Featured Application
The findings described in this paper could be treated as recommendations in a physical therapy program for DMD patients that focus on defining conditions along with the weight (light or heavy) that could give better muscle response in rehabilitation of DMD patients.
Abstract
(1) Background: This study presents a new method for the motion quantitative analysis of Duchenne muscular dystrophy patients (DMD) performing functional tasks in clinical conditions. (2) Methods: An experimental study was designed to define how different levels of external mass (light and heavy) influence the performance of the upper limbs of a tested DMD and reference subject (RS) during horizontal movements (level of the waist) under natural conditions (NC) and passive manipulator conditions (PMC); the kinematic data and muscle activity of four chosen superficial muscles (EMG) were recorded. (3) Results: A piecewise linear multi-regression analysis revealed high statistically significant results (R2 ≥ 0.75) between the tested muscle activities and kinematic data in the tested DMD patient and RS. These results were used to define whether the neural control of the four tested muscles (processed EMG data) was correlated more with the displacement of the wrist joint or the center of mass of the tested upper limb while performing functional tests with a given external weight in a horizontal plane under NC or PMC. (4) Conclusions: The proposed approach can be used to estimate the contributions of the tested muscles to patients’ motion performance and define whether this contribution is correlated with the kinematics or dynamics of the tested arm.
1. Introduction
Duchenne muscular dystrophy (DMD) is a genetic X-linked neuromuscular disorder characterized by progressive muscle weakness that step-by-step affects the lower limbs, upper limbs, and trunk, leading to progressive respiratory failure and cardiomyopathy [1,2,3,4]. To date, therapies for DMD patients have focused on delaying the disease’s progression in time because a cure does not yet exist for this illness. As the upper extremities get weaker, the patients’ activities of daily living (ADL) become difficult or impossible to perform and they lose their motor independence. To maintain this independence, customized external devices have to be designed to help perform ADL by using the upper limbs, e.g., passive or active end-effector devices, manipulators, or exoskeletons [5,6]. Additionally, this equipment may allow us to assess some parameters of motor function and adjust the level of therapy to the current condition of the patient [7,8,9].
To assess muscle function, surface electromyography (EMG) can be used for recording muscle activity while performing given physical activities. Even though DMD patients have high levels of fatty and connective tissues in their muscles, clinicians can reveal further muscle damage based on EMG data [10].
It is widely known that muscle forces are related to the functional ability of the upper limb in different external conditions [11,12,13,14]. According to [15], we should broaden our knowledge referring to muscle coordination in daily life and how perturbations influence the musculoskeletal system. However, most of the reported studies were conducted under laboratory conditions and mainly healthy subjects were tested for different biomechanical purposes.
Studies reported about correlation between the muscle activity and motor function of the upper extremity in tested DMD patients [15,16] and healthy patients equipped with an external device [17,18]. Moreover, a study [19] describes the values of the maximum joint angles of the upper limbs of DMD patients during passive and active motions along with the EMG data obtained during the motions performed in the given anatomical planes and axes. On the other hand, evidence of the force of the wrist extension and thumb adduction along with the range of motion of the upper limbs of DMD patients is presented in [20].
In the literature, one can find indexes (biomechanical metrics) used to assess the upper limb pathology [21] and disease progression [16,22], e.g., Pediatric Upper Limb Motion Index [23], Upper Limb Motion Deviation Index [24], and Upper Body Index, which assess the manipulative movements of upper limbs [19]. The reported indexes are mainly focused on the range of motion or difference between the maximum and minimum values of the chosen joint angles, the kinematics of the movement, or the duration of the tested phases.
The motion of a limb is performed by controlling the kinematics of this limb or its dynamics through muscle activations. By identifying the type of control that the tested patient uses to perform the motion, the clinician can design the most suitable strategy for the rehabilitation of the patient. Additionally, it is worth noting that kinematic assessments requiring repetitive movements are limited in DMD patients due to fatigue, which is a characteristic symptom of DMD [8,10]. That is why assessing the quality of motion in functional tasks is a key factor to diagnosing the condition of the DMD patient and planning a subsequent intervention to help them [25,26]. In this study, the quality of motion is treated as a collection of the following features. The first part of these features assesses the contribution of the tested muscles to controlling: (1) a displacement of the upper limb center of mass (COM) (describing the relation between dynamics of the limb and muscle activity); (2) a displacement of the upper-limb wrist joint (WJ) (describing the relation between the kinematics of the limb and muscle activity). The results of the contributions are assessed as: (a) distributions of the synergistic/antagonistic participation of the tested muscles at the time of the motion; (b) the accumulated synergistic/antagonistic participation of the tested muscles in the tested motion. The second part of the features defines whether: (1) the passive manipulator changes the muscle activity in the functional movement and the kinematics of this functional movement; (2) the muscle activities of the four tested muscles depend on an external weight which is moved in a horizontal plane (horizontal motion) at the level of the waist.
The motivation of this study was to answer the following questions: (1) How are the muscles of DMD patients activated in comparison to those of healthy subjects? (2) What movement strategies do DMD patients use to perform ADL functional tasks? (3) How does an external passive device influence the kinematics of the movements of DMD patients versus healthy ones in ADL functional tasks?
The purpose of this study was: (1) to propose a new approach to assess the quality of motion in ADL functional tasks under clinical conditions by revealing whether muscle activity is correlated more to the displacement of the upper limb COM or to the displacement of the upper-limb WJ; (2) to investigate whether different levels of external mass influence the performance of the dominant right upper limbs of DMD patients in natural conditions and using a passive lightweight manipulator that is a low-friction (non-resistant) device. In this study, the upper limb performance was examined by recording horizontal movements at the level of the waist and the muscle activity of four chosen superficial muscles and then comparing these results to those of a reference subject (a healthy adolescent).
The results of the assessment of the quality of motion are presented in two main parts. The first part involves the results of the application of a piecewise linear multi-regression to define the contribution of the tested muscles to controlling the displacement of the upper limb COM and the displacement of the upper-limb WJ. The second part involves the results of the chosen tests of significance aimed at identifying whether the use of a passive manipulator evoked changes in the muscle activity and changes in the kinematics of functional movements.
2. Materials and Methods
2.1. Participants
A control group composed of 12 healthy teenager/adolescent boys (14.33 ± 1.82 years, 66.87 ± 21.5 kg, 170.45 ± 10.66 cm) was analyzed to choose a reference subject. A group of 5 DMD patients was examined (15.20 ± 2.16 years, 73.60 ± 22.06 kg, 162.20 ± 3.49 cm) with a median of 3 on the Brooke Upper Extremity Scale (1–5). All selected DMD patients were non-ambulant boys with diagnosed DMD confirmed by genetic testing and/or muscle biopsy, without difficulties with cooperation (autism spectrum or concentration disorder). All volunteers (boys of the control group and DMD patients and their parents) provided written informed consent in accordance with procedures approved by the agreement of the Ethic Committee of Medical University of Gdansk NKBBN/23/2019, NKBBN/23-708/2019, NKBBN/23-409/2020.
2.2. Tasks and Organization of the Experiment
All tested subjects performed motions according to the protocol aimed to assess ADL functional movements of the upper limbs (Supplement S1). During a visit, each subject was assessed in the given conditions (to avoid fatigue which was detrimental for each DMD patient). For this study, one DMD patient and one reference subject were chosen. From the DMD patient group, the medical doctors selected the oldest one with the worst upper-limb motor function (Brooke 5). The reason for this choice was to focus on a less-independent DMD patient, i.e., the one with the worst motor activity. From the control group, the medical doctors selected one reference subject (RS) who fulfilled the posed criteria: (1) lack of postural disorders; (2) right-hand dominance; (3) anthropometric proportion close to that of the chosen DMD patient (DMD). The lengths of arm–forearm measured in (cm) were 32–27 (RS) and 30–25 (DMD). It is well known that there is an influence of the body’s morphological characteristics on the fitness and movement parameters [27]. Flow chart of patient quantification process and the choise of (DMD–RS) pair for comparison is described in Figure 1.
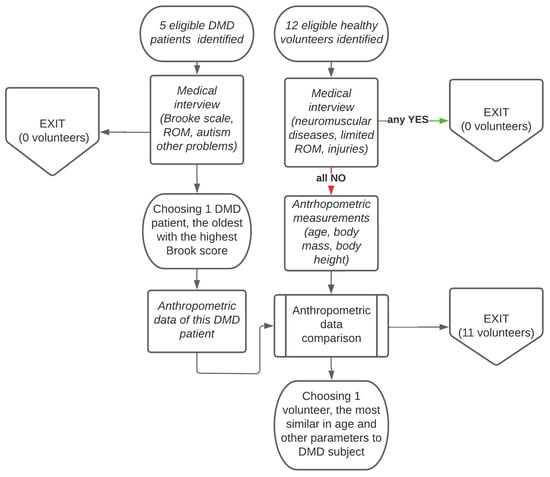
Figure 1.
Flow chart of patient quantification process and the pair (DMD–RS) chosen for comparison.
Performing motions according to the protocol, each tested subject from the control group sat on the stool without a backrest and each DMD patient used his wheelchair without armrests. To perform the motion, the table was adjusted to be even with the subject’s waist. Experimental study was designed to compare functional movements with two different masses (light and heavy): (1) under natural conditions (NC); (2) under passive manipulator conditions (PMC), i.e., by using a passive manipulator attached to the wrist. The scope of this study involved only horizontal-motion testing because these motions can be performed by DMD patients in the advanced stage of the disease. Functional activities were tested by moving a light weight (100 g, called motion1) and heavy weight (1000 g, called motion2) on the table (on the height of the tested subject’s waist) from the initial position localized on the right edge of the table to the end position localized at the midline of subject’s body (Figure 2). Each trial was performed after providing verbal instruction. For the reference subject, the series was composed of five trials and for the DMD patient, the series was composed of three trials. The number of trials was chosen after the recommendation given in the referenced publication [19,28]. Trials were performed naturally at the subject’s own pace without any initial learning and correcting. To avoid fatigue between each trial, there was a break for a few minutes.
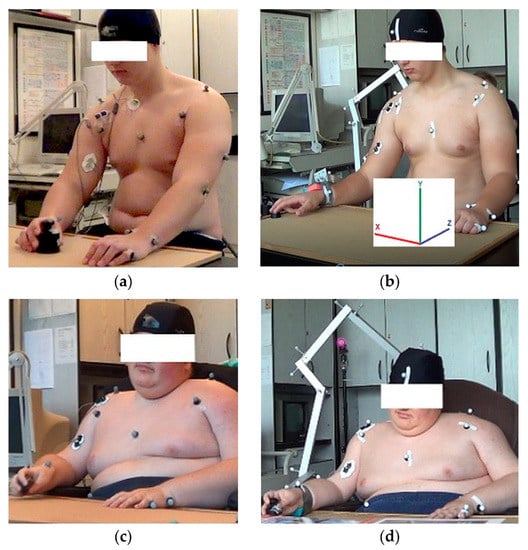
Figure 2.
(a) Reference subject (RS) under natural conditions (NC); (b) Reference subject (RS) with a passive manipulator (under passive manipulator conditions (PMC)); (c) DMD patient (DMD) under natural conditions (NC); (d) DMD patient (DMD) with a passive manipulator (under passive manipulator conditions (PMC)).
2.3. Measurement Technique and Equipment
Kinematic data were captured by using the OptiTrack system (6 cameras, 120 Hz). To capture the position of the right and left upper limbs (hand, forearm, and arm), head, neck, and torso, the passive markers were used and set according to the OptiTrack protocol to identify described segments of the body.
To collect EMG data, the Noraxon MyoTrace400 system (4 channels, 1000 Hz of sampling frequency) was used. This technique is well known and widely used in assessing muscle activity [29]. The reference electrode was attached to the medial clavicular head (RS in NC) or olecranon (RS in PMC, DMD in NC, and PMC). To collect data, the Noraxon dual EMG disposable electrodes were placed according to SENIAM recommendations [30] on the properly prepared skin above four muscle bellies: long head of biceps brachii (BB, EMG1), lateral head of triceps brachii (TB, EMG2), anterior deltoid (AD, EMG3), and upper trapezius (UT, EMG4). To reduce motion artefacts, all electrodes/cables were protected by using medical tape. The commercial MyoResearchXP software (Noraxon, Scottsdale, AZ, USA) and MATLAB R2020b (MathWorks, Natick, MA, USA) were used to collect raw data, record, and process these EMG data. The processing included filtering, rectification, and smoothing by using RootMeanSquare (RMS) algorithm with a 50 ms window (this algorithm was implemented in MyoResearchXP). Due to the fact that during one visit the chosen DMD patient was not able to perform maximum voluntary contraction (MVC) without fatigue, the processed EMG data were normalized with respect to the maximum value registered during the test (reference voluntary contraction). The activity of each examined muscle was assessed by applying our own codes written in MATLAB; the EMG threshold was equal to 0.005 and electromechanical delay was equal to 50 ms. The time scale was normalized to the motion timing and described as a percentage of motion.
To perform testing under PMC, a passive manipulator composed of four serially linked segments (the first shoulder joint, the second shoulder joint, the third shoulder joint and elbow joint) was used. All segments were fixed by low-friction pin connections allowing performing rotations (Figure 2b,d). This manipulator was manufactured from the aluminum alloy thin-walled beam and its weight was 357 g with the following mass distribution between segments: 26:16:26:32. The first shoulder joint segment was fixed to the backrest of the DMD patient’s wheelchair or back of the stool used to test the RS. The second point of the elbow segment (a collar) was fixed to the wrist joint of the tested subject by using medical tape and soft foam placed between the skin and the collar. The passive manipulator dimensions were designed to maintain the working space of the upper limb of each subject from the control group and DMD patient group.
2.4. Implementation of Motion Quantitative Analysis
During each test, a track of the wrist joint (WJ) was recorded with respect to x, y, and z axes (Figure 2b). The position of COM with respect to these x, y, and z axes was assessed by applying segmentation. Each displacement was calculated as a relation between the difference in current position and initial position divided by the maximum position estimated in the tested trial. In this study, we analyzed: (1) the x-th, y-th, and z-th displacement of the wrist joint (WJx, WJy, WJz) and absolute displacement of the wrist joint (ΔWJ) calculated as ; (2) the x-th, y-th, and z-th displacement of the upper limb COM (COMx, COMy, COMz) and absolute displacement of COM (ΔCOM) assessed as .
Each muscle activation was calculated as the difference between normalized processed EMG registered in the trial and normalized processed EMG registered at the initial stage of the trial.
A piecewise linear multi-regression (the first part of approach for assessment of quality of motion) was applied to correlate muscle activations (EMG1, EMG2, EMG3, EMG4) and the kinematic data of displacement of the wrist joint (WJx, WJy, WJz, ΔWJ) or upper limb COM (COMx, COMy, COMz, ΔCOM) in each tested window that was equal to 1% of the length of the motion fragment (composed of 10 frames). Assuming that muscle activations are independent variables (EMG1, EMG2, EMG3, EMG4) and each kinematic datum y (WJx, WJy, WJz, ΔWJ, COMx, COMy, COMz and ΔCOM) is dependent variable, the relation was formulated as:
where (for j = 0, 1, 2, 3, 4) describes the j-th participation (contribution) in the value of the tested kinematic data y; a0 describes the contribution of the motion of trunk and fingers, as well as pronation/supination of the wrist joint and/or upper limb passive structures and/or non-monitored active muscles (); (i = 1, …, 4) describes the coefficient of the i-th muscle activation (this coefficient depends on the muscle lever arm). The product between this coefficient ai and muscle activation EMGi defines the contribution of the i-th muscle to the motion performance, i.e., , , , .
Linear piecewise multi-regression analysis was performed in MATLAB by using our own codes and statistics toolbox. Assuming a threshold of statistical significance in which p was equal to 0.05 (p ≤ 0.05), only statistically significant results of relationships in multi-regression analysis (Equation (1)) with a coefficient of determination (R2) that was greater than 0.75 (R2 ≥ 0.75) were considered. Due to the fact that each muscle contribution (y1, y2, y3, y4) (Equation (1)) can be positive (synergistic participation), negative (antagonistic), or zero, the results of multi-regression analysis were presented as: (1) distributions of synergistic and antagonistic participation at the time of the motion; (2) summarizing synergistic and antagonistic participations. To consider all of the synergetic and antagonistic participations in each tested window, the i-th muscle contribution was normalized and presented in the form of participation:
Significance tests (the second part of approach for assessment of quality of motion) were performed in five arbitrarily chosen ranges for the performed motions: 2% (range 1), 10% (range 2), 38% (range 3), 66% (range 4), and 96% (range 5). The length of each range was equal to the tested window. These arbitrary ranges were chosen to test motor behavior in the initial phase (range 1), acceleration phase (range 2), phase of acceleration-to-middle transition (range 3), phase of middle-to-slowing down transition (range 4), and final phase related to the immobilization (range 5). Due to the fact that kinematic data and muscle activation did not have normal distributions (Shapiro–Wilk test), we used non-parametric tests of significance. To define the influence of the passive manipulator on the natural motions, the statistically significant differences were assessed by applying (threshold of statistical significance was assumed to be p ≤ 0.01): (1) Wilcoxon test between each kinematic data (WJx, WJy, WJz, ΔWJ, COMx, COMy, COMz, ΔCOM) obtained under NC and PMC for each tested subject, i.e., the RS and the DMD, respectively; (2) Mann–Whitney U test between each kinematic data (WJx, WJy, WJz, ΔWJ, COMx, COMy, COMz, ΔCOM) obtained for RS and DMD patients in each tested condition, i.e., under NC and PMC, respectively; (3) Wilcoxon test between each muscle activation (EMG1, EMG2, EMG3, EMG4) obtained under NC and PMC for each tested subject, i.e., the RS and the DMD patients, respectively; (4) ANOVA Kruskal–Wallis test between five sets of muscle activations (EMG1, EMG2, EMG3, EMG4) obtained under NC and PMC for each tested subject, i.e., the RS and the DMD patients, respectively. All tests of significance were performed in STATISTICA 13.1. Additionally, visual inspections have been conducted to analyze: (1) the normalized mean motion trajectories (Figure 3 and Figure S1 (Supplement S2)) and 3D mean trajectories (Figures S2 and S3 (Supplement S2)); (2) processed EMG amplitude obtained for RS and DMD patients under NC and PMC (Figure 4 and Figure S4 (Supplement S2)).
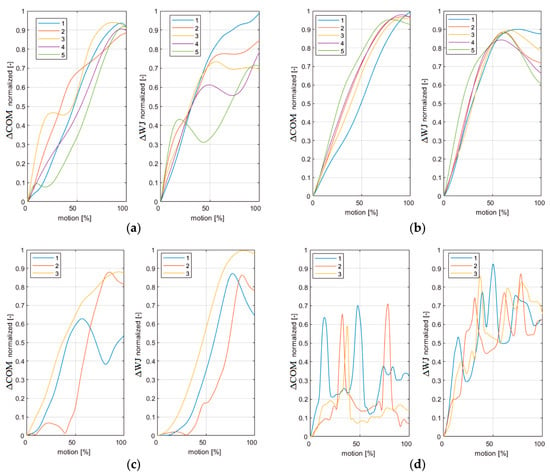
Figure 3.
(a) Reference subject in motion1 under natural conditions; (b) Reference subject in motion1 under passive manipulator conditions; (c) DMD subject in motion1 under natural conditions; (d) DMD subject in motion1 under passive manipulator conditions.
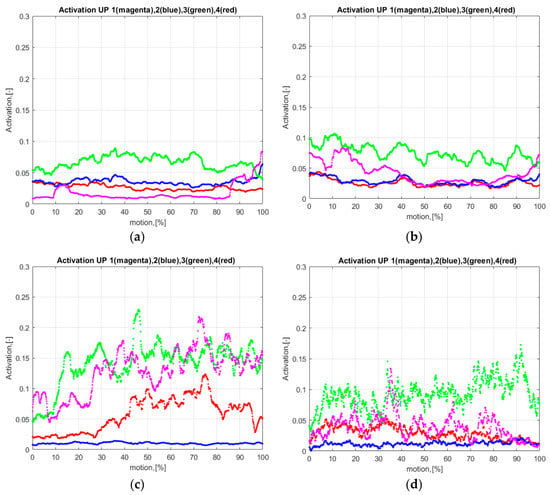
Figure 4.
EMG data processed (mean value of the RMS normalized data): BB (EMG1), TB (EMG2), AD (EMG3), and UT (EMG4): (a) Reference subject in motion1 under natural conditions; (b) Reference subject in motion1 under passive manipulator conditions; (c) DMD subject in motion1 under natural conditions; (d) DMD subject in motion1 under passive manipulator conditions.
3. Results
On the base of the normalized absolute displacements in the upper limb COM and wrist joint, one can state that the RS’s kinematics in all five trials in motion1 and motion2 show a higher repeatability under PMC (Figure 3a,b and Figure S1a,b). In contrast, the DMD’s kinematics in all three trials are less smooth and less repeatable for both motions (Figure 3c,d and Figure S1c,d). Note that the RS did not perform any trunk motions during testing. On the basis of the mean trajectories (Figures S2 and S3), the following observations were made: (1) under NC, the RS used greater movements of the shoulder, elbow, and wrist joints and had greater COM displacements (Figures S2a and S3a) in comparison to the movements of DMD patient (Figures S2c and S3c); (2) Under PMC, the DMD patient’s shoulder joint movements are greater with respect to the ones of the RS (Figures S2b,d and S3b,d).
By considering the processed EMG data (Figure 4 and Figure S4), increased amplitudes of EMG processed data were found in the UT, AD, and BB muscles of the DMD patient in comparison to those of the RS. Moreover, we revealed that the DMD patient’s muscle activity was increased in UT, AD, and BB from the beginning of the movement in motion2 in comparison to motion1 (Figure 4c and Figure S4c).
3.1. Results of Distributions of Synergistic and Antagonistic Participations at the Time of the Motion
Statistically significant results with a coefficient of determinacy R2 greater than 0.75 (R2 ≥ 0.75) are given in Figure 5a,b and Figures S5–S66 (Supplement S3). On the base of the visual inspection of the shapes of the kinematic parts (, , , , ), we identified three types of behavior: (1) the first type involves the antagonistic/synergetic dominance of ppart resented in the form of an arranged series (this part (y0 = a0) is the result of the accumulated contribution of non-monitored active muscles, the motions of the trunk and finger, and the pronation/supination of the wrist joint and upper-limb passive structures); (2) the second type describes the fully unarranged participation of all the tested parts (y0, y1, y2, y3, y4); (3) the third type defines the partly arranged participation of the kinematic parts related to the activity of the tested muscles (y1, y2, y3, y4) (these parts were presented in the form of a series arranged within some fragments). Identifying the type of behavior, we defined the contributions of the tested muscles to the motion performance. For the RS, we identified the following results:
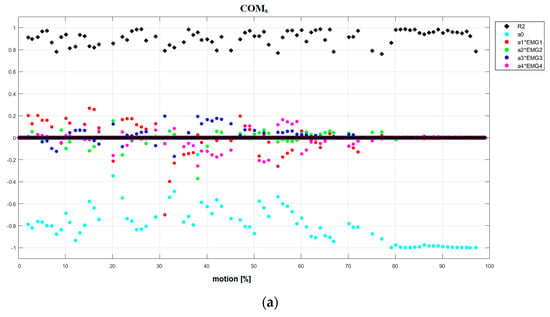
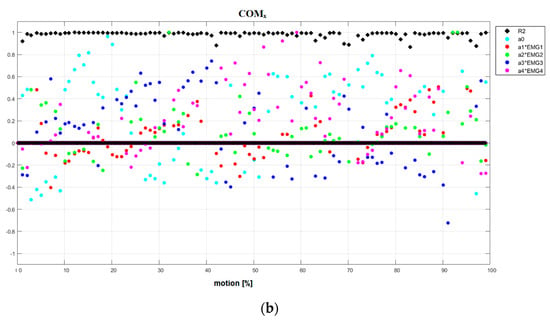
Figure 5.
(a) Results of linear piecewise multi-regression analysis performed in each tested fragment of the motion for reference subject in Motion1 under natural conditions. COMx. (b) Results of linear piecewise multi-regression analysis performed in each tested fragment of the motion for DMD patient in motion1 under natural conditions. COMx.
- NC Motion1 (Figure 5a and Figures S5–S11): the first type: the dominance of antagonistic part a0 (WJx, COMx, COMz) and synergetic part (ΔWJ); the second type: (WJy, COMy); and the third type: (WJz, ΔCOM).
- NC Motion2 (Figures S20–S27): the first type: the dominance of antagonistic part a0 (WJx, COMx, COMz) and synergetic part (ΔWJ, ΔCOM); the second type: (WJy, COMy), and the third type: (WJz).
- PMC Motion1 (Figures S12–S19): the first type: the dominance of antagonistic part a0 (WJx, WJz) and synergetic part (WJy, ΔWJ, COMy, ΔCOM); the third type: (COMx, COMz).
- PMC Motion2 (Figures S28–S35): the first type: the dominance of antagonistic part a0 (WJx, COMx) and synergetic part (ΔWJ, COMy, COMz, ΔCOM); the third type: (WJy, WJz).
For the DMD patient’s results, we identified the following:
- NC Motion1 (Figure 5b and Figures S36–S42): the second type (WJy, COMx, COMz, ΔCOM), the third type (WJx, WJz, ΔWJ, COMy).
- NC Motion2 (Figures S51–S58): the second type (COMy), the third type (WJx, WJy, WJz, ΔWJ, COMx, COMz, ΔCOM).
- PMC Motion1 (Figures S43–S50) and PMC Motion2 (Figures S59–S66): the second type (WJy, COMx, COMy, COMz, ΔCOM), the third type (WJx, WJz, ΔWJ).
3.2. Results of Accumulated Synergistic and Antagonistic Participations in Tested Motions
Aiming to define the summarized contributions of the tested muscles in each motion performance for each tested subject, we calculated the accumulated results of the kinematic parts related to the activity of the tested muscles (y1, y2, y3, y4) (Tables S5–S12 and Figures S67–S74 in Supplement S4). We identified the following largest parts for the RS:
- NC Motion1 (Table S5): two synergetic ( in COMy and WJy results) and three antagonistic parts ( in WJz, in WJy, in WJy results).
- NC Motion2 (Table S7): three synergetic ( in WJy and COMy, in WJz results) and three antagonistic parts ( in COMy and WJy, in WJz results).
- PMC Motion1 (Table S6): two synergetic ( in ΔCOM and WJz results) and two antagonistic parts ( in COMz, in WJx results).
- PMC Motion2 (Table S8): two synergetic ( in WJy, in COMz results) and one antagonistic part ( in WJy results).
For the DMD:
- NC Motion1 (Table S9): four synergetic ( in ΔCOM and WJz, in COMx and WJy results) and two antagonistic parts ( in COMz and WJx results).
- NC Motion2 (Table S11): one synergetic ( in COMy results) and three antagonistic parts ( in COMz, in WJz, in WJx results).
- PMC Motion1 (Table S10): two synergetic ( in COMy, in WJy results) and two antagonistic parts ( in the COMy, in COMz results).
- PMC Motion2 (Table S12): three synergetic ( in COMy, WJy, and ΔCOM results) and two antagonistic parts ( in WJx and COMx results).
To define whether PMC invokes different activities of the tested muscles with respect to the NC in motion1 and motion2, the accumulated results of the participation (p.3.1 and Tables S5–S12) were analyzed and we found that:
- In the case of motion1, the DMD patient under NC (Figure S71) has higher contributions of y3 (a3∙EMG3) and y4 (a4∙EMG4) parts with respect to those of the PMC (Figure S72). Under NC, the DMD patient has the largest antagonistic a3∙EMG3 part related to COMz. In PMC, the DMD patient also uses the synergistic a2∙EMG2 part related to COMy. Meanwhile, the RS under NC (Figure S67) has a higher participation of the tested muscles with respect to that of the PMC (Figure S68);
- In the case of motion2, the DMD patient under NC (Figure S73) has different muscle contributions (y1, y2, y3, y4) with respect to the PMC (Figure S74): while under NC the largest synergistic a4∙EMG4 part is related to COMy, thus under PMC the synergistic a1∙EMG1 part has the largest participation, in which COMy has the highest priority. Meanwhile, the RS under NC mainly activates the synergistic a3∙EMG3 and a4∙EMG4 parts related to WJy and COMy (Figure S69). However, under PMC the RS mostly activates the antagonistic a2∙EMG2 part related to WJy (Figure S70).
3.3. Results of Significance Tests
The statistically significant differences in the chosen motion ranges are shown in (Tables S1–S4 in Supplement S4) and include the following results:
- The kinematic data obtained under NC and PMC (Table S1) for the RS (87.5% in motion1 and 75.0% in motion2) and DMD patient (77.5% in motion1 and 75.0 % in motion2);
- The kinematic data obtained for the RS and DMD patient (Table S2) under NC (75% in motion1 and 80% in motion2) and PMC (80% in motion1 and 70% in motion2);
- Muscle activations obtained under NC and PMC (Table S3) for the RS (55% in motion1 and motion2) and DMD patient (60% in motion1 and 55% in motion2). The most similar muscle activations were obtained in motion2 for the EMG3 of the RS and the EMG2 of the DMD patient.
Analyzing ANOVA Kruskal–Wallis results obtained under NC and PMC for each tested subject (RS and DMD) in all the chosen motion ranges (Table S4), we revealed that there are the following similarities in muscle activations (i.e., the lowest number of statistically significant differences): (1) EMG1-EMG2-EMG3 set for the RS under PMC for both motions; (2) EMG1-EMG2-EMG4 set for the RS in motion1 under PMC; (3) EMG2-EMG3-EMG4 set for the RS in motion1 and DMD patient in motion2 under PMC; (4) EMG1-EMG3-EMG4 set for the RS in motion1 and DMD patient in motion2 under PMC; (5) EMG1-EMG2-EMG3-EMG4 set for the RS in motion1 under PMC.
4. Discussion
In the scope of the presented study, we focused on assessing the functional movements without giving any verbal or visual instructions related to the restricted path of the upper limb movement. The tested motions were natural reactions without the influence of the effects of learning related to the external passive manipulator used in this study. The analyzed motion occurred in horizontal plane XZ, where the x-th axis had the medio–lateral direction, the z-th axis had the anterior–posterior direction, and the y-th axis was parallel to the gravity force (Figure 2b).
4.1. First Part of Approach for Assessment of Quality of Motion
A piecewise linear multi-regression was used to assess the contributions of the five parts composing the kinematic data y (WJx, WJy, WJz, ΔWJ, COMx, COMy, COMz, ΔCOM): (1) the a0 part assesses the contribution of non-monitored active muscles, passive structures/tissues, and motion of the trunk and finger, and the supination–pronation of the wrist joint; (2) four muscle parts assess the contributions of four tested muscles (, , , ). Their contributions were assessed in two different external conditions (NC and PMC) by using two different external weights to perform the motion (light (motion1) and heavy (motion2)). We found highly statistically significant results (R2 ≥ 0.75) between the tested muscle activities (treated as independent variables) and the kinematic data (treated as dependent variables) in tested the RS and DMD patient and depict these results in Figure 5a,b and Supplement S3 and S4. Considering the shapes of the distributions of the synergistic/antagonistic contributions at the time of the motion (statistically significant results of the multi-regression analysis), we identified three types of behavior (p.3.1) that can be interpreted in the following ways: (1) the first type means that the kinematic data y is under the direct control of the visual and/or proprioception senses and the main participation has non-monitored active muscles and passive structures/tissues, motions of the trunk and finger, or pronation/supination of the wrist joint; (2) the second type means that tested kinematic data y is not under the control of visual nor proprioception senses; (3) the third type means that within some fragments of the motion, the considered kinematic data y (related to the activity of testing muscles (y1, y2, y3, y4)) is under the direct control of visual and/or proprioception senses.
We show that the revealed statistically significant correlations between muscle activity and: (1) the displacement of the wrist joint (ΔWJx, ΔWJy, ΔWJz, ΔWJ) is a manifestation of the kinematics control of the tested arm motion which may be mainly controlled by the visual sense and the proprioception sense (position sense); (2) the displacement of upper-limb COM is an indication of the dynamics control of the tested arm performance which could be mainly controlled by the proprioception sense. One can argue that WJ displacement mainly occurs due to the control of visual and proprioception senses. However, the COM displacement can be mainly controlled by the proprioception sense because the visual sense cannot identify the position of the COM in space (COM is a fictitious point assessed on the base of biomechanics).
Considering the results of the distributed participations in time (p.3.1), we revealed the following similarities between the RS and DMD patient. Both tested subjects used the third type of behavior to realize: (1) the dynamic control focused on the x-th coordinate (COMx) in motion2 under N; (2) the kinematic control focused on the z-th coordinate (WJz) in motion1 under NC, motion2 under NC, and in motion2 under PMC.
4.2. Second Part of Approach for Assessment of Quality of Motion
Analyzing the results obtained for the chosen motion ranges (Table S1), we revealed differences in the kinematic data of the RS as well as in the DMD patient under PMC. These results indicate that instead of achieving the same functional goal (moving the external weight 100 g/1000 g in the horizontal plane from the outside to the midline of the body), the passive manipulator changed the trajectory of movement. Moreover, the EMG analysis (Table S3) showed that in the chosen motion ranges, there are significant differences in the muscular activity under PMC in comparison with NC. However, the differences were not revealed in all muscles. We found that the movement pattern of the DMD patient is different in comparison to the movement of the RS (Table S2). However, there is a lack of statistically significant differences in some ranges: (1) in motion1 under NC in COMz, WJy, ΔCOM, and ΔWJ results; (2) in motion2 under NC in ΔCOM, WJy, and COMy results; (3) in motion1 under PMC in ΔCOM, WJy, and ΔWJ results; and (4) in motion2 under PMC in ΔWJ, WJx and ΔCOM results. We suggest that these similarities may be induced by the configuration of the tested upper limb to perform each functional task in a horizontal plane.
Based on the ANOVA Kruskal–Wallis results, we found that the RS and the DMD patient under NC used different muscle activities to perform each motion in nearly all of the tested ranges (Table S4). Analyzing the results under PMC, we defined that: (1) in case of the RS, the most similar muscular activity is observed in both motions in the EMG1-EMG2-EMG3 set and in motion1 in the EMG1-EMG2-EMG4 set; (2) in case of the DMD patient, the most similar muscular activity is observed in motion2 in the EMG1-EMG3-EMG4 and EMG2-EMG3-EMG4 sets.
Regarding the repeatability on the basis of the motion shape in the five RS trials and the three DMD patient trials (Figure 3 and Figure S1), we found that only in case of the RS did the passive manipulator facilitate motion and provide a more repeatable trajectory with a small variability. These findings agree with the evidence given in [26,30]. Comparing the shape of the motion of the RS and DMD patient under NC, we found no repeatability. Our findings are in contrast to the results of [27], which report a high similarity in shapes of the gestures performed by forearm and hand of the DMD patient and healthy subject.
In the case of the DMD patient, we observed compensation motions performed by his trunk. This behavior is reported in the literature, e.g., an analysis of kinematics revealed that DMD patients use increased trunk lateral bending and/or flexion-extension while performing reaching tasks and ADL tasks [20,25]. Additionally, comparing the mean motion trajectories of the tested subjects under NC, we revealed very small motions of DMD displacements related to the upper-limb COM, shoulder joint, elbow joint, and wrist joint. This behavior was caused by the compensation of the hand joints and fingers (in this study we did not analyze the kinematic data of these segments). However, under PMC this DMD patient performed more motions in his shoulder joint with respect to those of the RS. It is worth noting that the DMD patient examined in our study had a score of 5 on the Brooke scale. This is the lowest Brooke score, which indicates very little mobility in the upper limb. Our findings agree with ones described in [29], which states that the decrease in the active range of motion of the limb leads to its long static positioning (immobilization). This results in the development of contractures in joints of the upper limbs and compensation motions.
By analyzing muscle activity of the DMD patient in motion with the light and heavy weights, we revealed that the amplitude of the EMG data is increased. These findings agree with those previously published [31]. In motion2, increased muscle activity was observed during the beginning phase of this movement. This observation may suggest that the DMD patient tried to prepare his upper limb to perform a motion with greater resistance.
5. Conclusions
The approach presented in this study can be applied to estimate the contributions of the tested muscles to motion performance and to define whether the tested muscles contribute more to the control of the displacement of the upper-limb COM (dynamics relation) or to the control of displacement of the wrist joint (kinematics relation). By examining the defined contributions and type of control (kinematics and dynamics) along with the type of behavior (the first, second, or third) in each tested direction (x, y, z), clinicians can deduce about the disease’s progress and follow a suitable rehabilitation strategy. Moreover, the proposed method can be used to reveal: (1) whether PMC can evoke different activity of the tested muscles with respect to the NC in the tested motions; (2) whether the tested horizontal motion is mainly caused by the tested muscles or other factors, including the motion of the trunk.
The findings described in this paper could be treated as a recommendation in a physical therapy program for DMD patients that focusing on defining conditions (NC or PMC) along with the weight (light or heavy) that could give better muscle response. However, two scenarios should be considered. First, if the tested subject treats a passive manipulator as a facilitating device, then they reduce their muscle activity (the subject will be trying to unload themselves and load more onto this external device). Second, the tested subject associates this passive manipulator with resistance/obstacles and changes his/her muscle activity in two ways: (1) increasing (if the subject is determined to succeed in performing a task by using a passive manipulator); (2) decreasing (if the subject is determined to fail a task by applying this passive manipulator). It should be emphasized that there is still a lack of knowledge concerning the amount, intensity, and weight that has to be used to enhance the motor skills of DMD patients [25,26]. Our study provides grounds for further studies of the factors influencing muscle activity increases.
The presented results indicate that functional motions should be analyzed as a multidimensional task. The success of the functional task performance strongly depends on the subject’s perception of the external device used (especially the lightweight passive manipulator). Further studies should focus on revealing how the trajectory of motion is changing while learning, especially while moving different weights without any learning nor adaptation to the applied passive manipulator.
We believe that the proposed method for the analysis of functional tasks is relevant to communicate to encourage similar studies aimed at analyzing DMD patient motion performance and to find ways of maintaining motor abilities despite the progression of muscle weakness.
5.1. Study Limitations
In this paper, we presented the results of a pilot study involving testing one reference subject (a healthy teenager/adolescent) and one chosen DMD patient (a young adult) in two functional horizontal motions (with light and heavy weights) under two different conditions (NC and PMC). A study limitation is the lack of normalization due to the MVC method, which makes the results less comparable to those of other studies—we decided not to use maximal contractures because the DMD patients are not used to performing such activities that might provoke fatigue and result in a worse performance of tested tasks.
5.2. Recommendation
Using a passive manipulator, DMD patients can learn to use an active exoskeleton in a guidance mode. Our study can be used to address the main question in the field of DMD physiotherapy: whether the application of a passive manipulator can evoke a neural command which can better control muscles and move the arm of a DMD patient in a smoother way [32,33].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122312247/s1. Supplement S1—motion protocol to ADL functional motion test; Supplement S2—(Figures S1–S4); Supplement S3—(Figures S5–S66); Supplement S4—(Tables S1–S12, Figures S67–S74).
Author Contributions
Conceptualization, W.W. and A.S.-R.; methodology, W.W. and A.S.-R.; software, W.W.; validation, A.S.-R., B.Z. and M.L.; formal analysis, W.W. and A.S.-R.; investigation, W.W., A.S.-R., B.Z., M.L., J.J.-B. and K.F.; resources, A.S.-R. and J.J.-B.; data curation, W.W., B.Z. and M.L.; writing—original draft preparation, W.W. and A.S.-R.; writing—review and editing, W.W. and A.S.-R.; visualization, M.L.; supervision, B.Z.; project administration, W.W. and A.S.-R.; funding acquisition, W.W. and A.S.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project “The e-Pionier-using tertiary education institutions’ potential to boost ICT solutions in the public sector-, No. WG-POPC.03.03.00-00-0008/16-00, Custom-made device to assist the motor functions of the upper limb with muscular dystrophy”. This paper was also supported by the Grant for Young Scientists from Lodz University of Technology.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Ethic Committee of Medical University of Gdansk NKBBN/23/2019, NKBBN/23-708/2019, NKBBN/23-409/2020.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We would like to thank our colleagues for taking part in development of this project: Dominika Szalewska (Medical University of Gdansk), Marek Chodnicki, Michał Mazur, Maciej Kaczmarczyk, and Anna Jednachowska (Gdansk University of Technology). The calculations were carried out at the Academic Computer Centre in Gdańsk (TASK), Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Nomenclature | |
| DMD (Duchenne muscular dystrophy) | UT (upper trapezius) |
| ADL (Activities of daily living) | RMS (Root Mean Square) |
| EMG (surface electromyography) | MVC (maximum voluntary contraction) |
| COM (center of mass) | x, y, z (the x-th, y-th and z-th axis in XYZ coordinate system) |
| WJ (wrist joint) | EMG1 (EMG collected from BB) |
| RS (reference subject) | EMG2 (EMG collected from TB) |
| NCS (natural conditions) | EMG3 (EMG collected from AD) |
| PMCS (passive manipulator conditions, i.e., passive manipulator is attached to the wrist joint) | EMG4 (EMG collected from UT) |
| BB (biceps brachii) | ΔCOM (absolute displacement of COM) |
| TB (lateral head of triceps brachii) | R2 (coefficient of determination) |
| AD (anterior deltoid) | p (threshold of statistical significance) |
References
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Neuromuscular, Rehabilitation, Endocrine, and Gastrointestinal and Nutritional Management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Wasilewska, E.; Sobierajska-Rek, A.; Małgorzewicz, S.; Soliński, M.; Szalewska, D.; Jassem, E. Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the Covid-19 Pandemic?—A One Center Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 8967. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, E.; Sobierajska-rek, A.; Śledzińska, K.; Małgorzewicz, S.; Jassem, E.; Wierzba, J. Morbidity, Clinical Course and Vaccination against Sars-cov-2 Virus in Patients with Duchenne Muscular Dystrophy: A Patient Reported Survey. Int. J. Environ. Res. Public Health 2022, 19, 406. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska-Rek, A.; Mański, Ł.; Jabłońska-Brudło, J.; Śledzińska, K.; Wasilewska, E.; Szalewska, D. Respiratory Telerehabilitation of Boys and Young Men with Duchenne Muscular Dystrophy in the Covid-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 6179. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Prat, J.; Bergsma, A.; Vroom, E. 2nd Workshop on Upper-Extremity Assistive Technology for People with Duchenne: Effectiveness and Usability of Arm Supports. Neuromuscul. Disord. 2019, 29, 651–656. [Google Scholar] [CrossRef]
- Pirondini, E.; Coscia, M.; Marcheschi, S.; Roas, G.; Salsedo, F.; Frisoli, A.; Bergamasco, M.; Micera, S. Evaluation of the Effects of the Arm Light Exoskeleton on Movement Execution and Muscle Activities: A Pilot Study on Healthy Subjects. J. Neuroeng. Rehabil. 2016, 13, 9. [Google Scholar] [CrossRef]
- Lo, H.S.; Xie, S.Q. Medical Engineering & Physics Exoskeleton Robots for Upper-Limb Rehabilitation: State of the Art and Future Prospects. Med. Eng. Phys. 2012, 34, 261–268. [Google Scholar] [CrossRef]
- Jansen, M.; van Alfen, N.; Geurts, A.C.; de Groot, I.J. Assisted Bicycle Training Delays Functional Deterioration in Boys with Duchenne Muscular Dystrophy: The Randomized Controlled Trial “No Use Is Disuse”. Neurorehabil. Neural Repair 2013, 27, 816–827. [Google Scholar] [CrossRef]
- Kong, D.; Wang, W.; Guo, D.; Shi, Y. RBF Sliding Mode Control Method for an Upper Limb Rehabilitation Exoskeleton Based on Intent Recognition. Appl. Sci. 2022, 12, 4993. [Google Scholar] [CrossRef]
- Janssen, M. Upper Extremity Function in Duchenne Muscular Dystrophy—Mechanisms of Declined Task Performance. Ph.D. Thesis, Radboud University Medical Centre (Radboudumc), Nijmegen, The Netherlands, 2017. [Google Scholar]
- Harris, J.E.; Eng, J.J.; Ot, P.T. Strength Training Improves Upper-Limb Function in Individuals With Stroke A Meta-Analysis. Stroke 2010, 41, 136–140. [Google Scholar] [CrossRef]
- Merlini, L.; Bertini, E.; Minetti, C.; Mongini, T.; Morandi, L.; Angelini, C.; Vita, G. Motor Function-Muscle Strength Relationship in Spinal Muscular Atrophy. Muscle Nerve 2004, 29, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Assila, N.; Goubault, E.; Begon, M. Sex Differences in Upper Limb Musculoskeletal Biomechanics during a Lifting Task. Appl. Ergon. 2020, 86, 103106. [Google Scholar] [CrossRef] [PubMed]
- Sänger, J.; Yao, Z.; Schubert, T.; Wolf, A.; Molz, C.; Miehling, J.; Wartzack, S.; Gwosch, T.; Matthiesen, S.; Weidner, R. Evaluation of Active Shoulder Exoskeleton Support to Deduce Application-Oriented Optimization Potentials for Overhead Work. Appl. Sci. 2022, 12, 10805. [Google Scholar] [CrossRef]
- Nunes, M.F.; Hukuda, M.E.; Favero, F.M.; Oliveira, A.B.; Voos, M.C.; Caromano, F.A. Relationship between Muscle Strength and Motor Function in Duchenne Muscular Dystrophy. Arq. Neuro-Psiquiatr. 2016, 74, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Pangalila, R.; Bartels, B.; Bergen, M.; Cobben, N.; Stam, H.; Roebroeck, M. Upper Limb Function in Adults with Duchenne Muscular Dystrophy. J. Rehabil. Med. 2011, 43, 770–775. [Google Scholar] [CrossRef]
- Harith, H.H.; Mohd, M.F.; Nai Sowat, S. A Preliminary Investigation on Upper Limb Exoskeleton Assistance for Simulated Agricultural Tasks. Appl. Ergon. 2021, 95, 103455. [Google Scholar] [CrossRef]
- Zhu, Y.; Weston, E.B.; Mehta, R.K.; Marras, W.S. Neural and Biomechanical Tradeoffs Associated with Human-Exoskeleton Interactions. Appl. Ergon. 2021, 96, 103494. [Google Scholar] [CrossRef]
- Janssen, M.M.H.P.; Harlaar, J.; Koopman, B.; de Groot, I.J.M. Dynamic Arm Study: Quantitative Description of Upper Extremity Function and Activity of Boys and Men with Duchenne Muscular Dystrophy. J. Neuroeng. Rehabil. 2017, 14, 45. [Google Scholar] [CrossRef]
- Peeters, L.H.C.; Kingma, I.; van Dieën, J.H.; de Groot, I.J.M. Don’t Forget the Trunk in Duchenne Muscular Dystrophy Patients: More Muscle Weakness and Compensation than Expected. J. Neuroeng. Rehabil. 2019, 16, 44. [Google Scholar] [CrossRef]
- Guzik-Kopyto, A.; Nowakowska-Lipiec, K.; Krysiak, M.; Jochymczyk-Woźniak, K.; Jurkojć, J.; Wodarski, P.; Gzik, M.; Michnik, R. Selection of Kinematic and Temporal Input Parameters to Define a Novel Upper Body Index Indicator for the Evaluation of Upper Limb Pathology. Appl. Sci. 2022, 12, 11634. [Google Scholar] [CrossRef]
- Khallaf, M.E.; Ameer, M.A.; Fayed, E.E. Effect of Task Specific Training and Wrist-Fingers Extension Splint on Hand Joints Range of Motion and Function after Stroke. NeuroRehabilitation 2017, 41, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.E.; Rose, J. The Pediatric Upper Limb Motion Index and a Temporal–Spatial Logistic Regression: Quantitative Analysis of Upper Limb Movement Disorders during the Reach & Grasp Cycle. J. Biomech. 2012, 45, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Jurkojć, J.; Wodarski, P.; Michnik, R.; Nowakowska, K.; Bieniek, A.; Gzik, M. The Upper Limb Motion Deviation Index: A New Comprehensive Index of Upper Limb Motion Pathology. Acta Bioeng. Biomech. 2017, 19, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Bulut, N.; Alemdaroğlu-Gürbüz, I.; Topaloğlu, H.; Yılmaz, Ö.; Karaduman, A. The Association between Trunk Control and Upper Limb Functions of Children with Duchenne Muscular Dystrophy. Physiother. Theory Pract. 2022, 38, 46–54. [Google Scholar] [CrossRef]
- Huysamen, K.; Bosch, T.; de Looze, M.; Stadler, K.S.; Graf, E.; O’Sullivan, L.W. Evaluation of a Passive Exoskeleton for Static Upper Limb Activities. Appl. Ergon. 2018, 70, 148–155. [Google Scholar] [CrossRef]
- Lockie, R.G.; Bloodgood, A.; Moreno, M.; McGuire, M.; Balfany, K.; Dawes, J. Training Load Demands Measured by Surface Electromyography Wearable Technology When Performing Law Enforcement-Specific Body Drags. Facta Univ. Ser. Phys. Educ. Sport 2020, 1–12. [Google Scholar] [CrossRef]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Kinematic Variables Quantifying Upper-Extremity Performance After Stroke During Reaching and Drinking From a Glass. Neurorehabil. Neural Repair 2011, 25, 71–80. [Google Scholar] [CrossRef]
- Stegeman, D.F. Standards for Suface Electromyography: The European Project Surface EMG for Standards for Surface Electromyography: The European Project “Surface EMG for Non-Invasive Assessment of Muscles (SENIAM)”. Enschede Roessingh Res. Dev. 2007, 10, 8–12. [Google Scholar]
- Iranzo, S.; Piedrabuena, A.; Iordanov, D.; Martinez-Iranzo, U.; Belda-Lois, J.M. Ergonomics Assessment of Passive Upper-Limb Exoskeletons in an Automotive Assembly Plant. Appl. Ergon. 2020, 87, 103120. [Google Scholar] [CrossRef]
- Janssen, M.M.H.P.; Harlaar, J.; Groot, I.J.M. De Surface EMG to Assess Arm Function in Boys with DMD: A Pilot Study. J. Electromyogr. Kinesiol. 2015, 25, 323–328. [Google Scholar] [CrossRef]
- Wang, J.-S.; Lee, S.-B.; Moon, S.-H. The Immediate Effect of PNF Pattern on Muscle Tone and Muscle Stiffness in Chronic Stroke Patient. J. Phys. Ther. Sci. 2016, 28, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Kasai, T. Effects of Proprioceptive Neuromuscular Facilitation on the Initiation of Voluntary Movement and Motor Evoked Potentials in Upper Limb Muscles. Hum. Mov. Sci. 2002, 21, 101–113. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).