Oncological Applications of Photodynamic Therapy in Dogs and Cats
Abstract
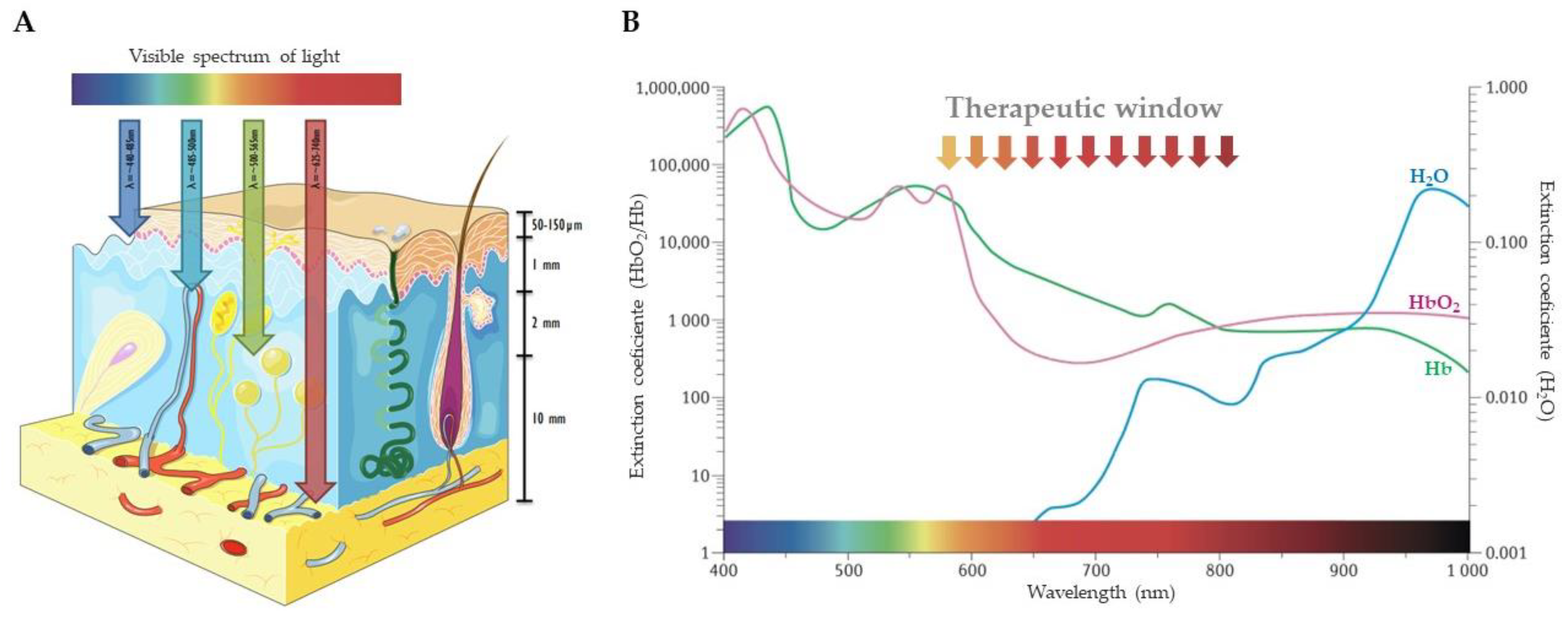
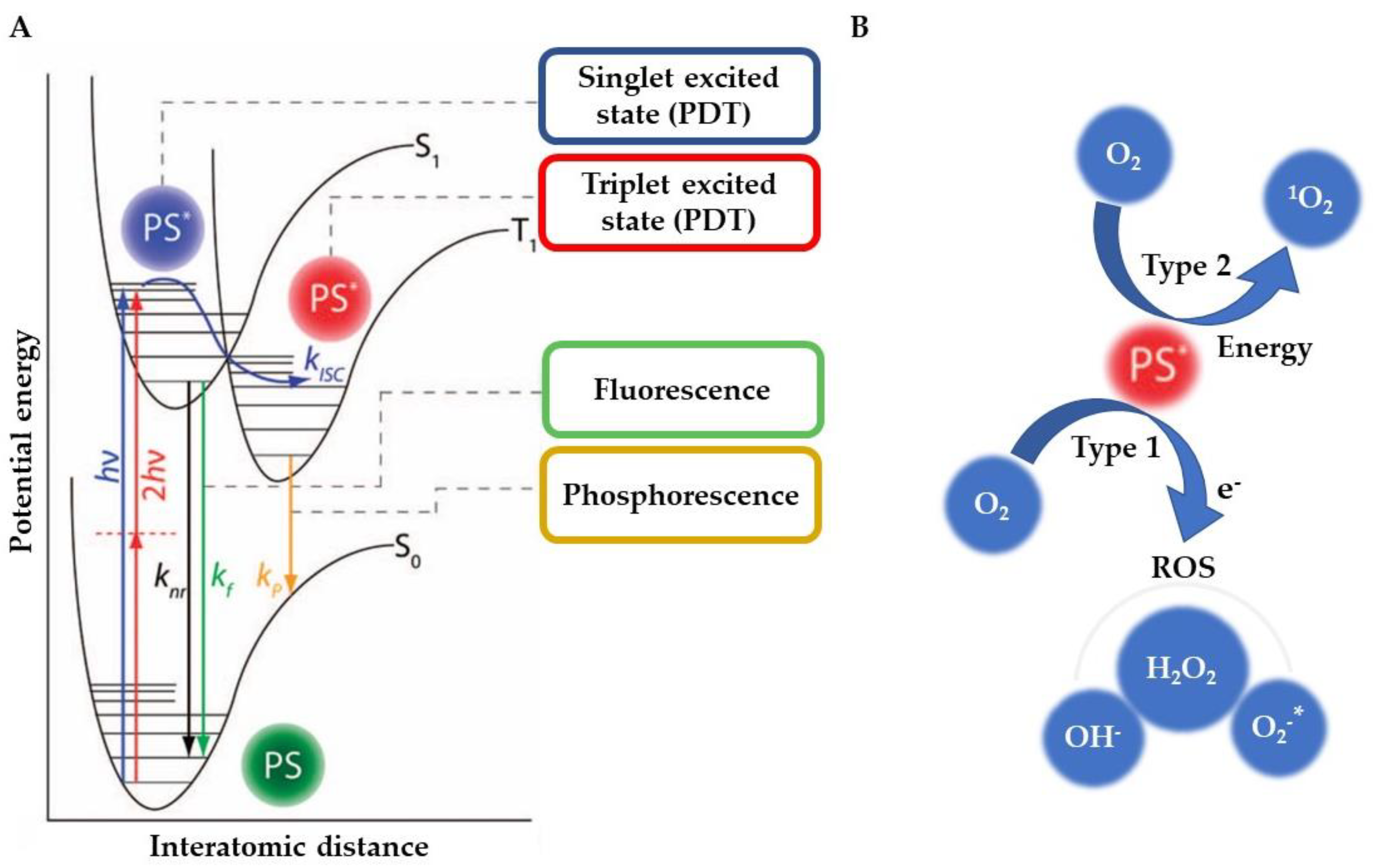
1. Introduction
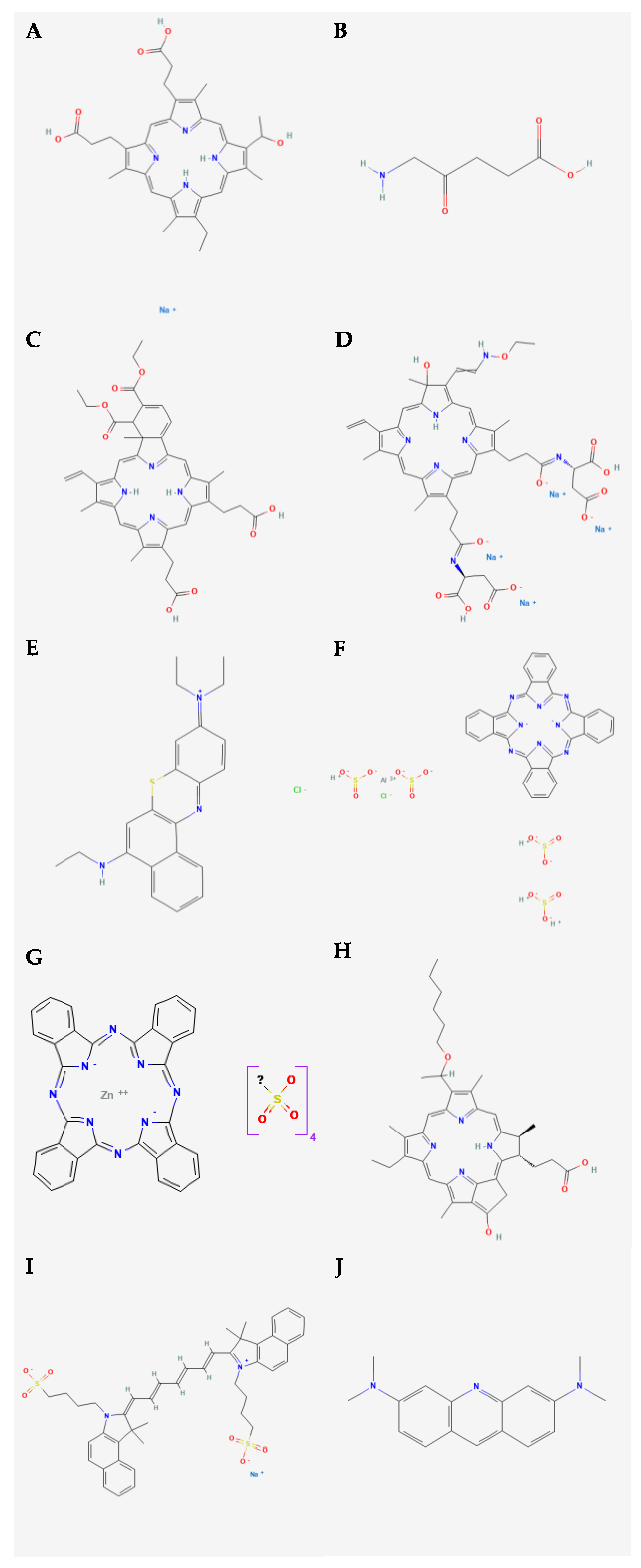
2. Photosensitizers
3. PDT in Veterinary Oncology
3.1. Carcinomas
3.2. Mastocytomas
3.3. Sarcomas
3.4. Melanomas
3.5. Other Tumors
4. Preclinical Studies
4.1. In Vivo Studies
4.2. In Vitro Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biller, B.; Berg, J.; Garrett, L.; Ruslander, D.; Wearing, R.; Abbott, B.; Patel, M.; Smith, D.; Bryan, C. 2016 AAHA Oncology Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2016, 52, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.; Kaplan, L. Canine and Feline Geriatric Oncology; Blackwell Publishing Ltd.: Oxford, UK, 2017; ISBN 9780470344446. [Google Scholar]
- Lieshchova, M.O.; Shuleshko, O.O.; Balchuhov, V.O. The incidence and structure of neoplasms in animals in Dnipro city. Sci. Technol. Bull. SRC Biosaf. Environ. Control AIC 2018, 6, 30–37. [Google Scholar]
- Buchholz, J.; Ludewig, E.; Brühschwein, A.; Nitzl, D.; Sumova, A.; Kaser-Hotz, B. Radiation therapy planning using MRI-CT fusion in dogs and cats with brain tumors. Tierärztliche Prax. Ausgabe K Kleintiere/Heimtiere 2019, 47, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Meuten, D.J. Tumors in Domestic Animals, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781119181194. [Google Scholar]
- Moreira, L.; Kinappe, L.; Duhart, D.; De Souza Da Motta, A. Canine geriatrics and management of neoplastic diseases: Review. Pubvet 2018, 12, 147. [Google Scholar] [CrossRef]
- Buchholz, J. Clinical Applications of Cancer PDT. In Photodynamic Therapy in Veterinary Medicine: From Basics to Clinical Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–155. [Google Scholar]
- Dougherty, T.J.; Thoma, R.E.; Boyle, D.G.; Weishaupt, K.R. Interstitial Photoradiation Therapy for Primary Solid Tumors in Pet Cats and Dogs. CANCER Res. 1981, 41, 401–404. [Google Scholar]
- Nascimento, C.L.; Sellera, F.P.; Ribeiro, M.S. How to Enter PDT in Clinical Practice? In Photodynamic Therapy in Veterinary Medicine: From Basics to Clinical Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 111–123. [Google Scholar]
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef]
- Couto, G.K.; Seixas, F.K.; Iglesias, B.A.; Collares, T. Perspectives of photodynamic therapy in biotechnology. J. Photochem. Photobiol. B Biol. 2020, 213, 112051. [Google Scholar] [CrossRef]
- Pereira, N.A.M.; Laranjo, M.; Nascimento, B.F.O.; Simões, J.C.S.; Pina, J.; Costa, B.D.P.; Brites, G.; Braz, J.; Seixas de Melo, J.S.; Pineiro, M.; et al. Novel fluorinated ring-fused chlorins as promising PDT agents against melanoma and esophagus cancer. RSC Med. Chem. 2021, 12, 615–627. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jiang, X.; Wei, Y.; Wang, Q.; Cui, K.; Xu, X.; Wang, F.; Zhang, L. Application of phototherapeutic-based nanoparticles in colorectal cancer. Int. J. Biol. Sci. 2021, 17, 1361–1381. [Google Scholar] [CrossRef]
- Guimarães, T.G.; Menezes Cardoso, K.; Tralhão, P.; Marto, C.M.; Alexandre, N.; Botelho, M.F.; Laranjo, M. Current Therapeutics and Future Perspectives to Ocular Melanocytic Neoplasms in Dogs and Cats. Bioengineering 2021, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, T.G.; Marto, C.M.; Cardoso, K.M.; Alexandre, N.; Botelho, M.F.; Laranjo, M. Evaluation of eye melanoma treatments in rabbits: A systematic review. Lab. Anim. 2021, 56, 002367722110393. [Google Scholar] [CrossRef] [PubMed]
- Winifred Nompumelelo Simelane, N.; Abrahamse, H. Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 12405. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, I.; Gasymova, E.; Dietiker-Moretti, S.; Tichy, A.; Rohrer Bley, C. Evaluation of long-term outcome and prognostic factors of feline squamous cell carcinomas treated with photodynamic therapy using liposomal phosphorylated meta-tetra(hydroxylphenyl)chlorine. J. Feline Med. Surg. 2018, 20, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Sobhani, N.; Samadani, A.A. Implications of photodynamic cancer therapy: An overview of PDT mechanisms basically and practically. J. Egypt. Natl. Canc. Inst. 2021, 33, 34. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Pereira, N.A.M.M.; Laranjo, M.; Pina, J.; Oliveira, A.S.R.R.; Ferreira, J.D.; Sánchez-Sánchez, C.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Piñeiro, M.; et al. Advances on photodynamic therapy of melanoma through novel ring-fused 5,15-diphenylchlorins. Eur. J. Med. Chem. 2018, 146, 395–408. [Google Scholar] [CrossRef]
- Teixo, R.; Laranjo, M.; Abrantes, A.M.; Brites, G.; Serra, A.; Proença, R.; Botelho, M.F. Retinoblastoma: Might photodynamic therapy be an option? Cancer Metastasis Rev. 2015, 34, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Qin, Y.; Feng, X. Advances in the Genetically Engineered KillerRed for Photodynamic Therapy Applications. Int. J. Mol. Sci. 2021, 22, 10130. [Google Scholar] [CrossRef] [PubMed]
- Cerman, E.; Çekiç, O. Clinical use of photodynamic therapy in ocular tumors. Surv. Ophthalmol. 2015, 60, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Yow, C.; Huang, Z. Combination of photodynamic therapy and immunomodulation: Current status and future trends. Med. Res. Rev. 2008, 28, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.A.; Nam, G.-H.; Hong, Y.; Kim, G.B.; Choi, Y.; Lee, S.; Cho, Y.; Kwon, M.; Jeong, C.; et al. In situ immunogenic clearance induced by a combination of photodynamic therapy and rho-kinase inhibition sensitizes immune checkpoint blockade response to elicit systemic antitumor immunity against intraocular melanoma and its metastasis. J. Immunother. Cancer 2021, 9, e001481. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Allison, R.R. Photodynamic therapy: Oncologic horizons. Futur. Oncol. 2014, 10, 123–124. [Google Scholar] [CrossRef]
- Pereira, N.A.M.M.; Laranjo, M.; Pineiro, M.; Serra, A.C.; Santos, K.; Teixo, R.; Abrantes, A.M.; Gonçalves, A.C.; Sarmento Ribeiro, A.B.S.; Casalta-Lopes, J.; et al. Novel 4,5,6,7-tetrahydropyrazolo[1,5-a]pyridine fused chlorins as very active photodynamic agents for melanoma cells. Eur. J. Med. Chem. 2015, 103, 374–380. [Google Scholar] [CrossRef]
- Serra, A.; Pineiro, M.; Pereira, N.; Rocha Gonsalves, A.; Laranjo, M.; Abrantes, M.; Botelho, F. A look at clinical applications and developments of photodynamic therapy. Oncol. Rev. 2008, 2, 235–249. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.S.T.; Lucci, C.M.; dos Santos, J.A.M.; Longo, J.P.F.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic therapy for cutaneous hemangiosarcoma in dogs. Photodiagn. Photodyn. Ther. 2019, 27, 39–43. [Google Scholar] [CrossRef]
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Rong, P.; Yang, K.; Srivastan, A.; Kiesewetter, D.O.; Yue, X.; Wang, F.; Nie, L.; Bhirde, A.; Wang, Z.; Liu, Z.; et al. Photosensitizer Loaded Nano-Graphene for Multimodality Imaging Guided Tumor Photodynamic Therapy. Theranostics 2014, 4, 229. [Google Scholar] [CrossRef]
- Fragola, J.A.; Dubielzig, R.R.; Bentley, E.; Teixeira, L.B.C. Iridociliary cysts masquerading as neoplasia in cats: A morphologic review of 14 cases. Vet. Ophthalmol. 2018, 21, 125–131. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Cienc. 2018, 90, 993–1026. [Google Scholar] [CrossRef]
- Senge, M.O. mTHPC—A drug on its way from second to third generation photosensitizer? Photodiagn. Photodyn. Ther. 2012, 9, 170–179. [Google Scholar] [CrossRef]
- Ferreira, I.; Rahal, S.C.; Rocha, N.S.; Gouveia, A.H.; Corrêa, T.P.; Carvalho, Y.K.; Bagnato, V.S. Hematoporphyrin-based photodynamic therapy for cutaneous squamous cell carcinoma in cats. Vet. Dermatol. 2009, 20, 174–178. [Google Scholar] [CrossRef]
- de Oliveira, K.T.; de Souza, J.M.; da Gobo, N.R.S.; de Assis, F.F.; Brocksom, T.J. Basic Concepts and Applications of Porphyrins, Chlorins and Phthalocyanines as Photosensitizers in Photonic Therapies. Rev. Virtual Química 2015, 7, 310–335. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Yong-gang, Q.; Xiu-ping, Z.; Jian, L.; Zheng, H.; Qiang, Y.; Zhang, X.; Li, J.; Huang, Z. Photodynamic therapy for malignant and non-malignant diseases: Clinical investigation and application. Chin. Med. J. 2006, 119, 845–857. [Google Scholar]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-Tetra(m-hydroxyphenyl)chlorin)-A Second-generation Photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Minamide, T.; Takashima, K.; Nakajo, K.; Kadota, T.; Yoda, Y. Clinical Practice of Photodynamic Therapy Using Talaporfin Sodium for Esophageal Cancer. J. Clin. Med. 2021, 10, 2785. [Google Scholar] [CrossRef]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-l-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic Therapy (PDT) for Lung Cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef]
- Krammer, B.; Plaetzer, K. ALA and its clinical impact, from bench to bedside. Photochem. Photobiol. Sci. 2008, 7, 283–289. [Google Scholar] [CrossRef]
- Bartosińska, J.; Szczepanik-Kułak, P.; Raczkiewicz, D.; Niewiedzioł, M.; Gerkowicz, A.; Kowalczuk, D.; Kwaśny, M.; Krasowska, D. Topical Photodynamic Therapy with Different Forms of 5-Aminolevulinic Acid in the Treatment of Actinic Keratosis. Pharmaceutics 2022, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Zeitouni, N.C.; Bhatia, N.; Ceilley, R.I.; Cohen, J.L.; Del Rosso, J.Q.; Moore, A.Y.; Munavalli, G.; Pariser, D.M.; Schlesinger, T.; Siegel, D.M.; et al. Photodynamic Therapy with 5-aminolevulinic Acid 10% Gel and Red Light for the Treatment of Actinic Keratosis, Nonmelanoma Skin Cancers, and Acne: Current Evidence and Best Practices. J. Clin. Aesthet. Dermatol. 2021, 14, E53–E65. [Google Scholar] [PubMed]
- Vallecorsa, P.; Di Venosa, G.; Gola, G.; Sáenz, D.; Mamone, L.; MacRobert, A.J.; Ramírez, J.; Casas, A. Photodynamic therapy of cutaneous T-cell lymphoma cell lines mediated by 5-aminolevulinic acid and derivatives. J. Photochem. Photobiol. B Biol. 2021, 221, 112244. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Hasunuma, Y.; Kikuchi, E.; Ishii, T.; Ishizuka, M.; Tokuoka, Y. Accumulation of porphyrins in Propionibacterium acnes by 5-aminolevulinic acid and its esters. Photodiagn. Photodyn. Ther. 2017, 19, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H.; Downie, G.H.; Cuenca, R.E. A clinical review of PDT for cutaneous malignancies. Photodiagn. Photodyn. Ther. 2006, 3, 214–226. [Google Scholar] [CrossRef]
- Maruo, T.; Fukuyama, Y.; Nishiyama, Y.; Nemoto, Y.; Kanai, E.; Kawarai, S.; Kayanuma, H.; Orito, K. Recurrence analysis of intraoperative acridine orange-photodynamic therapy for dogs with intranasal tumors. Can. Vet. J. 2021, 62, 1117–1122. [Google Scholar]
- Zhang, Y.; Yang, Z.; Zheng, X.; Chen, L.; Xie, Z. Highly efficient near-infrared BODIPY phototherapeutic nanoparticles for cancer treatment. J. Mater. Chem. B 2020, 8, 5305–5311. [Google Scholar] [CrossRef]
- Mai, D.K.; Kim, C.; Lee, J.; Vales, T.P.; Badon, I.W.; De, K.; Cho, S.; Yang, J.; Kim, H.J. BODIPY nanoparticles functionalized with lactose for cancer-targeted and fluorescence imaging-guided photodynamic therapy. Sci. Rep. 2022, 12, 2541. [Google Scholar] [CrossRef]
- Kwon, N.; Kim, K.H.; Park, S.; Cho, Y.; Park, E.-Y.Y.; Lim, J.; Çetindere, S.; Tümay, S.O.; Kim, W.J.; Li, X.; et al. Hexa-BODIPY-cyclotriphosphazene based nanoparticle for NIR fluorescence/photoacoustic dual-modal imaging and photothermal cancer therapy. Biosens. Bioelectron. 2022, 216, 114612. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Sabino, C.P. Photosensitizers. In Photodynamic Therapy in Veterinary Medicine: From Basics to Clinical Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–43. [Google Scholar]
- Iyer, A.K.; Greish, K.; Seki, T.; Okazaki, S.; Fang, J.; Takeshita, K.; Maeda, H. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J. Drug Target. 2007, 15, 496–506. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Yokoe, I.; Ogura, S.; Takahashi, K.; Murakami, K.; Inoue, K.; Ishizuka, M.; Tanaka, T.; Li, L.; Sugiyama, A.; et al. Photodynamic detection of canine mammary gland tumours after oral administration of 5-aminolevulinic acid. Vet. Comp. Oncol. 2017, 15, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Cabon, Q.; Sayag, D.; Texier, I.; Navarro, F.; Boisgard, R.; Virieux-Watrelot, D.; Ponce, F.; Carozzo, C. Evaluation of intraoperative fluorescence imaging–guided surgery in cancer-bearing dogs: A prospective proof-of-concept phase II study in 9 cases. Transl. Res. 2016, 170, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.B.; Dai, H.; Tan, X.; Li, H.; Liang, H.; Hu, C.; Huang, M.; Lee, J.Y.; Zhao, J.; Zhou, L.; et al. A Facile, Protein-Derived Supramolecular Theranostic Strategy for Multimodal-Imaging-Guided Photodynamic and Photothermal Immunotherapy In Vivo. Adv. Mater. 2022, 34, 2109111. [Google Scholar] [CrossRef]
- Osaki, T.; Yokoe, I.; Sunden, Y.; Ota, U.; Ichikawa, T.; Imazato, H.; Ishii, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; et al. Efficacy of 5-Aminolevulinic Acid in Photodynamic Detection and Photodynamic Therapy in Veterinary Medicine. Cancers 2019, 11, 495. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef]
- Adamson, D.C.; Halani, S. Clinical utility of 5-aminolevulinic acid HCl to better visualize and more completely remove gliomas. Onco. Targets. Ther. 2016, 9, 5629–5642. [Google Scholar] [CrossRef]
- Pereira, N.A.M.M.; Laranjo, M.; Casalta-Lopes, J.; Serra, A.C.; Piñeiro, M.; Pina, J.; Seixas de Melo, J.S.; Senge, M.O.; Botelho, M.F.; Martelo, L.; et al. Platinum(II) Ring-Fused Chlorins as Near-Infrared Emitting Oxygen Sensors and Photodynamic Agents. ACS Med. Chem. Lett. 2017, 8, 310–315. [Google Scholar] [CrossRef]
- Laranjo, M.; Aguiar, M.C.; Pereira, N.A.M.; Brites, G.; Nascimento, B.F.O.; Brito, A.F.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Pineiro, M.; et al. Platinum(II) ring-fused chlorins as efficient theranostic agents: Dyes for tumor-imaging and photodynamic therapy of cancer. Eur. J. Med. Chem. 2020, 200, 112468. [Google Scholar] [CrossRef]
- Jiménez, J.; Prieto-Montero, R.; Maroto, B.L.; Moreno, F.; Ortiz, M.J.; Oliden-Sánchez, A.; López-Arbeloa, I.; Martínez-Martínez, V.; Moya, S. Manipulating Charge-Transfer States in BODIPYs: A Model Strategy to Rapidly Develop Photodynamic Theragnostic Agents. Chem.—A Eur. J. 2020, 26, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C.R.; Calienni, M.N.; Rivas Aiello, B.; Prieto, M.J.; Rodriguez Sartori, D.; Tuninetti, J.; Toledo, P.; del Alonso, S.V.; Moya, S.; Gonzalez, M.C.; et al. BSA-capped gold nanoclusters as potential theragnostic for skin diseases: Photoactivation, skin penetration, in vitro, and in vivo toxicity. Mater. Sci. Eng. C 2020, 112, 110891. [Google Scholar] [CrossRef] [PubMed]
- Cheli, R.; Addis, F.; Mortellaro, C.M.; Fonda, D.; Andreoni, A.; Cubeddu, R. HpD Phototherapy on Spontaneous Tumors in Dog and Cat. In Porphyrins in Tumor Phototherapy; Springer: Boston, MA, USA, 1984; pp. 251–258. [Google Scholar]
- Cheli, R.; Addis, F.; Mortellaro, C.M.; Fonda, D.; Cubeddu, R. Photodynamic therapy of spontaneous animal tumors using the active component of hematoporphyrin derivative (DHE) as photosensitizing drug: Clincal results. Cancer Lett. 1987, 38, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tochner, Z.; Mitchell, J.B.; Hoekstra, H.J.; Smith, P.; Deluca, A.M.; Barnes, M.; Harrington, F.; Manyak, M.; Russo, D.; Russo, A. Photodynamic therapy of the canine peritoneum: Normal tissue response to intraperitoneal and intravenous photofrin followed by 630 nm light. Lasers Surg. Med. 1991, 11, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Panjehpour, M.; Overholt, B.F.; Denovo, R.C.; Petersen, M.G.; Sneed, R.E. Comparative study between pulsed and continuous wave lasers for Photofrin® photodynamic therapy. Lasers Surg. Med. 1993, 13, 296–304. [Google Scholar] [CrossRef]
- Musani, A.I.; Veir, J.K.; Huang, Z.; Lei, T.; Groshong, S.; Worley, D. Photodynamic therapy via navigational bronchoscopy for peripheral lung cancer in dogs. Lasers Surg. Med. 2018, 50, 483–490. [Google Scholar] [CrossRef]
- Peavy, G.M.; Klein, M.K.; Newman, H.C.; Roberts, W.G.; Berns, M.W. Use of Chloro-Aluminum Sulfonated Phthalocyanine as a Photosensitizer in the Treatment of Malignant Tumors in Dogs and Cats; O’Brien, S.J., Dederich, D.N., Wigdor, H., Trent, A.M., Eds.; International Society for Optics and Photonics: Bellingham, WC, USA, 1991; Volume 1424, p. 171. [Google Scholar]
- Borgatti-Jeffreys, A.; Hooser, S.B.; Miller, M.A.; Lucroy, M.D. Phase I clinical trial of the use of zinc phthalocyanine tetrasulfonate as a photosensitizer for photodynamic therapy in dogs. Am. J. Vet. Res. 2007, 68, 399–404. [Google Scholar] [CrossRef]
- Bexfield, N.H.; Stell, A.J.; Gear, R.N.; Dobson, J.M. Photodynamic Therapy of Superficial Nasal Planum Squamous Cell Carcinomas in Cats: 55 Cases. J. Vet. Intern. Med. 2008, 22, 1385–1389. [Google Scholar] [CrossRef]
- Stell, A.J.; Dobson, J.M.; Langmack, K. Photodynamic therapy of feline superficial squamous cell carcinoma using topical 5-aminolaevulinic acid. J. Small Anim. Pract. 2001, 42, 164–169. [Google Scholar] [CrossRef]
- Ridgway, T.D.; Lucroy, M.D. Phototoxic effects of 635-nm light on canine transitional cell carcinoma cells incubated with 5-aminolevulinic acid. Am. J. Vet. Res. 2003, 64, 131–136. [Google Scholar] [CrossRef]
- Svaasand, L.O.; Wyss, P.; Wyss, M.-T.; Tadir, Y.; Tromberg, B.J.; Berns, M.W. Dosimetry model for photodynamic therapy with topically administered photosensitizers. Lasers Surg. Med. 1996, 18, 139–149. [Google Scholar] [CrossRef]
- Buchholz, J.; Walt, H. Veterinary photodynamic therapy: A review. Photodiagn. Photodyn. Ther. 2013, 10, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; Lyon, J.P. Photodynamic Therapy in Veterinary Medicine: Applications in dogs and cats. Pubvet 2022, 16, 180. [Google Scholar] [CrossRef]
- Sellera, F.P.; Gargano, R.G.; Pogliani, F.C. Terapia fotodinâmica: Revisão de literatura. Rev. Educ. Contin. Med. Veterinária Zootec. Do CRMV-SP 2014, 12, 5–13. [Google Scholar] [CrossRef]
- Tibbitts, J.; Fike, J.R.; Lamborn, K.R.; Bollen, A.W.; Kahl, S.B. Toxicology of a boronated porphyrin in dogs. Photochem. Photobiol. 1999, 69, 587–594. [Google Scholar] [CrossRef]
- Buchholz, J.; Wergin, M.; Walt, H.; Gräfe, S.; Bley, C.R.; Kaser-Hotz, B. Photodynamic therapy of feline cutaneous squamous cell carcinoma using a newly developed liposomal photosensitizer: Preliminary results concerning drug safety and efficacy. J. Vet. Intern. Med. 2007, 21, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. ANZ J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lucroy, M.D.; Ridgway, T.D.; Peavy, G.M.; Krasieva, T.B.; Higbee, R.G.; Campbell, G.A.; Blaik, M.A. Preclinical evaluation of 5-aminolevulinic acid-based photodynamic therapy for canine transitional cell carcinoma. Vet. Comp. Oncol. 2003, 1, 76–85. [Google Scholar] [CrossRef]
- Frimberger, A.E.; Moore, A.S.; Cincotta, L.; Cotter, S.M.; Foley, J.W. Photodynamic therapy of naturally occurring tumors in animals using a novel benzophenothiazine photosensitizer. Clin. Cancer Res. 1998, 4, 2207–2218. [Google Scholar]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Laranjo, M. Fotossensibilizadores Para Terapia e Imagem em Oncologia; University of Coimbra: Coimbra, Portugal, 2014. [Google Scholar]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Lucroy, M.D.; Magne, M.L.; Peavy, G.M.; Madewell, B.R.; Edwards, B.F. Photodynamic Therapy in Veterinary Medicine: Current Status and Implications for Applications in Human Disease. J. Clin. Laser Med. Surg. 1996, 14, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Cheli, R.; Addis, F.; Mortellaro, C.M.; Fonda, D.; Andreoni, A.; Cubeddu, R. Hematoporphyrin derivative photochemotherapy of spontaneous animal tumors: Clinical results with optimized drug dose. Cancer Lett. 1984, 23, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Rosen, G. Photodynamic therapy as a treatment for esophageal squamous cell carcinoma in a dog. J. Am. Anim. Hosp. Assoc. 2000, 36, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Lucroy, M.D.; Bowles, M.H.; Higbee, R.G.; Blaik, M.A.; Ritchey, J.W.; Ridgway, T.D. Photodynamic Therapy for Prostatic Carcinoma in a Dog. J. Vet. Intern. Med. 2003, 17, 235–237. [Google Scholar] [CrossRef] [PubMed]
- L’Eplattenier, H.F.; Klem, B.; Teske, E.; van Sluijs, F.J.; van Nimwegen, S.A.; Kirpensteijn, J. Preliminary results of intraoperative photodynamic therapy with 5-aminolevulinic acid in dogs with prostate carcinoma. Vet. J. 2008, 178, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Hoshino, S.; Hoshino, Y.; Takagi, S.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Clinical Pharmacokinetics of Anti-angiogenic Photodynamic Therapy with Benzoporphyrin Derivative Monoacid Ring-A in Dogs Having Naturally Occurring Neoplasms. J. Vet. Med. Ser. A 2006, 53, 108–112. [Google Scholar] [CrossRef]
- Osaki, T.; Takagi, S.; Hoshino, Y.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Efficacy of Antivascular Photodynamic Therapy Using Benzoporphyrin Derivative Monoacid Ring A (BPD-MA) in 14 Dogs with Oral and Nasal Tumors. J. Vet. Med. Sci. 2009, 71, 125–132. [Google Scholar] [CrossRef]
- Tanabe, S.; Yamaguchi, M.; Iijima, M.; Nakajima, S.; Sakata, I.; Miyaki, S.; Takemura, T.; Furuoka, H.; Kobayashi, Y.; Matsui, T.; et al. Fluorescence detection of a new photosensitizer, PAD-S31, in tumour tissues and its use as a photodynamic treatment for skin tumours in dogs and a cat: A preliminary report. Vet. J. 2004, 167, 286–293. [Google Scholar] [CrossRef]
- Ishigaki, K.; Nariai, K.; Izumi, M.; Teshima, K.; Seki, M.; Edamura, K.; Takahashi, T.; Asano, K. Endoscopic photodynamic therapy using talaporfin sodium for recurrent intranasal carcinomas after radiotherapy in three dogs. J. Small Anim. Pract. 2018, 59, 128–132. [Google Scholar] [CrossRef]
- Lucroy, M.D.; Long, K.R.; Blaik, M.A.; Higbee, R.G.; Ridgway, T.D. Photodynamic Therapy for the Treatment of Intranasal Tumors in 3 Dogs and 1 Cat. J. Vet. Intern. Med. 2003, 17, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Magne, M.L.; Rodriguez, C.O.; Autry, S.A.; Edwards, B.F.; Theon, A.P.; Madewell, B.R. Photodynamic therapy of facial squamous cell carcinoma in cats using a new photosensitizer. Lasers Surg. Med. 1997, 20, 202–209. [Google Scholar] [CrossRef]
- Reeds, K.B.; Ridgway, T.D.; Higbee, R.G.; Lucroy, M.D. Non-coherent light for photodynamic therapy of superficial tumours in animals. Vet. Comp. Oncol. 2004, 2, 157–163. [Google Scholar] [CrossRef] [PubMed]
- McCaw, D.L.; Pope, E.R.; Payne, J.T.; West, M.K.; Tompson, R.V.; Tate, D. Treatment of canine oral squamous cell carcinomas with photodynamic therapy. Br. J. Cancer 2000, 82, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.G.; Klein, M.K.; Loomis, M.; Weldy, S.; Berns, M.W. Photodynamic Therapy of Spontaneous Cancers in Felines, Canines, and Snakes With Chloro-aluminium Sulfonated Phthalocyanine. JNCI J. Natl. Cancer Inst. 1991, 83, 18–23. [Google Scholar] [CrossRef]
- Hahn, K.A.; Panjehpour, M.; Legendre, A.M. Photodynamic therapy response in cats with cutaneous squamous cell carcinoma as a function of fluence. Vet. Dermatol. 1998, 9, 3–7. [Google Scholar] [CrossRef]
- Buchholz, J.; Kaser-Hotz, B.; Khan, T.; Rohrer Bley, C.; Melzer, K.; Schwendener, R.A.; Roos, M.; Walt, H. Optimizing Photodynamic Therapy: In vivo Pharmacokinetics of Liposomal meta -(Tetrahydroxyphenyl)Chlorin in Feline Squamous Cell Carcinoma. Clin. Cancer Res. 2005, 11, 7538–7544. [Google Scholar] [CrossRef]
- Ohlerth, S.; Laluhová, D.; Buchholz, J.; Roos, M.; Walt, H.; Kaser-Hotz, B. Changes in vascularity and blood volume as a result of photodynamic therapy can be assessed with power Doppler ultrasonography. Lasers Surg. Med. 2006, 38, 229–234. [Google Scholar] [CrossRef]
- Maruo, T.; Nagata, K.; Fukuyama, Y.; Nemoto, Y.; Kawarai, S.; Fujita, Y.; Nakayama, T. Intraoperative acridine orange photodynamic therapy and cribriform electron-beam irradiation for canine intranasal tumors: A pilot study. Can. Vet. J. 2015, 56, 1232–1238. [Google Scholar]
- Maruo, T.; Fukuyama, Y.; Nagata, K.; Yoshioka, C.; Nishiyama, Y.; Kawarai, S.; Kayanuma, H.; Orito, K.; Nakayama, T. Intraoperative acridine orange photodynamic therapy and cribriform electron-beam irradiation for canine intranasal carcinomas: 14 cases. Can. Vet. J. 2019, 60, 509–513. [Google Scholar]
- Bisland, S.K.; Burch, S. Photodynamic therapy of diseased bone. Photodiagn. Photodyn. Ther. 2006, 3, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Burch, S.; London, C.; Seguin, B.; Rodriguez, C.; Wilson, B.C.; Bisland, S.K. Treatment of canine osseous tumors with photodynamic therapy: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- McCaw, D.L.; Payne, J.T.; Pope, E.R.; West, M.K.; Tompson, R.V.; Tate, D. Treatment of canine hemangiopericytomas with photodynamic therapy. Lasers Surg. Med. 2001, 29, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Onoyama, M.; Tsuka, T.; Imagawa, T.; Osaki, T.; Minami, S.; Azuma, K.; Kawashima, K.; Ishi, H.; Takayama, T.; Ogawa, N.; et al. Photodynamic hyperthermal chemotherapy with indocyanine green: A novel cancer therapy for 16 cases of malignant soft tissue sarcoma. J. Vet. Sci. 2014, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Martano, M.; Morello, E.; Avnet, S.; Costa, F.; Sammartano, F.; Kusuzaki, K.; Baldini, N. Photodynamic Surgery for Feline Injection-Site Sarcoma. Biomed Res. Int. 2019, 2019, 8275935. [Google Scholar] [CrossRef] [PubMed]
- Gloi, A.M.; Beck, E. Threshold dose of three photosensitizers in dogs with spontaneous tumors. Vet. Ther. 2003, 4, 269–278. [Google Scholar]
- Hori, H.; Teramoto, Y.; Fukuyama, Y.; Maruo, T. Marginal Resection and Acridine Orange Photodynamic Therapy in a Cat with Recurrent Cutaneous Malignant Melanoma. Int. J. Appl. Res. Vet. Med. 2014, 12, 181–185. [Google Scholar]
- Huang, Z.; Chen, Q.; Luck, D.; Beckers, J.; Wilson, B.C.; Trncic, N.; LaRue, S.M.; Blanc, D.; Hetzel, F.W. Studies of a vascular-acting photosensitizer, Pd-bacteriopheophorbide (Tookad), in normal canine prostate and spontaneous canine prostate cancer. Lasers Surg. Med. 2005, 36, 390–397. [Google Scholar] [CrossRef]
- Laranjo, M.; Serra, A.C.A.C.; Abrantes, M.; Pineiro, M.; Goncalves, A.C.; Casalta-Lopes, J.J.; Carvalho, L.; Sarmento-Ribeiro, A.B.; Rocha-Gonsalves, A.A.; Botelho, F.; et al. 2-Bromo-5-hydroxyphenylporphyrins for photodynamic therapy: Photosensitization efficiency, subcellular localization and in vivo studies. Photodiagn. Photodyn. Ther. 2013, 10, 51–61. [Google Scholar] [CrossRef]
- Perlmann, E.; Sá, M.B.P.B.; Squarzoni, R. Ocular ultrasonography as a diagnostic tool in the Veterinary Medicine. Rev. Científica Med. Veterinária—Pequenos Animais Animais Estimação 2012, 10, 204–211. [Google Scholar]
- Whelan, H.T.; Schmidt, M.H.; Segura, A.D.; McAuliffe, T.L.; Bajic, D.M.; Murray, K.J.; Moulder, J.E.; Strother, D.R.; Thomas, J.P.; Meyer, G.A. The role of photodynamic therapy in posterior fossa brain tumors. J. Neurosurg. 1993, 79, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Overholt, B.F.; DeNovo, R.C.; Panjehpour, M.; Petersen, M.G. A centering balloon for photodynamic therapy of esophageal cancer tested in a canine model. Gastrointest. Endosc. 1993, 39, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Overholt, B.F.; Panjehpour, M.; Denovo, R.C.; Petersen, M.G. Photodynamic therapy for esophageal cancer using a 180° windowed esophageal balloon. Lasers Surg. Med. 1994, 14, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Overholt, B.F.; Panjehpour, M.; DeNovo, R.C.; Peterson, M.G.; Jenkins, C. Balloon photodynamic therapy of esophageal cancer: Effect of increasing balloon size. Lasers Surg. Med. 1996, 18, 248–252. [Google Scholar] [CrossRef]
- Nseyo, U.O.; Kim, H.; DeBord, J.; Tate, K.; DeHaven, J. Sequential whole bladder photodynamic therapy treatments: A preclinical study. Urol. Oncol. Semin. Orig. Investig. 1997, 3, 27–30. [Google Scholar] [CrossRef]
- Tochner, Z.A.; Pass, H.I.; Smith, P.D.; Delaney, T.F.; Sprague, M.; Deluca, A.M.; Harrington, F.; Thomas, G.F.; Terrill, R.; Bacher, J.D.; et al. Intrathoracic photodynamic therapy: A canine normal tissue tolerance study and early clinical experience. Lasers Surg. Med. 1994, 14, 118–123. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hirano, T.; Yamaguchi, N. Novel After-loading Interstitial Photodynamic Therapy of Canine Transmissible Sarcoma with Photofrin II and Excimer Dye Laser. Jpn. J. Cancer Res. 1995, 86, 239–244. [Google Scholar] [CrossRef]
- Abramson, A.L.; Barrezueta, N.X.; Shikowitz, M.J. Thermal Effects of Photodynamic Therapy on the Larynx: Experimental Study. Arch. Otolaryngol.—Head Neck Surg. 1987, 113, 854–858. [Google Scholar] [CrossRef]
- Lee, L.K.; Whitehurst, C.; Chen, Q.; Pantelides, M.L.; Hetzel, F.W.; Moore, J.V. Interstitial photodynamic therapy in the canine prostate. BJU Int. 1997, 80, 898–902. [Google Scholar] [CrossRef]
- Chang, S.-C.; Buonaccorsi, G.A.; MacRobert, A.J.; Brown, S.G. Interstitial photodynamic therapy in the canine prostate with disulfonated aluminum phthalocyanine and 5-aminolevulinic acid-induced protoporphyrin IX. Prostate 1997, 32, 89–98. [Google Scholar] [CrossRef]
- Chang, S.C.; Chern, I.F.; Hsu, Y.H. Biological responses of dog prostate and adjacent structures after meso-tetra-(m-hydroxyphenyl) chlorin and aluminum disulfonated phthalocyanine based photodynamic therapy. Proc. Natl. Sci. Counc. Repub. China. B 1999, 23, 158–166. [Google Scholar] [PubMed]
- Swartling, J.; Höglund, O.V.; Hansson, K.; Södersten, F.; Axelsson, J.; Lagerstedt, A.-S. Online dosimetry for temoporfin-mediated interstitial photodynamic therapy using the canine prostate as model. J. Biomed. Opt. 2016, 21, 028002. [Google Scholar] [CrossRef] [PubMed]
- Panjehpour, M.; DeNovo, R.C.; Petersen, M.G.; Overholt, B.F.; Bower, R.; Rubinchik, V.; Kelly, B. Photodynamic therapy using Verteporfin (benzoporphyrin derivative monoacid ring A, BPD-MA) and 630 nm laser light in canine esophagus. Lasers Surg. Med. 2002, 30, 26–30. [Google Scholar] [CrossRef]
- Cramer, G.; Lewis, R.; Gymarty, A.; Hagan, S.; Mickler, M.; Evans, S.; Punekar, S.R.; Shuman, L.; Simone, C.B.; Hahn, S.M.; et al. Preclinical Evaluation of Cetuximab and Benzoporphyrin Derivative-Mediated Intraperitoneal Photodynamic Therapy in a Canine Model. Photochem. Photobiol. 2020, 96, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Horimatsu, T.; Muto, M.; Yoda, Y.; Yano, T.; Ezoe, Y.; Miyamoto, S.; Chiba, T. Tissue Damage in the Canine Normal Esophagus by Photoactivation with Talaporfin Sodium (Laserphyrin): A Preclinical Study. PLoS ONE 2012, 7, e38308. [Google Scholar] [CrossRef]
- Ross, H.M.; Smelstoys, J.A.; Davis, G.J.; Kapatkin, A.S.; Del Piero, F.; Reineke, E.; Wang, H.; Zhu, T.C.; Busch, T.M.; Yodh, A.G.; et al. Photodynamic Therapy with Motexafin Lutetium for Rectal Cancer: A Preclinical Model in the Dog. J. Surg. Res. 2006, 135, 323–330. [Google Scholar] [CrossRef]
- Griffin, G.M.; Zhu, T.; Solonenko, M.; Del Piero, F.; Kapakin, A.; Busch, T.M.; Yodh, A.; Polin, G.; Bauer, T.; Fraker, D.; et al. Preclinical evaluation of motexafin lutetium-mediated intraperitoneal photodynamic therapy in a canine model. Clin. Cancer Res. 2001, 7, 374–381. [Google Scholar]
- Hsi, R.A.; Kapatkin, A.; Strandberg, J.; Zhu, T.; Vulcan, T.; Solonenko, M.; Rodriguez, C.; Chang, J.; Saunders, M.; Mason, N.; et al. Photodynamic therapy in the canine prostate using motexafin lutetium. Clin. Cancer Res. 2001, 7, 651–660. [Google Scholar]
- Zhu, T.C.; Hahn, S.M.; Kapatkin, A.S.; Dimofte, A.; Rodriguez, C.E.; Vulcan, T.G.; Glatstein, E.; Hsi, R.A. In vivo Optical Properties of Normal Canine Prostate at 732 nm Using Motexafin Lutetium-mediated Photodynamic Therapy. Photochem. Photobiol. 2007, 77, 81–88. [Google Scholar] [CrossRef]
- Du, K.L.; Mick, R.; Busch, T.M.; Zhu, T.C.; Finlay, J.C.; Yu, G.; Yodh, A.G.; Malkowicz, S.B.; Smith, D.; Whittington, R.; et al. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg. Med. 2006, 38, 427–434. [Google Scholar] [CrossRef]
- Lucroy, M.D.; Edwards, B.F.; Peavy, G.M.; Krasieva, T.B.; Griffey, S.M.; Stiles, J.B.; Madewell, B.R. Preclinical study in cats of the pro-photosensitizer 5-aminolevulinic acid. Am. J. Vet. Res. 1999, 60, 1364–1370. [Google Scholar] [PubMed]
- Chen, Q.; Huang, Z.; Luck, D.; Beckers, J.; Brun, P.-H.; Wilson, B.C.; Scherz, A.; Salomon, Y.; Hetzel, F.W. Preclinical Studies in Normal Canine Prostate of a Novel Palladium-Bacteriopheophorbide (WST09) Photosensitizer for Photodynamic Therapy of Prostate Cancer. Photochem. Photobiol. 2002, 76, 438. [Google Scholar] [CrossRef] [PubMed]
- Dole, K.C.; Chen, Q.; Hetzel, F.W.; Whalen, L.R.; Blanc, D.; Huang, Z. Effects of Photodynamic Therapy on Peripheral Nerve: In Situ Compound-Action Potentials Study in a Canine Model. Photomed. Laser Surg. 2005, 23, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Anidjar, M.; Scarlata, E.; Hamel, L.; Scherz, A.; Ficheux, H.; Borenstein, N.; Fiette, L.; Elhilali, M. Preclinical Study of the Novel Vascular Occluding Agent, WST11, for Photodynamic Therapy of the Canine Prostate. J. Urol. 2011, 186, 302–309. [Google Scholar] [CrossRef]
- Liu, W.; Chen, N.; Jin, H.; Huang, J.; Wei, J.; Bao, J.; Li, C.; Liu, Y.; Li, X.; Wang, A. Intravenous repeated-dose toxicity study of ZnPcS2P2-based-photodynamic therapy in beagle dogs. Regul. Toxicol. Pharmacol. 2007, 47, 221–231. [Google Scholar] [CrossRef]
- Selman, S.H.; Albrecht, D.; Keck, R.W.; Brennan, P.; Kondo, S. Studies of tin ethyl etiopurpurin photodynamic therapy of the canine prostate. J. Urol. 2001, 165, 1795–1801. [Google Scholar] [CrossRef]
- Selman, S.H.; Keck, R.W.; Hampton, J.A. Transperineal photodynamic ablation of the canine prostate. J. Urol. 1996, 156, 258–260. [Google Scholar] [CrossRef]
- Aniola, J.; Selman, S.H.; Lilge, L.; Keck, R.; Jankun, J. Spatial distribution of liposome encapsulated tin etiopurpurin dichloride (SnET2) in the canine prostate: Implications for computer simulation of photodynamic therapy. Int. J. Mol. Med. 2003, 11, 287–291. [Google Scholar] [CrossRef]
- Xiao, Z.; Owen, R.J.; Liu, W.; Tulip, J.; Brown, K.; Woo, T.; Moore, R.B. Lipophilic photosensitizer administration via the prostate arteries for photodynamic therapy of the canine prostate. Photodiagn. Photodyn. Ther. 2010, 7, 106–114. [Google Scholar] [CrossRef]
- Lin, N.; Li, C.; Wang, Z.; Zhang, J.; Ye, X.; Gao, W.; Wang, A.; Jin, H.; Wei, J. A safety study of a novel photosensitizer, sinoporphyrin sodium, for photodynamic therapy in Beagle dogs. Photochem. Photobiol. Sci. 2015, 14, 815–832. [Google Scholar] [CrossRef]
- Osaki, T.; Hibino, S.; Yokoe, I.; Yamaguchi, H.; Nomoto, A.; Yano, S.; Mikata, Y.; Tanaka, M.; Kataoka, H.; Okamoto, Y. A Basic Study of Photodynamic Therapy with Glucose-Conjugated Chlorin e6 Using Mammary Carcinoma Xenografts. Cancers 2019, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H.; Reichert II, K.W.; Ozker, K.; Meyer, G.A.; Donohoe, D.L.; Bajic, D.M.; Whelan, N.T.; Whelan, H.T. Preclinical Evaluation of Benzoporphyrin Derivative Combined with a Light-Emitting Diode Array for Photodynamic Therapy of Brain Tumors. Pediatr. Neurosurg. 1999, 30, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.Q.; Jin, P.; Li, H.T.; Zhang, R.R.; Ren, X.L.; Wang, H.B.; Tang, D.; Tian, W.R. Apoptosis induced by hematoporphyrin monomethyl ether combined with He–Ne laser irradiation in vitro on canine breast cancer cells. Vet. J. 2011, 188, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Song, X.Y.; Yang, C.; Li, Q.; Tang, D.; Tian, W.R.; Liu, Y. Effect of hematoporphyrin monomethyl ether-mediated PDT on the mitochondria of canine breast cancer cells. Photodiagn. Photodyn. Ther. 2013, 10, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tong, J.; Bao, J.; Tang, D.; Tian, W.; Liu, Y. Hematoporphyrin monomethyl ether combined with He-Ne laser irradiation-induced apoptosis in canine breast cancer cells through the mitochondrial pathway. J. Vet. Sci. 2016, 17, 235–242. [Google Scholar] [CrossRef]
- Rocha, M.S.T.; Lucci, C.M.; Longo, J.P.F.; Galera, P.D.; Simioni, A.R.; Lacava, Z.G.M.; Tedesco, A.C.; Azevedo, R.B. Aluminum-Chloride-Phthalocyanine Encapsulated in Liposomes: Activity Against Naturally Occurring Dog Breast Cancer Cells. J. Biomed. Nanotechnol. 2012, 8, 251–257. [Google Scholar] [CrossRef]
- Narumi, A.; Rachi, R.; Yamazaki, H.; Kawaguchi, S.; Kikuchi, M.; Konno, H.; Osaki, T.; Okamoto, Y.; Shen, X.; Kakuchi, T.; et al. Maltotriose–Chlorin e6 Conjugate Linked via Tetraethyleneglycol as an Advanced Photosensitizer for Photodynamic Therapy. Synthesis and Antitumor Activities against Canine and Mouse Mammary Carcinoma Cells. ACS Omega 2021, 6, 7023–7033. [Google Scholar] [CrossRef]
- Turna, O.; Baykal, A.; Sozen Kucukkara, E.; Ozten, O.; Deveci Ozkan, A.; Guney Eskiler, G.; Kamanli, A.F.; Bilir, C.; Yildiz, S.Z.; Kaleli, S.; et al. Efficacy of 5-aminolevulinic acid-based photodynamic therapy in different subtypes of canine mammary gland cancer cells. Lasers Med. Sci. 2022, 37, 867–876. [Google Scholar] [CrossRef]
- Osaki, T.; Kunisue, N.; Ota, U.; Imazato, H.; Ishii, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; Okamoto, Y. Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Cancers 2021, 13, 4174. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Gomes, A.T.P.C.; Moura, N.M.M. Chlorins in Photodynamic Therapy—Synthesis and applications. Rev. Virtual Química 2009, 1, 92–103. [Google Scholar] [CrossRef]
| Ref. | Neoplasm | Patients | PDT Protocol | Main Results |
|---|---|---|---|---|
| [44] | SCC | 12 Cats | PS: HPD, 1–5 mg/mg, iv Light: LED, 300 J/cm2, 630 nm, 1 session |
|
| [101] | SCC | 4 Dogs | PS: HPD, 5 mg/kg, iv Light: Argon laser, 293–900 J/cm2, 631 nm, 1–3 sessions |
|
| [101] | Circumanal gland carcinoma | 1 Dog | PS: HPD, 5 mg/kg, iv Light: Argon laser, 144–400 J/cm2, 631 nm, 1 session |
|
| [79] | SCC | 1 Dog | PS: HPD, 2.5 mg/kg, iv Light: Argon laser, 348 J/cm2, 631 nm, 1 session |
|
| [79] | Adenocarcinoma, oral cavity | 1 Dog | PS: HPD, 2.5 mg/kg, iv Light: Argon laser, 100 J/cm2, 631 nm, 1 session |
|
| [79] | SCC, gastric mucosa | 1 Dog | PS: HPD, 2.5 mg/kg, iv Light: Argon laser, 240 J/cm2, 631 nm, 1 session |
|
| [102] | Esophageal SCC | 1 Dog | PS: Porfimer sodium, 2.7 mg/kg, iv Light: Argon laser, 200–250 J/cm3, 630 nm, 3 sessions |
|
| [82] | Lung carcinoma | 3 Dogs | PS: Porfimer sodium, 2 mg/kg, oral Light: Diode laser, 200 J/cm, 630 nm, 1 session |
|
| [86] | SCC | 11 Cats | PS: 5-ALA, cream 20%, topical Light: LED, 12 J/cm2, 635 nm, 1 session |
|
| [85] | SCC | 55 Cats | PS: 5-ALA, cream 20%, topical Light: LED, 12 J/cm2, 635 nm, 1 session |
|
| [95] | Transitional cell carcinoma | 6 Dogs | PS: 5-ALA, 60 mg/kg, oral Light: Diode laser, 100 J/cm−2, 635 nm, 1–3 sessions |
|
| [71] | Transitional cell carcinoma | 2 Dogs | PS: 5-ALA, 40 mg/kg, oral Light: LED, 300 J/cm2, 635 nm, 3 sessions; Diode laser, 270 J/cm2, 630 nm, 15 sessions |
|
| [71] | Adenocarcinoma | 2 Dogs | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 270 J/cm2, 700 J/cm, 630 nm, 15–19 sessions |
|
| [71] | Sebaceous gland carcinoma | 1 Cat | PS: 5-ALA, 40 mg/kg, oral Light: LED, 120 J/cm2, 635 nm, 4 sessions |
|
| [103] | Transitional cell carcinoma of the prostate | 1 Dog | PS: 5-ALA, 60 mg/kg Light: Diode laser, 100 J/cm2, 75 mW/cm2, 635 nm, 1 session |
|
| [104] | Prostate Carcinoma | 6 Dogs | PS: 5-ALA, intratumoral Light: Halogen lamp, 75 J/cm2, 570-670 nm, 1 session |
|
| [105] | SCC | 4 Dogs | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 40–90 J/cm2, 690 nm, 1 session |
|
| [106] | SCC | 3 Dogs | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 80–600 J/cm2, 690 nm, 1–2 sessions |
|
| [106] | Adenocarcinoma | 3 Dogs | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 50–350 J/cm2, 200–300 J/cm, 690 nm, 1–2 sessions |
|
| [107] | Basal cell carcinoma | 1 Cat | PS: PAD-S31, 15 mg/kg, iv Light: Argon laser, 150 J/cm2, 670 nm, 3 sessions |
|
| [108] | Intranasal carcinoma | 3 Dogs | PS: Talaporfin sodium, 5.0 mg/kg, iv Light: Diode laser, 100 J/cm2, 665 nm, 1–3 sessions |
|
| [96] | SCC | 8 Cats | PS: EtNBS, 5 mg/kg, iv Light: Diode laser, 400–800 J/cm2, 652 nm, 1–2 sessions |
|
| [96] | SCC | 2 Dogs | PS: EtNBS, 2.0–2.5 mg/kg, iv Light: Diode laser, 303–400 J/cm2, 652 nm, 1–2 sessions |
|
| [109] | Intranasal carcinoma | 2 Dogs | PS: HPPH, 0.3 mg/kg, iv Light: Potassium titanyl phosphate–pumped dye laser, 100 J/cm2, 665 nm, 1 session |
|
| [109] | Intranasal carcinoma | 1 Cat | PS: HPPH, 0.3 mg/kg, iv Light: Potassium titanyl phosphate–pumped dye laser, 100 J/cm2, 665 nm, 1 session |
|
| [110] | SCC (facial lesions) | 51 Cats | PS: HPPH, 0.3 mg/kg, iv Light: Argon laser, 100 J/cm2, 665 nm, 1–3 sessions |
|
| [111] | SCC | 3 Dogs | PS: HPPH, 0.15 mg/mg, iv Light: LED, 100 J/cm2, 665 nm, 1 session |
|
| [111] | Adenocarcinoma apocrine gland | 1 Dog | PS: HPPH, 0.15 mg/mg, iv Light: LED, 100 J/cm2, 665 nm, 1 session |
|
| [111] | SCC | 3 Cats | PS: HPPH, 0.15 mg/mg, iv Light: LED, 100 J/cm2, 665 nm, 1 session |
|
| [112] | Oral SCC | 11 Dogs | PS: HPPH, 0.3 mg/kg, iv Light: Argon laser, 100 J/cm2, 665 nm, 1 session |
|
| [113] | SCC | 8 Cats | PS: CASPc, 1 mg/kg, iv Light: Argon laser, 100–150 J/cm2, 675 nm, 1–2 sessions |
|
| [113] | Carcinoma in situ | 3 Cats | PS: CASPc, 1.0 mg/kg, iv Light: Argon laser, 100 J/cm2, 675 nm, 1–2 sessions |
|
| [83] | SCC | 15 Cats | PS: CASPc, 1 mg/kg, iv Light: Argon laser, 50–150 J/cm2, 675 nm, 1 session |
|
| [114] | Cutaneous SCC | 10 Cats | PS: CASPc, 1.0 mg/kg, iv Light: Argon laser, 100–200 J/cm2, 675 nm, 1 session |
|
| [84] | SCC | 6 Dogs | PS: ZnPcS4, 1–4 mg/mg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| [93] | SCC | 18 Cats | PS: Liposomal mTHPC, 0.15 mg/mg, iv Light: Diode laser, 10 J/cm2, 652 nm, 1 session |
|
| [18] | SCC | 38 Cats | PS: Liposomal mTHPC, 0.15 mg/mg, iv Light: Diode laser, 10–20 J/cm2, 652 nm, 1 session |
|
| [115] | SCC | 10 Cats | PS: Liposomal and lipophilic mTHPC, 0.15 mg/mg, iv Light: Diode laser, 10 J/cm2, 652 nm, 1 session |
|
| [116] | SCC | 6 Cats | PS: Liposomal and lipophilic mTHPC, 0.15 mg/mg, iv Light: Diode laser, 10 J/cm2, 652 nm, 1 session |
|
| [117] | Adenocarcinoma | 3 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [117] | Carcinoma transitional | 1 Dogs | PS: AO, 1 μg/mL, tumor bed and 0.1 mg/kg, iv Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [117] | Carcinoma undifferentiated | 1 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [118] | Intranasal carcinoma | 14 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | Adenocarcinomas | 14 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | Transitional cell carcinomas | 10 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | SCC | 2 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | Adenosquamous carcinomas | 2 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | Carcinoma | 3 Dogs | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | Undifferentiated adenocarcinomas | 1 Dog | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| [60] | Undifferentiated carcinomas | 1 Dog | PS: AO, 1 μg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 1 session |
|
| Ref. | Neoplasm | Patients | PDT Protocol | Main Results |
|---|---|---|---|---|
| [101] | Mastocytoma (Lips) | 1 Cat | PS: HPD, 5.0 mg/kg, iv Light: Argon laser, 324 J/cm2, 631 nm, 2 sessions |
|
| [101] | Mastocytoma (Lips) | 1 Dog | PS: HPD, 5.0 mg/kg, iv Light: Argon laser, 103 J/cm2, 631 nm, 1 session |
|
| [71] | Mast cell tumor | 1 Dog | PS: 5-ALA, 40 mg/kg, oral Light: LED, 60 J/cm2, 635 nm, 1 session |
|
| [107] | Mast cell tumor | 2 Dogs | PS: PAD-S31, 15 mg/kg, iv Light: Argon laser, 150 J/cm2, 670 nm, 1 session |
|
| [96] | Mast cell tumor | 2 Dogs | PS: EtNBS, 2.0 mg/kg, iv Light: Diode laser, 100–400 J/cm2, 652 nm, 1–2 sessions |
|
| [113] | Mast cell tumor | 1 Dog | PS: CASPc, 1.0 mg/kg, iv Light: Argon laser, 50–100 J/cm2, 675 nm, 1 session |
|
| [84] | Mast cell tumor | 1 Dog | PS: ZnPcS4, 4.0 mg/kg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| Ref. | Neoplasm | Patients | PDT Protocol | Main Results |
|---|---|---|---|---|
| [101] | Reticulum cell sarcoma | 1 Dog | PS: HPD, 5 mg/kg, iv Light: Argon laser, 120/585 J/cm2, 631 nm, 1 session |
|
| [101] | Fibrosarcoma | 1 Dog | PS: HPD, 0.5 mg/kg, iv Light: Argon laser, 300 J/cm2, 631 nm, 1 session |
|
| [71] | Histiocytoma | 1 Dog | PS: 5-ALA, 40 mg/kg, oral Light: LED, 240 J/cm2, 635 nm, 5 sessions |
|
| [71] | Chondrosarcoma | 1 Dog | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 800 J/cm, 630 nm, 16 sessions |
|
| [71] | Hemangiopericytoma | 1 Dog | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 200 J/cm, 630 nm, 11 sessions |
|
| [71] | Fibrosarcoma | 1 Cat | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 270 J/cm2, 630 nm, 5 sessions |
|
| [119] | Osteosarcoma | 7 Dogs | PS: BPD-MA, 0.4 mg/kg, iv Light: 690 ± 5 nm, 500 J, 1 session |
|
| [120] | Osteosarcoma | 7 Dogs | PS: BPD-MA, 0.4 mg/kg, iv Light: Diode laser, 500 J/cm, 690 nm, 1 session |
|
| [105] | Fibrosarcoma | 3 Dog | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 525 J/cm, 600–1275 J/cm2, 690 nm, 1 session |
|
| [105] | Osteosarcoma | 1 Dog | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 800 J/cm2, 690 nm, 1 session |
|
| [106] | Fibrosarcoma | 5 Dogs | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 300–600 J/cm2, 450–525 J/cm, 690 nm, 1–2 sessions |
|
| [106] | Osteosarcoma | 1 Dog | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 150–950 J/cm2, 690 nm, 5 sessions |
|
| [106] | Sarcoma | 1 Dog | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 800 J/cm2, 690 nm, 1 session |
|
| [121] | Hemangiopericytoma | 16 Dogs | PS: HPPH, 0.3 mg/kg, iv Light: Argon laser dye, 100 J/cm2, 665 nm, 1 session |
|
| [109] | Chondrosarcoma | 1 Dog | PS: HPPH, 0.3 mg/kg, iv Light: Potassium titanyl phosphate–pumped dye laser, 100 J/cm2, 665 nm, 2 sessions |
|
| [83] | Fibrosarcoma | 3 Dogs | PS: CASPc, 1 mg/kg, iv Light: Argon laser, 50–150 J/cm2, 675 nm, 1 session |
|
| [83] | Hemangiopericytoma | 4 Dogs | PS: CASPc, 1 mg/kg, iv Light: Argon laser, 50–150 J/cm2, 675 nm, 1 session |
|
| [113] | Sarcoma | 1 Dog | PS: CASPc, 1 mg/kg, iv Light: Argon laser, 100 J/cm2, 675 nm, 1 session |
|
| [84] | Histiocytoma | 1 Dog | PS: ZnPcS4, 0.5 mg/kg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| [84] | Spindle cell Sarcoma | 1 Dog | PS: ZnPcS4, 2 mg/kg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| [84] | Undifferentiated Sarcoma | 1 Dog | PS: ZnPcS4, 4.0 mg/kg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| [37] | Hemangiosarcoma | 8 Dogs | PS: AlClPc-nano, 13.3 μM, intra/peritumoral Light: LED, 120 J/cm2, 658–662 nm, 1–4 sessions |
|
| [122] | Malignant soft tissue sarcoma | 10 Dog | PS: ICG, 5 mg/9 mL, tumor bed Light: Broadband light, 48 J/cm2, 600–800 nm, 3–21 sessions |
|
| [122] | Malignant soft tissue sarcoma | 6 Cats | PS: ICG, 5 mg/9 mL, tumor bed Light: Broadband light, 48 J/cm2, 600–800 nm, 3–21 sessions |
|
| [123] | Injection-site sarcoma | 37 Cats | PS: AO, 1 µg/mL Light: Surgical light, 24 V/250 Watt, 80,000 Lux, 1 session |
|
| [124] | Sarcomas | 12 Dogs | PS: Porfimer sodium, 3.0 mg/kg, iv; AlClPc, 0.175–0.589 mg/kg, iv; SnET2, 0.83 mg/kg, iv Light: Argon laser, 150–280 J/cm2, 1 session |
|
| Ref. | Neoplasm | Patients | PDT Protocol | Main Results |
|---|---|---|---|---|
| [79] | Melanoma, hard palate | 1 Dog | PS: HPD, 2.5 mg/kg, iv Light: Argon laser, 528–960 J/cm2, 631 nm, 3 sessions |
|
| [96] | Melanoma, sclera | 1 Dog | PS: EtNBS, 2.0 mg/kg, iv Light: Diode laser, 200 J/cm2, 652 nm, 1 session |
|
| [84] | Melanoma, soft palate | 1 Dog | PS: ZnPcS4, 0.25 mg/kg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| [125] | Melanoma, eyelid | 1 Cat | PS: AO, 1 µg/mL, tumor bed Light: Xenon light, 20.7 mW/cm2, 400–700 nm, 2 sessions |
|
| Ref. | Neoplasm | Patients | PDT Protocol | Main Results |
|---|---|---|---|---|
| [79] | Eosinophilic granuloma | 1 Cat | PS: HPD, 2.5 mg/kg, iv Light: Argon laser, 480 J/cm2, 631 nm, 1 session |
|
| [79] | Sebaceous glands adenoma | 1 Dog | PS: HPD, 2.5 mg/kg, iv Light: Argon laser, 288 J/cm2, 631 nm, 1 session |
|
| [71] | Mammary gland tumor | 2 Cats | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 20 J/cm2, 200 J/cm, 630 nm, 3 sessions |
|
| [71] | Inflammatory mammary gland tumor | 1 Dog | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 1.000 J/cm, 630 nm, 5 sessions |
|
| [71] | Myeloma | 1 Dog | PS: 5-ALA, 40 mg/kg, oral Light: Diode laser, 270 J/cm2, 630 nm, 16 sessions |
|
| [105] | Ameloblastoma | 2 Dogs | PS: BPD-MA, 0.5 mg/mg, iv Light: Diode laser, 1.275 J/cm2, 75 J/cm, 690 nm, 1 session |
|
| [84] | Viral papilloma | 1 Dog | PS: ZnPcS4, 4 mg/kg, iv Light: Diode laser, 100 J/cm2, 675 ± 0.2 nm, 1 session |
|
| [126] | Spontaneous advanced primary prostate cancer | 1 Dog | PS: WST09, 2.0 mg/kg, iv Light: Diode laser, 100–150 J/cm, 763 nm, 1 session |
|
| Ref. | Disease, Model | Animals | PDT Protocol | Main Results |
|---|---|---|---|---|
| [129] | Glioma, cell inoculation in the brain | 8 Dogs | PS: Porfimer sodium, 0.75–4.0 mg/kg, iv Light: Laser light, 1800 J, 630 nm, 1 session |
|
| [130] | Esophageal cancer, normal tissue | 11 Dogs | PS: Porfimer sodium, 2–4 mg/kg, iv Light: Argon laser, 75–600 J/cm, 630 nm, 1 session |
|
| [131] | Esophageal cancer, normal tissue | 8 Dogs | PS: Porfimer sodium, 4 mg/kg, iv Light: Argon laser, 300–600 J/cm, 630 nm, 1 session |
|
| [132] | Esophageal cancer, normal tissue | 10 Dogs | PS: Porfimer sodium, 4 mg/kg, iv Light: Argon laser, 300 J/cm, 630 nm, 1 session |
|
| [81] | Esophageal cancer, normal tissue | 7 Dogs | PS: Porfimer sodium, 4 mg/kg, iv Light: Argon laser, 300 J/cm, 630 nm; KTP/532 laser, 300 J/cm, 532 nm, 1 session |
|
| [133] | Bladder cancer, normal tissue | 13 Dogs | PS: Porfimer sodium, 1.5 mg/kg, iv Light: Red light, 15 J/cm2, 630 nm, 1–3 sessions |
|
| [134] | Pleural cancers, normal tissue | 15 Dogs | PS: HPD, 6 mg/kg, iv Light: Argon laser, 5–40 J/cm2, 630 nm, 1 session |
|
| [135] | Sarcoma, cell inoculation in the inguinal region | 5 dogs | PS: HPD, 5 mg/kg; iv Light: Excimer dye laser; 0–1200 J/cm, 630 nm, 1 session |
|
| [136] | Laryngeal cancer, normal tissue | 11 Dogs | PS: HPD, 3.0 mg/kg, iv Light: Argon laser, 10–150 J/cm2, 630 nm, 1 session |
|
| [137] | Prostate cancer, normal tissue | 7 Dogs | PS: HPD, 2.0 mg/kg, iv Light: Argon laser, 450 J/cm, 630 nm, 1 session |
|
| [95] | Bladder cancer, normal tissue | 9 Dogs | PS: 5-ALA, 30–90 mg/kg, oral Light: Tunable dye laser with titanyl potassium phosphate, 100 J cm−2, 635 nm, 1 session |
|
| [138] | Prostate cancer, normal tissue | 11 dogs | PS: 5-ALA, 100–200 mg/kg, iv; AlS2Pc, 1 mg/kg, iv Light: Diode laser, 100–1080 J, 630 nm, 1 session |
|
| [139] | Prostate cancer, normal tissue | 15 Dogs | PS: mTHPC, 0.3 mg/kg, iv; AlS2Pc, 1.0 mg/kg, iv Light: KTP/532 and Argon laser, 100–200 J/cm, 650 nm, 1 session |
|
| [140] | Prostate cancer, normal tissue | 9 Dogs | PS: mTHPC, 0.15 mg/kg, iv Light: Diode laser, 10–20 J/cm2, 652 nm, 1 session |
|
| [141] | Esophageal cancer, normal tissue | 24 Dog | PS: BPD-MA, 0.75 mg/kg, iv Light: Laser, 40–200 J/cm, 630 nm, 1 session |
|
| [142] | Peritoneal carcinomatosis, normal tissue | 15 Dogs | PS: BPD-MA, 0.125–0.25 mg/kg, iv Light: Diode laser, 690 nm, 2–10 J/cm2, 1 session |
|
| [143] | Esophageal cancer, normal tissue | 9 Dogs | PS: Talaporfin sodium, 20 mg/kg, iv Light: Diode laser, 25–100 J/cm2, 664 nm, 1 session |
|
| [144] | Rectal cancer, normal tissue | 6 Dogs | PS: MoLut, 2.0 mg/kg, iv Light: Quartz tungsten halogen lamp, 0.5–10 J/cm2, 730 nm, 1 session |
|
| [145] | Peritoneal carcinomatosis and sarcomatosis, normal tissue | 11 Dogs | PS: MoLut, 0.2–2.0 mg/kg, iv Light: Diode laser, 0.5–2.0 J/cm2, 730 nm, 1 session |
|
| [146] | Prostate cancer, normal tissue | 25 Dogs | PS: MoLut, 2.0–6.0 mg/kg, iv Light: KTP/532 and Diode laser, 75–150 J/cm, 732 nm, 1 session |
|
| [147] | Prostate cancer, normal tissue | 12 Dogs | PS: MoLut, 2.0–6.0 mg/kg, iv Light: Diode laser, 100–150 J/cm, 732 nm, 1 session |
|
| [148] | Prostate cancer, normal tissue | 16 Dogs | PS: MoLut, 2–6 mg/kg, iv Light: 75–150 J/cm, 732 nm, 1 session |
|
| [150] | Prostate cancer, normal tissue | 16 Dogs | PS: WST09, 2.0 mg/kg, iv Light: Diode laser, 100–200 J/cm2, 50–200 J/cm, 763 nm, 1 session |
|
| [126] | Prostate cancer, normal tissue | 19 Dogs | PS: WST09, 0.25–2.0 mg/kg, iv Light: Diode laser, 50–300 J/cm, 763 nm, 1–2 sessions |
|
| [151] | Prostate cancer, normal tissue | 9 Dogs | PS: WST09, 1.0–2.0 mg/kg, iv Light: Diode laser, 50–200 J/cm2, 763 nm, 1 session |
|
| [152] | Prostate cancer, normal tissue | 37 Dogs | PS: WST09, 2.0 mg/kg, iv; WST11, 2.0–30 mg/kg, iv Light: Diode laser, 100–400 J/cm, 753–763 nm, 1 session |
|
| [153] | Leukemia, normal tissue | 24 Dogs | PS: ZnPcS2P2, 0.5–4.5 mg/kg, iv; Light: Red light laser, 7 J/cm2, 670 nm, 10 sessions |
|
| [154] | Prostate cancer, normal tissue | 15 Dogs | PS: SnET2, 0.5–1.0 mg/kg, iv Light: Diode laser, 100–200 J/cm, 664 ± 7 nm, 1 session |
|
| [155] | Prostate cancer, normal tissue | 9 Dogs | PS: SnET2, 1.0 mg/kg, iv Light: KTP/YAG laser, 200 J/cm, 660 nm, 1 session |
|
| [156] | Prostate cancer, normal tissue | 5 Dogs | PS: SnET2, 1.0 mg/kg, iv Light: Diode laser, 200 J/cm, 665 nm, 1 session |
|
| [157] | Prostate cancer, normal tissue | 20 Dogs | PS: SL052, 0.75–18 mg/kg, iv, ia Light: Diode laser, 200–600 J/cm3, 635 nm, 1 session |
|
| [158] | Sarcoma, normal tissue | 48 Dogs | PS: Sinoporphyrin sodium, 1–9 mg/kg, iv Light: Red light, 76 J/cm2, 630 nm, 5 sessions |
|
| [159] | Canine mammary carcinoma, cells were inoculated subcutaneously | 6 Mice | PS: G-Ce6, 10 mg/kg, iv Light: Diode laser, 100 J/cm3, 677 nm, 1 session |
|
| Photosensitizer | Carcinomas | Mastocytomas | Sarcomas | Melanomas | ||||
|---|---|---|---|---|---|---|---|---|
| Dogs | Cats | Dogs | Cats | Dogs | Cats | Dogs | Cats | |
| HPD | 8 | 12 | 1 | 1 | 2 | - | 1 | - |
| Porfimer sodium | 4 | - | - | - | 12 | - | - | - |
| 5-ALA | 17 | 67 | 1 | - | 3 | 1 | - | - |
| BPD-MA | 10 | - | - | - | 25 | - | - | - |
| PAD-S31 | - | 1 | 2 | - | - | - | - | - |
| Talaporfin sodium | 3 | - | - | - | - | - | - | - |
| EtNBS | 2 | 8 | 2 | - | - | - | 1 | - |
| HPPH | 17 | 55 | - | - | 17 | - | - | - |
| CASPc | - | 36 | 1 | - | 8 | - | - | - |
| mTHPC | - | 72 | - | - | - | - | - | - |
| AO | 52 | - | - | - | - | 37 | - | 1 |
| ZnPcS4 | 6 | - | 1 | - | 3 | - | 1 | - |
| AlClPc-nano | - | - | - | - | 8 | - | - | - |
| ICG | - | - | - | - | 10 | 6 | - | - |
| Total | 119 | 251 | 8 | 1 | 88 | 44 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, T.G.; Cardoso, K.M.; Marto, C.M.; Teixo, R.; Serambeque, B.; Silva, F.C.e.; Alexandre, N.; Botelho, M.F.; Laranjo, M. Oncological Applications of Photodynamic Therapy in Dogs and Cats. Appl. Sci. 2022, 12, 12276. https://doi.org/10.3390/app122312276
Guimarães TG, Cardoso KM, Marto CM, Teixo R, Serambeque B, Silva FCe, Alexandre N, Botelho MF, Laranjo M. Oncological Applications of Photodynamic Therapy in Dogs and Cats. Applied Sciences. 2022; 12(23):12276. https://doi.org/10.3390/app122312276
Chicago/Turabian StyleGuimarães, Tarcísio Guerra, Karla Menezes Cardoso, Carlos Miguel Marto, Ricardo Teixo, Beatriz Serambeque, Fernando Capela e Silva, Nuno Alexandre, Maria Filomena Botelho, and Mafalda Laranjo. 2022. "Oncological Applications of Photodynamic Therapy in Dogs and Cats" Applied Sciences 12, no. 23: 12276. https://doi.org/10.3390/app122312276
APA StyleGuimarães, T. G., Cardoso, K. M., Marto, C. M., Teixo, R., Serambeque, B., Silva, F. C. e., Alexandre, N., Botelho, M. F., & Laranjo, M. (2022). Oncological Applications of Photodynamic Therapy in Dogs and Cats. Applied Sciences, 12(23), 12276. https://doi.org/10.3390/app122312276










