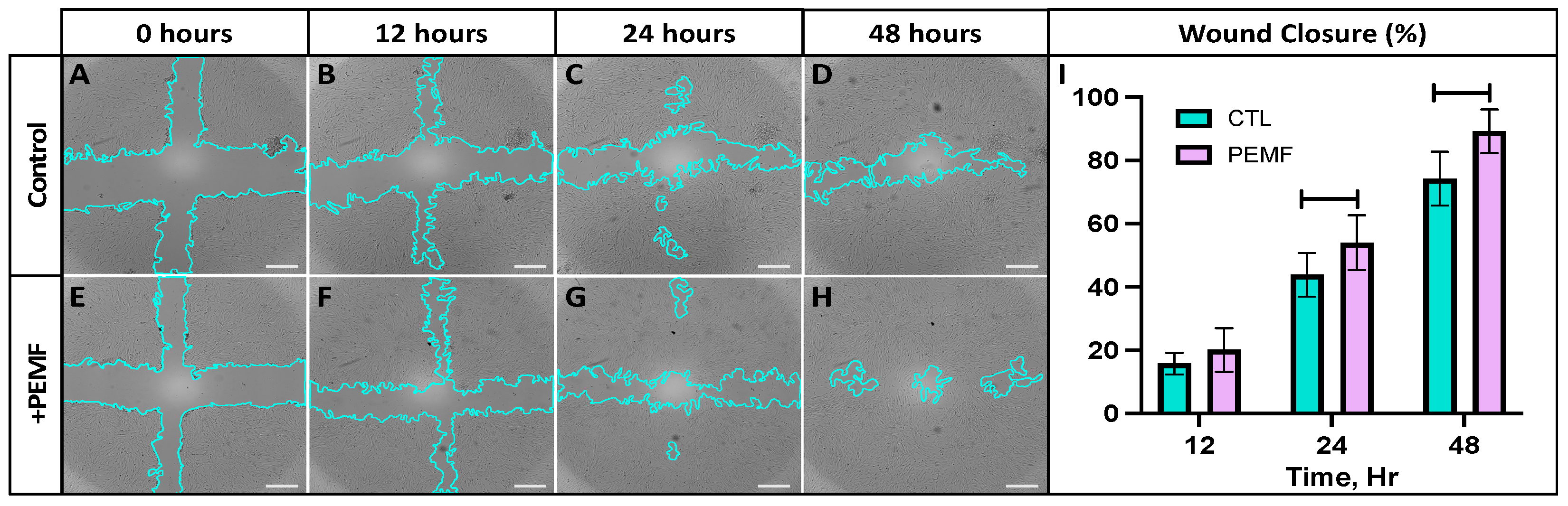
2.6. Wound Closure Assay with PEMF Stimulation
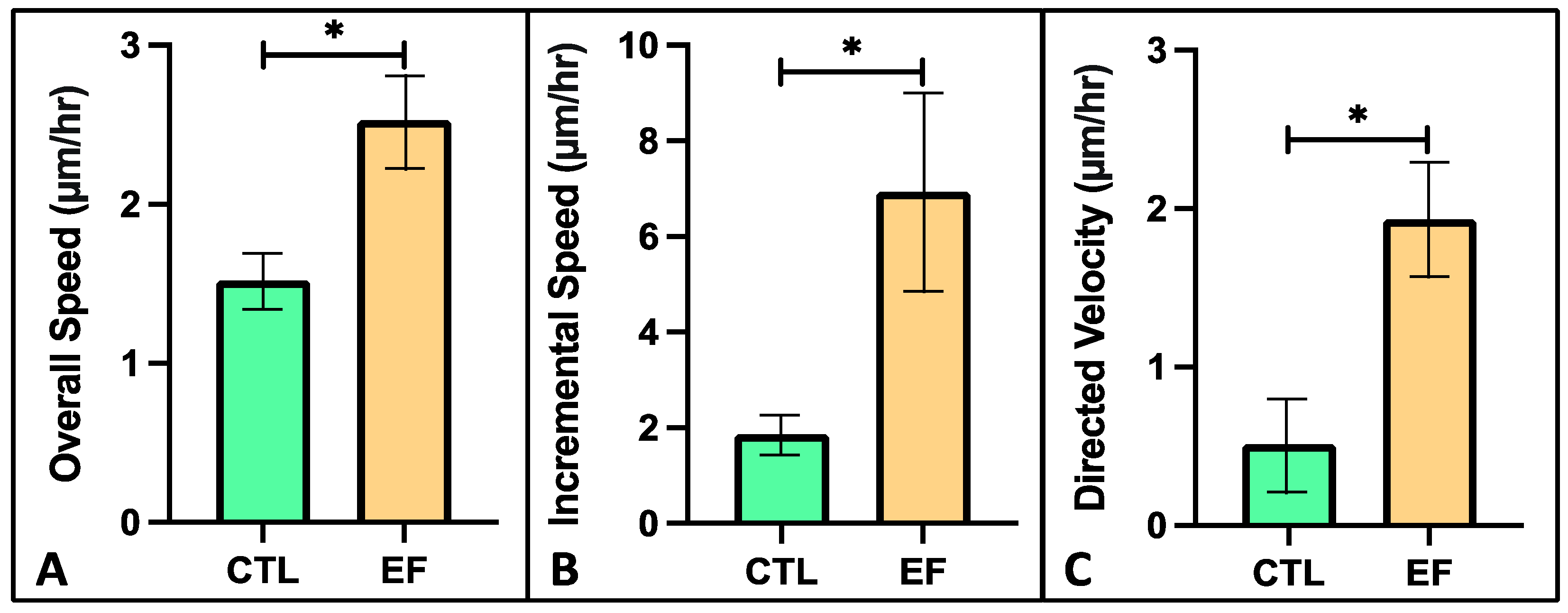
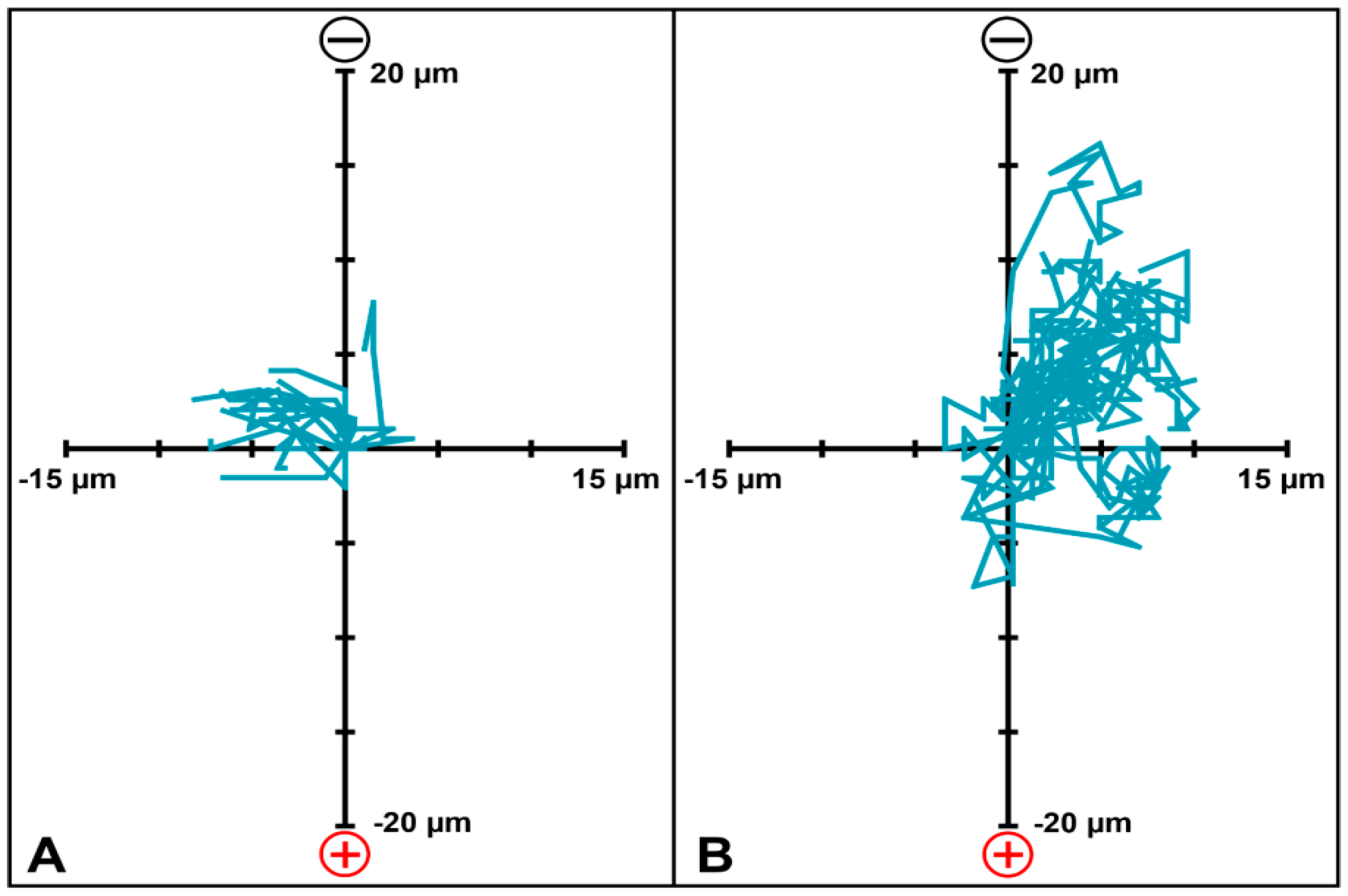
2.7. FLS Migration in Collagen Hydrogel with DC EF Stimulation
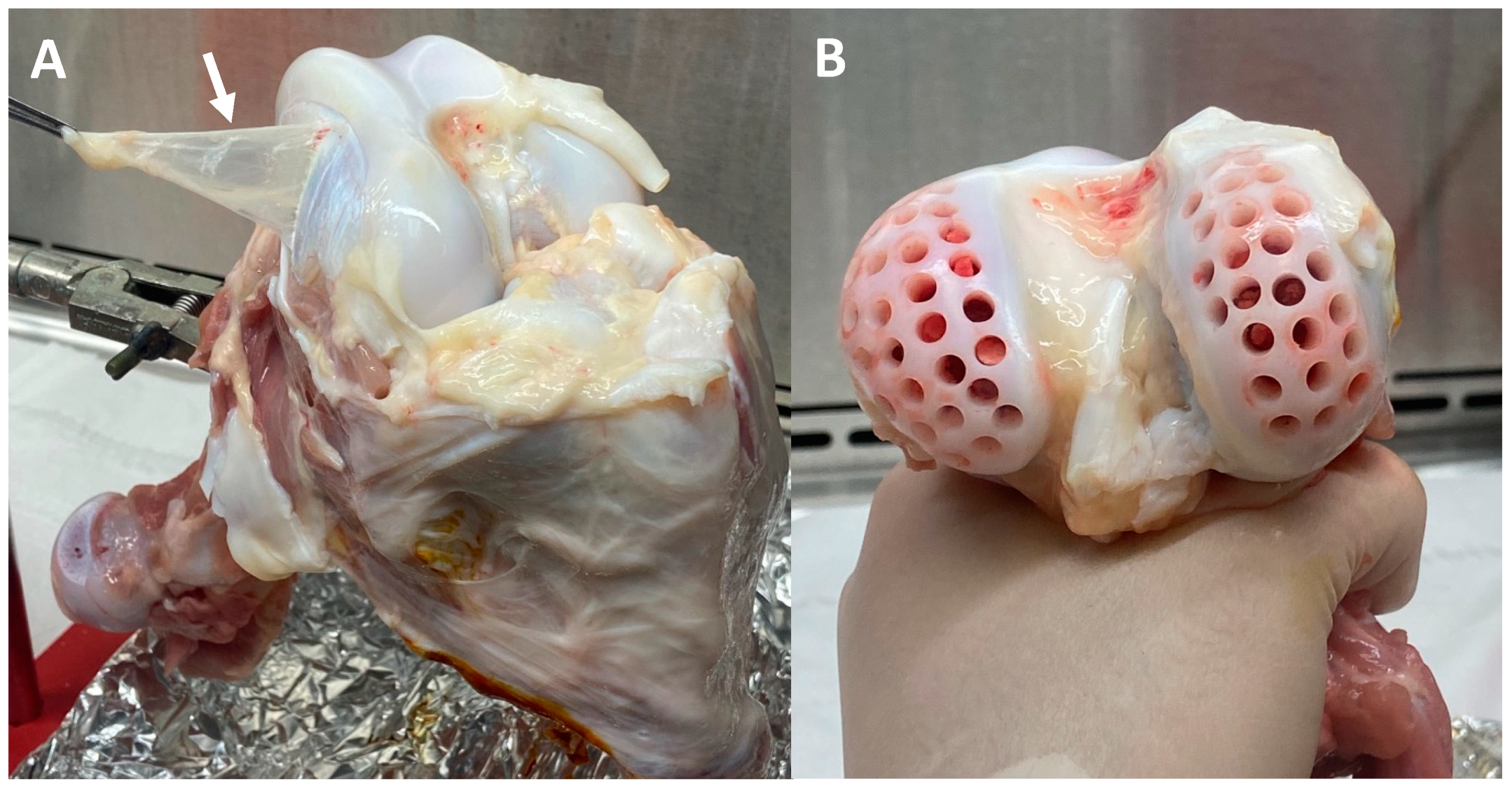
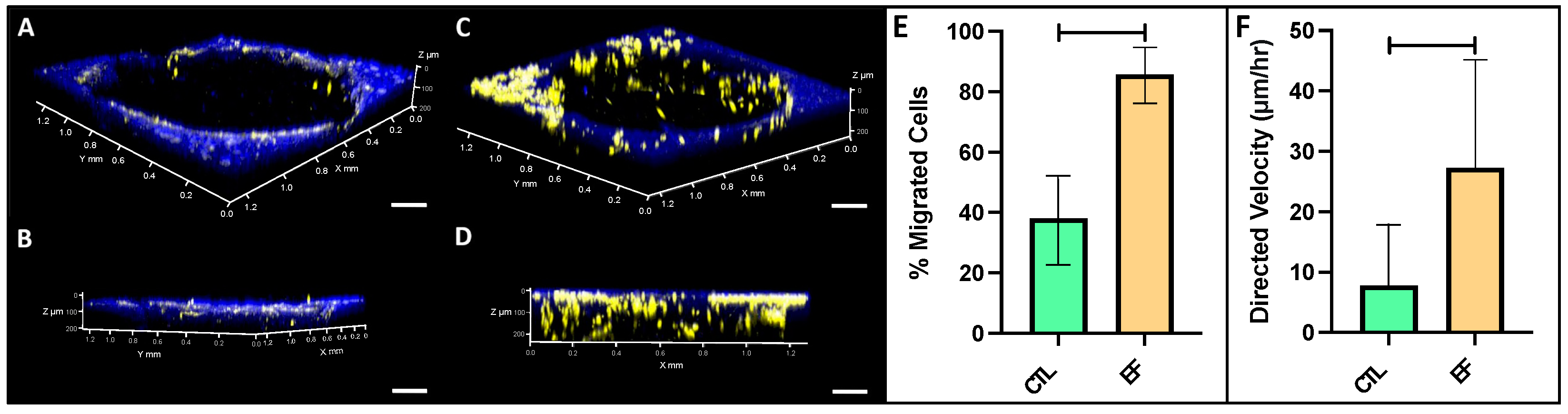
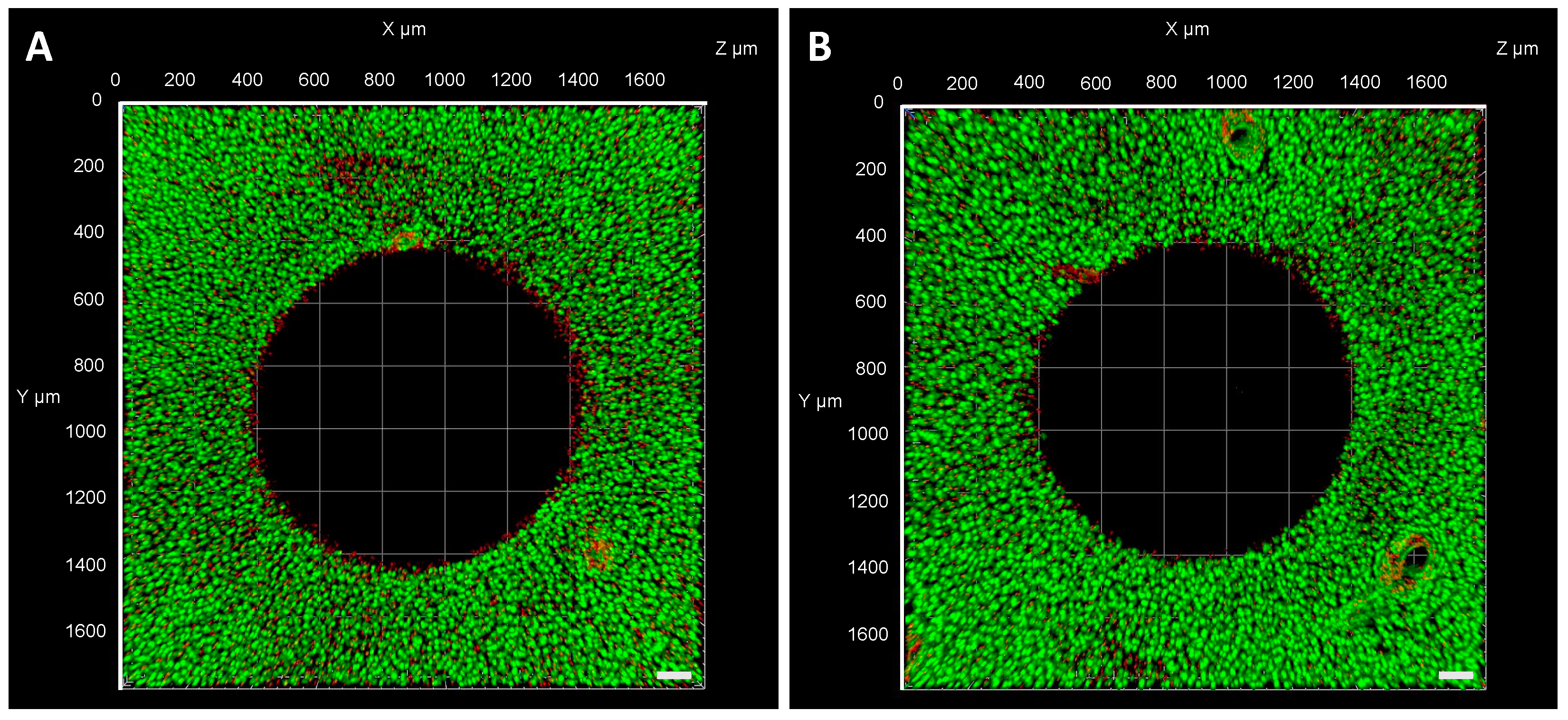
2.8. FLS Migration into a Cartilage Defect
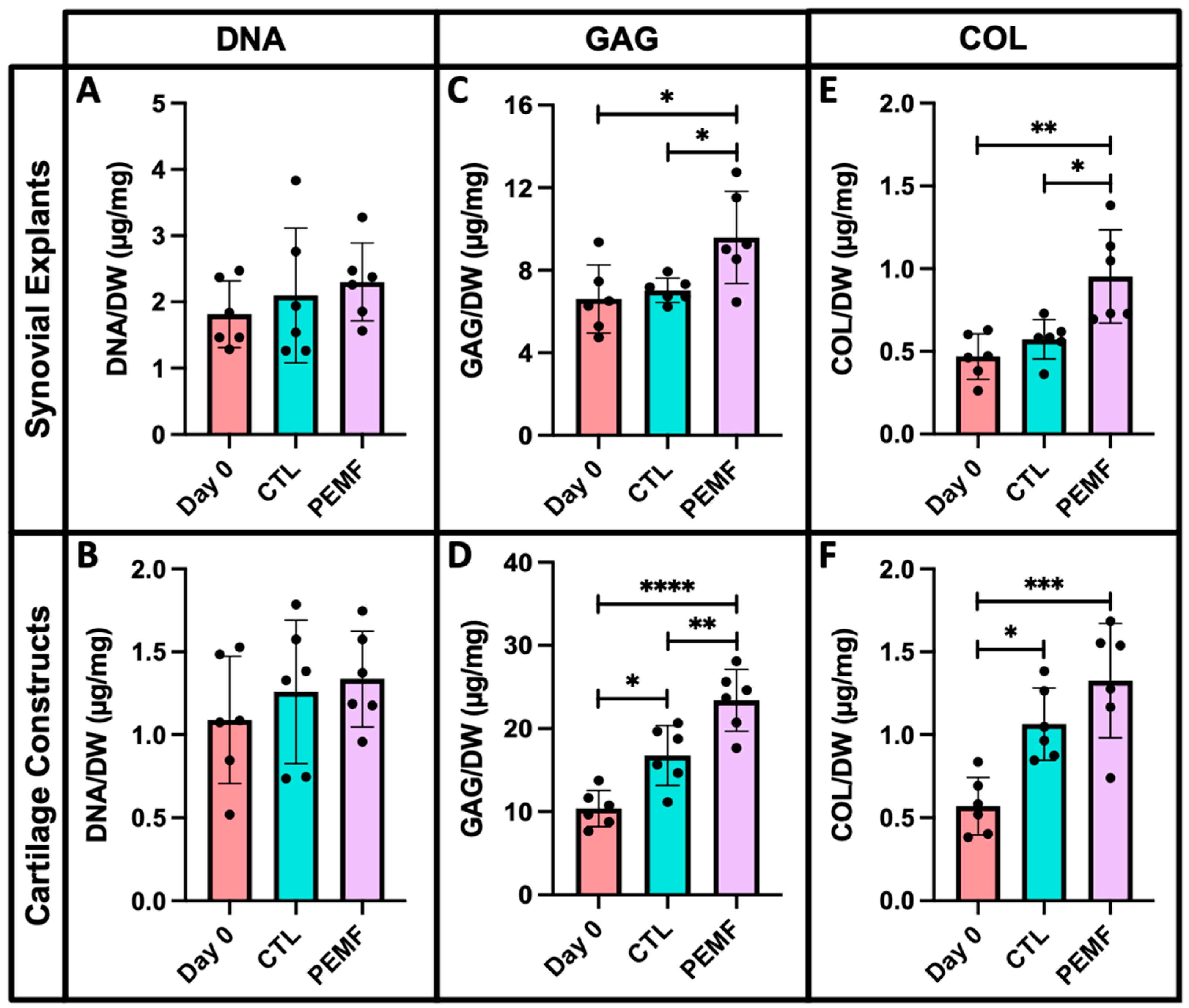
2.9. Biochemistry
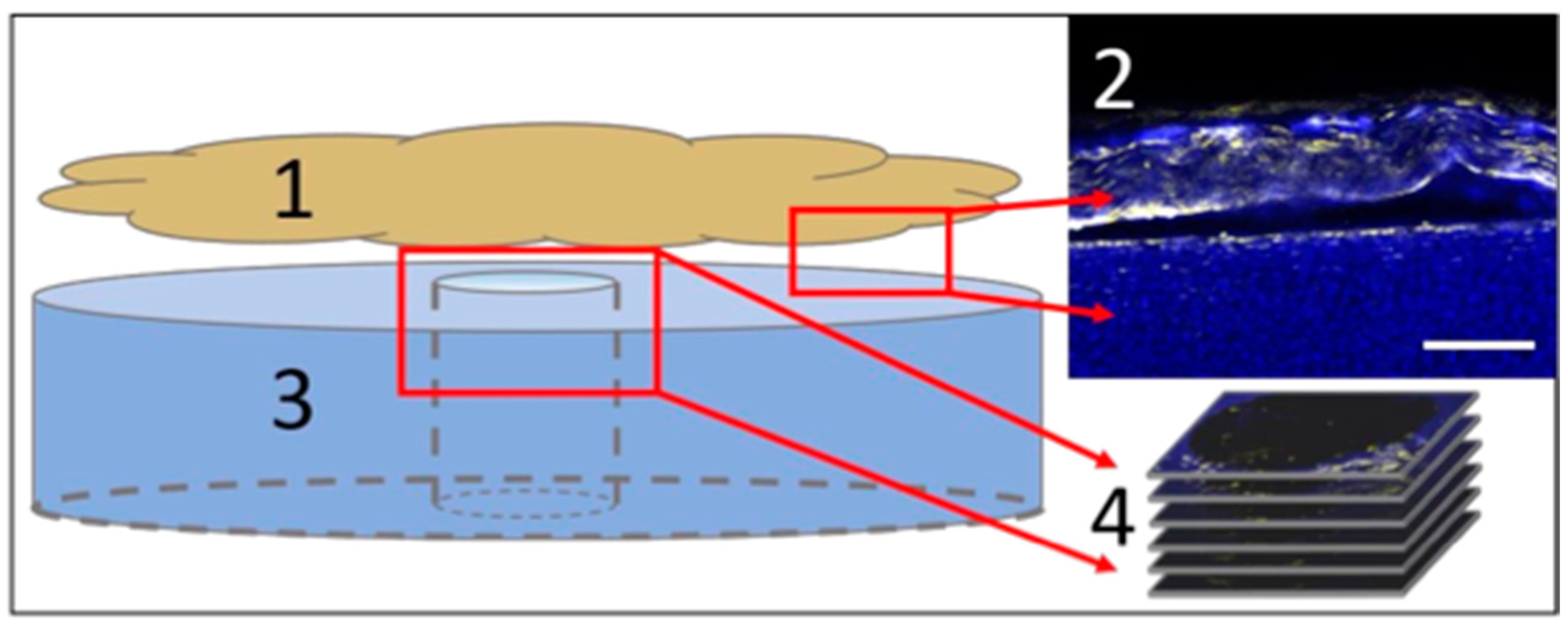
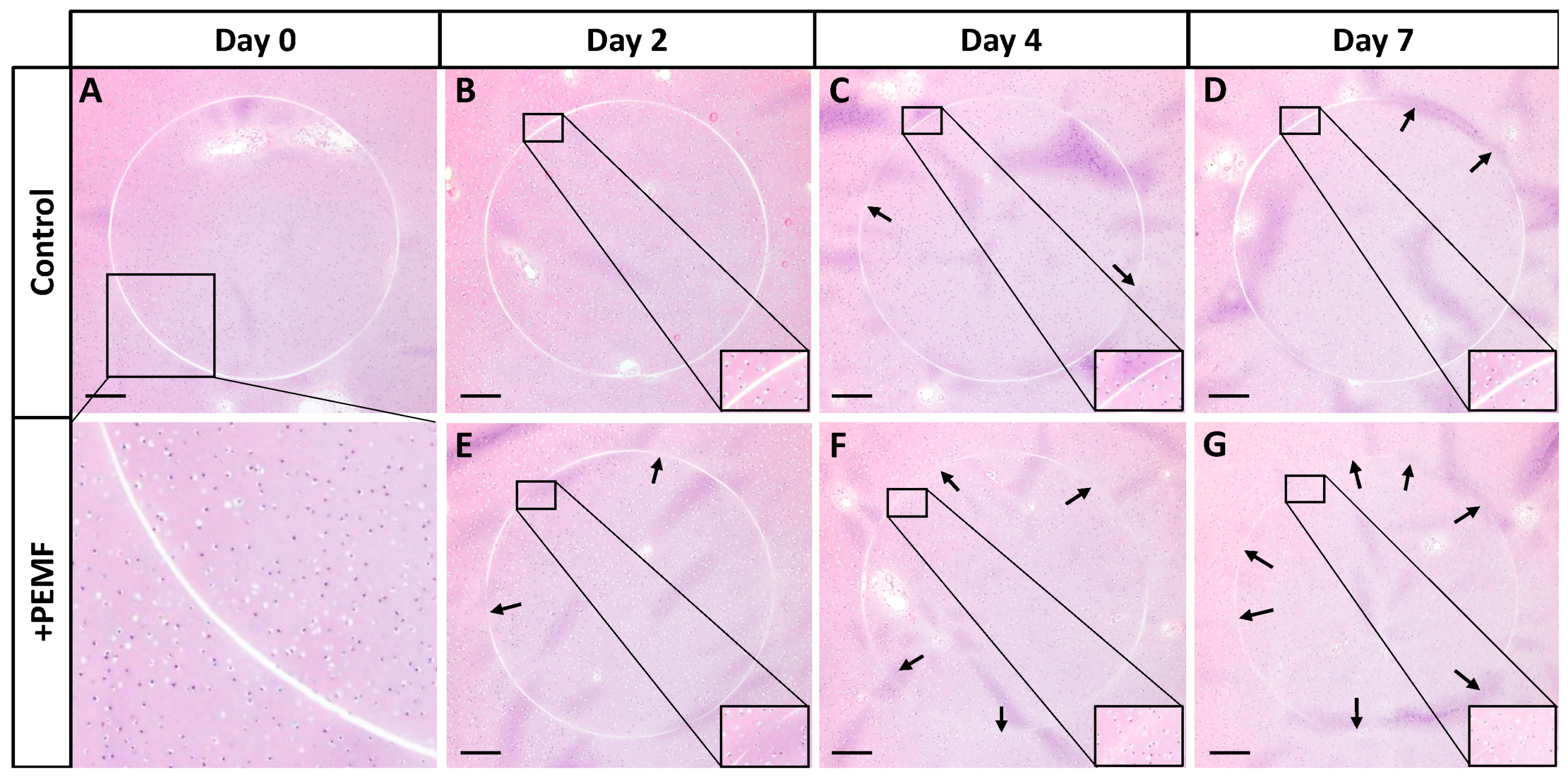
2.10. Histological Characterization
2.11. qPCR Preparation
2.12. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health. 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front Bioeng Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Blalock, D.; Miller, A.; Tilley, M.; Wang, J. Joint Instability and Osteoarthritis. Clin. Med. Insights Arthritis Musculoskelet Disord. 2015, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Hudelmaier, H.; Putz, R. The Effects of Exercise on Human Articular Cartilage. J. Anat. 2006, 208, 491–512. [Google Scholar] [CrossRef]
- Karuppal, R. Current Concepts in the Articular Cartilage Repair and Regeneration. J. Orthop. 2017, 14, A1–A3. [Google Scholar] [CrossRef]
- Borrelli, J.; Olson, S.A.; Godbout, C.; Schemitsch, E.H.; Stannard, J.P.; Giannoudis, P.V. Understanding Articular Cartilage Injury and Potential Treatments. J. Orthop. Trauma. 2019, 33, S6–S12. [Google Scholar] [CrossRef]
- Christensen, B.B.; Olesen, M.L.; Hede, K.T.C.; Bergholt, N.L.; Foldager, C.B.; Lind, M. Particulated Cartilage for Chondral and Osteochondral Repair: A Review. Cartilage 2021, 13, 1047S–1057S. [Google Scholar] [CrossRef]
- Wang, Z.; Le, H.; Wang, Y.; Liu, H.; Li, Z.; Yang, X.; Wang, C.; Ding, J.; Chen, X. Instructive Cartilage Regeneration Modalities with Advanced Therapeutic Implantations under Abnormal Conditions. Bioact. Mater. 2021, 11, 317–338. [Google Scholar] [CrossRef]
- Trengove, A.; Bella, C.D.; O’Connor, A.J. The Challenge of Cartilage Integration: Understanding a Major Barrier to Chondral Repair. Tissue Eng. Part B Rev. 2022, 28, 114–128. [Google Scholar] [CrossRef]
- von Rechenberg, B.; Akens, M.K.; Nadler, D.; Bittmann, P.; Zlinszky, K.; Kutter, A.; Poole, A.R.; Auer, J.A. Changes in Subchondral Bone in Cartilage Resurfacing--an Experimental Study in Sheep using Different Types of Osteochondral Grafts. Osteoarthr. Cartil. 2003, 11, 265–277. [Google Scholar] [CrossRef]
- Arshi, A.; Petrigliano, F.A.; Williams, R.J.; Jones, K.J. Stem Cell Treatment for Knee Articular Cartilage Defects and Osteoarthritis. Curr. Rev. Musculoskelet Med. 2020, 13, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Q.; Xia, J.; Chen, X.; Xiong, J.; Yang, L.; Liang, Y. Modification of Mesenchymal Stem Cells for Cartilage-Targeted Therapy. J. Transl. Med. 2022, 20, 515. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells for Cartilage Regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Z.; Ma, N.; Hao, C.; Guo, W.; Zou, G.; Zhang, Y.; Chen, M.; Gao, S.; Peng, J.; et al. Advances and Prospects in Stem Cells for Cartilage Regeneration. Stem Cells Int. 2017, 2017, 4130607. [Google Scholar] [CrossRef]
- Pareek, A.; Carey, J.L.; Reardon, P.J.; Peterson, L.; Stuart, M.J.; Krych, A.J. Long-Term Outcomes after Autologous Chondrocyte Implantation. Cartilage 2016, 7, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Xiao, R.; Li, J.B.; Zhu, Q. Regenerative Approaches for Cartilage Repair in the Treatment of Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; El Haj, A.J.; Alini, M.; Stoddart, M.J. Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration. J. Funct. Morphol. Kinesiol. 2021, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Shintani, N.; Haspl, M.; Lippuner, K.; Vögelin, E.; Keel, M. The Synovium of Human Osteoarthritic Joints Retains Its Chondrogenic Potential Irrespective of Age. Tissue Eng. Part A 2022, 28, 283–295. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Varshney, R.R.; Zhou, R.; Hao, J.; Yeo, S.S.; Chooi, W.H.; Fan, J.; Wang, D.A. Chondrogenesis of Synovium-derived Mesenchymal Stem Cells in Gene-transferred Co-culture System. Biomaterials 2010, 31, 6876–6891. [Google Scholar] [CrossRef]
- Sampat, S.R.; O’Connell, G.D.; Fong, J.V.; Alegre-Aguarón, E.; Ateshian, G.A.; Hung, C.T. Growth Factor Priming of Synovium-derived Stem Cells for Cartilage Tissue Engineering. Tissue Eng. Part A 2011, 17, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S.; Um, H.B.; Dyment, N.A.; Cottingham, N.; Usami, Y.; Enomoto-Iwamoto, M.; Kronenberg, M.S.; Maye, P.; Rowe, D.W.; Koyama, E.; et al. Cell Origin, Volume and Arrangement are Drivers of Articular Cartilage Formation, Morphogenesis and Response to Injury in Mouse Limbs. Dev. Biol. 2017, 426, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.K.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint Morphogenetic Cells in the Adult Mammalian Synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef]
- Tan, A.R.; Alegre-Aguarón, E.; O’Connell, G.D.; VandenBerg, C.D.; Aaron, R.K.; Vunjak-Novakovic, G.; Bulinski, J.C.; Ateshian, G.A.; Hung, C.T. Passage-dependent Relationship Between Mesenchymal Stem Cell Mobilization and Chondrogenic Potential. Osteoarthr. Cartil. 2015, 23, 319–327. [Google Scholar] [CrossRef]
- Betti, E.; Marchetti, S.; Cadossi, R.; Faldini, C.; Faldini, A. Effect of Stimulation by Low- Frequency Pulsed Electromagnetic Fields in Subjects with Fracture of the Femoral Neck. In Electricity and Magnetism in Biology and Medicine; Springer: Boston, MA, USA, 1999; pp. 853–855. [Google Scholar] [CrossRef]
- Zorzi, C.; Dall’Oca, C.; Cadossi, R.; Setti, S. Effects of Pulsed Electromagnetic Fields on Patients’ Recovery After Arthroscopic Surgery: Prospective, Randomized and Double-blind Study. Knee Surg. Sports Traumatol. Arthr. 2007, 15, 830–834. [Google Scholar] [CrossRef]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In Vitro Electrical-stimulated Wound-healing Chip for Studying Electric Field-assisted Wound-healing Process. Biomicrofluidics 2012, 6, 34117. [Google Scholar] [CrossRef]
- Zhao, M.; Penninger, J.; Isseroff, R.R. Electrical Activation of Wound-Healing Pathways. Adv. Skin Wound Care 2010, 1, 567–573. [Google Scholar] [CrossRef]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e1900155. [Google Scholar] [CrossRef]
- Cadossi, M.; Buda, R.E.; Ramponi, L.; Sambri, A.; Natali, S.; Giannini, S. Bone Marrow–derived Cells and Biophysical Stimulation for Talar Osteochondral Lesions: A Randomized Controlled Study. Foot Ankle Int. 2014, 35, 981–987. [Google Scholar] [CrossRef]
- Collarile, M.; Sambri, A.; Lullini, G.; Cadossi, M.; Zorzi, C. Biophysical Stimulation Improves Clinical Results of Matrix-assisted Autologous Chondrocyte Implantation in the Treatment of Chondral Lesions of the Knee. Knee Surg Sports Traumatol. Arthrosc. 2018, 26, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, R.; Ursino, C.; Setti, S.; Scelsi, M.; Ursino, N. Pulsed Electromagnetic Fields Improve Pain Management and Clinical Outcomes After Medial Unicompartmental Knee Arthroplasty: A Prospective Randomised Controlled Trial. J. ISAKOS 2022, 7, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Reddi, A.H. Pulsed Electromagnetic Fields and Tissue Engineering of the Joints. Tissue Eng. Part B Rev. 2018, 24, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, J.M.; Johnson, M.I.; Lopes-Martins, R.A.B.; Bogen, B.; Chow, R.; Ljunggren, A.E. Short-term Efficacy of Physical Interventions in Osteoarthritic Knee Pain. A Systematic Review and Meta-analysis of Randomised Placebo-controlled Trials. BMC Musculoskelet Disord. 2007, 8, 51. [Google Scholar] [CrossRef]
- Gobbi, A.; Lad, D.; Petrera, M.; Karnatzikos, G. Symptomatic Early Osteoarthritis of the Knee Treated with Pulsed Electromagnetic Fields: Two-Year Follow-up. Cartilage 2014, 5, 78–85. [Google Scholar] [CrossRef]
- Stefani, R.M.; Barbosa, S.; Tan, A.R.; Setti, S.; Stoker, A.M.; Ateshian, G.A.; Cadossi, R.; Vunjak-Novakovic, G.; Aaron, R.K.; Cook, J.L.; et al. Pulsed Electromagnetic Fields Promote Repair of Focal Articular Cartilage Defects with Engineered Osteochondral Constructs. Biotechnol. Bioeng. 2020, 117, 1584–1596. [Google Scholar] [CrossRef]
- Chao, P.H.; Roy, R.; Mauck, R.L.; Liu, W.; Valhmu, W.B.; Hung, C.T. Chondrocyte Translocation Response to Direct Current Electric Fields. J. Biomech. Eng. 2000, 122, 261–267. [Google Scholar] [CrossRef]
- Chao, P.G.; Lu, H.H.; Hung, C.T.; Nicoll, S.B.; Bulinski, J.C. Effects of Applied DC Electric Field on Ligament Fibroblast Migration and Wound Healing. Connect Tissue Res. 2007, 48, 188–197. [Google Scholar] [CrossRef]
- Gunja, N.J.; Dujari, D.; Chen, A.; Luengo, A.; Fong, J.V.; Hung, C.T. Migration Responses of Outer and Inner Meniscus Cells to Applied Direct Current Electric Fields. J. Orthop. Res. 2011, 30, 103–111. [Google Scholar] [CrossRef]
- Tan, A.R.; Alegre-Aguarón, E.; Dujari, D.N.; Sampat, S.R.; Bulinski, J.C.; Ateshian, G.A.; Hung, C.T. Effects of Passaging on the Migration Response of Synovium-Derived Stem Cells to an Applied DC Electric Field. Summer Bioeng. Conf. Parts A B 2011, 54587, 401–402. [Google Scholar] [CrossRef]
- Braddock, M.; Campbell, C.J.; Zuder, D. Current Therapies for Wound Healing: Electrical Stimulation, Biological Therapeutics, and the Potential for Gene Therapy. Int. J. Dermatol. 1999, 38, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I. Electrical Stimulation as a Novel Tool for Regulating Cell Behavior in Tissue Engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Matsukura, Y.; Muneta, T.; Ozeki, N.; Mizuno, M.; Katano, H.; Sekiya, I. Fibrous Synovium Releases Higher Numbers of Mesenchymal Stem Cells than Adipose Synovium in a Suspended Synovium Culture Model. Arthroscopy 2017, 33, 800–810. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Minas, T. The Quality of Healing: Articular Cartilage: Articular Cartilage. Wound Repair Regen. 2014, 1, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Stefani, R.M.; Halder, S.S.; Estell, E.G.; Lee, A.J.; Silverstein, A.M.; Sobczak, E.; Chahine, N.O.; Ateshian, G.A.; Shah, R.P.; Hung, C.T. A Functional Tissue-Engineered Synovium Model to Study Osteoarthritis Progression and Treatment. Tissue Eng. Part A 2019, 25, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Sakhrani, N.; Lee, A.J.; Murphy, L.A.; Kenawy, H.M.; Visco, C.J.; Ateshian, G.A.; Shah, R.P.; Hung, C.T. Toward Development of a Diabetic Synovium Culture Model. Front Bioeng. Biotechnol. 2022, 10, 825046. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Short-term Gene Expression Changes in Cartilage Explants Stimulated with Interleukin Beta Plus Glucosamine and Chondroitin Sulfate. J. Rheumatol. 2006, 33, 1329–1340. [Google Scholar]
- Tan, A.R.; Dong, E.Y.; Ateshian, G.A.; Hung, C.T. Response of Engineered Cartilage to Mechanical Insult Depends on Construct Maturity. Osteoarthr. Cartil. 2010, 18, 1577–1585. [Google Scholar] [CrossRef][Green Version]
- Caldwell, K.L.; Wang, J. Cell-based Articular Cartilage Repair: The Link Between Development and Regeneration. Osteoarthr. Cartil. 2015, 23, 351–362. [Google Scholar] [CrossRef]
- Kubosch, E.J.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.P.; Schmal, H. The Potential for Synovium-derived Stem Cells in Cartilage Repair. Curr. Stem Cell Res. Ther. 2018, 13, 174–184. [Google Scholar] [CrossRef]
- To, K.; Zhang, B.; Romain, K.; Mak, C.; Khan, W. Synovium-Derived Mesenchymal Stem Cell Transplantation in Cartilage Regeneration: A PRISMA Review of in vivo Studies. Front. Bioeng. Biotechnol. 2019, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Ang, D.C.; Almeida-Porada, G. Targeting Mesenchymal Stromal Cells/Pericytes (MSCs) With Pulsed Electromagnetic Field (PEMF) Has the Potential to Treat Rheumatoid Arthritis. Front. Immunol. 2019, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Feng, L.; Zhang, X.; Wang, H.; Zhang, N.; Viohl, I.; Li, G. Pulsed Electromagnetic Field Enhances Healing of a Meniscal Tear and Mitigates Posttraumatic Osteoarthritis in a Rat Model. Am. J. Sports Med. 2022, 50, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, Q.; Sun, Y.; Tian, J. Synergistic Effect of a LPEMF and SPIONs on BMMSC Proliferation, Directional Migration, and Osteoblastogenesis. Am. J. Transl. Res. 2018, 10, 1431–1443. [Google Scholar]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.H.; Franco-Obregón, A.; Yang, Z. Pulsed Electromagnetic Fields Potentiate the Paracrine Function of Mesenchymal Stem Cells for Cartilage Regeneration. Stem Cell Res. Ther. 2020, 11, 46. [Google Scholar] [CrossRef]
- Qu, F.; Guilak, F.; Mauck, R.L. Cell Migration: Implications for Repair and Regeneration in Joint Disease. Nat. Rev. Rheumatol. 2019, 15, 167–179. [Google Scholar] [CrossRef]
- Sun, S.; Titushkin, I.; Cho, M. Regulation of Mesenchymal Stem Cell Adhesion and Orientation in 3D Collagen Scaffold by Electrical Stimulus. Bioelectrochemistry 2006, 69, 133–141. [Google Scholar] [CrossRef]
- Mow, V.C.; Huiskes, R. Basic Orthopaedic Biomechanics & Mechano-Biology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Iwamoto, N.; Fukui, S.; Takatani, A.; Shimizu, T.; Umeda, M.; Nishino, A.; Igawa, T.; Koga, T.; Kawashiri, S.; Ichinose, K.; et al. Osteogenic Differentiation of Fibroblast-like Synovial Cells in Rheumatoid Arthritis is Induced by MicroRNA-218 Through a ROBO/Slit Pathway. Arthritis Res. Ther. 2018, 20, 189. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Vunjak-Novakovic, G. Synovium-derived Stem Cell-based Chondrogenesis. Differentiation 2008, 76, 1044–1056. [Google Scholar] [CrossRef]
- Ryan, C.; Doulgkeroglou, M.N.; Zeugolis, D.I. Electric Field Stimulation for Tissue Engineering Applications. BMC Biomed. Eng. 2021, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, Y.B.; Tjörnstrand, J.; Tiderius, C.J.; Dahlberg, L.E. Relationship Between Cartilage Glycosaminoglycan Content (assessed with dGEMRIC) and OA Risk Factors in Meniscectomized Patients. Osteoarthr. Cartil. 2009, 17, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Lullini, G.; Cammisa, E.; Setti, S.; Sassoli, I.; Zaffagnini, S.; Marcheggiani Muccioli, G.M. Role of Pulsed Electromagnetic Fields After Joint Replacements. World J. Orthop. 2020, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Nam, J. The Role of Changes in Extracellular Matrix of Cartilage in the Presence of Inflammation on the Pathology of Osteoarthritis. BioMed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II Collagen Degradation and its Regulation in Articular Cartilage in Osteoarthritis. Ann. Rheum Dis. 2002, 61, 78–81. [Google Scholar] [CrossRef]
- Zhou, X.; Haudenschild, A.K.; Sherlock, B.E.; Hu, J.C.; Leach, J.K.; Athanasiou, K.A.; Marcu, L. Detection of Glycosaminoglycan Loss in Articular Cartilage by Fluorescence Lifetime Imaging. J. Biomed. Opt. 2018, 23, 126002. [Google Scholar] [CrossRef]



| Gene | FWD Primer Sequence | REV Primer Sequence |
|---|---|---|
| GAPDH | GTC ATC ATC TCT GCA CCT TCT G | GGA GGC ATT GCT GAC AAT CT |
| COL10A1 | CTG CCC GAG GAC TTT GTA A | GGG TAA GCT TTG GAG AGG ATA A |
| COL2A1 | GGC CTG TCT GCT TCT TGT AA | CTA GAG TGA CTG GGA TTG GAA AG |
| COL1A2 | TCC AGA AGG CTC TAG GAA GAA | CTT GGT TAG GGT CAA TCC AGT AG |
| COL11A2 | CCT GGA TGA GGA AGT CTT TGA G | CTT CTG GTC ACA GGA TTC GTA G |
| ACAN | CCT CAG GGT TTC CTG ACA TTA G | GCT CAG TCA CGC CAG ATA TT |
| COL3A1 | TGG TAT TCC TGG GCG AAA TG | CCA CCA GTA GGA CAT GAT TCA C |
| COL1A1 | CAG ACT GGC AAC CTC AAG AA | TAG GTG ACG CTG TAG GTG AA |
| COL4A1 | CAC GGC TAC TCT TTG CTC TAC | GAA GGG CAT GGT ACT GAA CTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakhrani, N.; Stefani, R.M.; Setti, S.; Cadossi, R.; Ateshian, G.A.; Hung, C.T. Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro. Appl. Sci. 2022, 12, 12406. https://doi.org/10.3390/app122312406
Sakhrani N, Stefani RM, Setti S, Cadossi R, Ateshian GA, Hung CT. Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro. Applied Sciences. 2022; 12(23):12406. https://doi.org/10.3390/app122312406
Chicago/Turabian StyleSakhrani, Neeraj, Robert M. Stefani, Stefania Setti, Ruggero Cadossi, Gerard A. Ateshian, and Clark T. Hung. 2022. "Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro" Applied Sciences 12, no. 23: 12406. https://doi.org/10.3390/app122312406
APA StyleSakhrani, N., Stefani, R. M., Setti, S., Cadossi, R., Ateshian, G. A., & Hung, C. T. (2022). Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro. Applied Sciences, 12(23), 12406. https://doi.org/10.3390/app122312406








