Abstract
Cartilage conduction hearing aids (CC-HA), bone anchored hearing aids (Baha), and ADHEAR are good choices to improve hearing in patients who cannot use air conduction hearing aids because of aural atresia or continuous otorrhea. As there are many overlaps in the characteristics of these devices, we conducted a comparative trial of CC-HA, Baha, and ADHEAR. We provided patients with the opportunity to select their devices. The data of 6 patients who underwent comparative trials in our department between October 2021 and August 2022 were retrospectively examined. The gains of Baha and CC-HA outweighed those of ADHEAR. Regarding the sound localization test, there was no significant tendency for any of the hearing devices. Regarding the Glasgow Benefit Inventory, there was no clear tendency among the three devices in the quality of life evaluation. The most satisfactory model was the one subjectively chosen by the patient, regardless of the gain and score of the sound source localization test. Therefore, we believe that it is necessary to provide an opportunity for comparative trials and a consultation with each patient in the process of selecting a device.
1. Introduction
Cartilage conduction hearing aids (CC-HA) or bone conduction hearing aids are the treatments of choice to improve hearing in patients who cannot use air conduction hearing aids (AC-HA) because of aural atresia or continuous otorrhea [1,2]. In regards to bone conduction hearing aids, the conventional eyeglass and headband types are large and are problematic in terms of aesthetics, as well as comfortability due to the compression of the vibrator [3]. In recent years, however, bone conduction implants such as bone anchored hearing aids (Baha; Cochlea Corp., Sydney, NSW, Australia) [4] and bone conduction hearing aids with an adhesive adapter sticking to the skin, such as the ADHEAR system (MED-EL, Innsbruck, Austria), are available in Japan, and the range of choices for the above cases are broad.
CC-HA is a device that utilizes cartilage conduction which has been in use in Japan since 2017 [5]. Cartilage conduction is the third type of hearing conduction pathway proposed by Hosoi [6], followed by air and bone conduction [7]. It is preferred in patients who cannot use AC-HA and whose hearing level cannot be improved with appropriate surgery, and moreover, there are no restrictions on hearing level. Aural atresia is a good indication for CC-HAs and they are also expected to be effective and preferred in patients with persistent otorrhea due to chronic otitis media [8]. We previously reported that CC-HA is effective in pediatric cases [9]. We also reported that CC-HA enabled a novel noninvasive therapeutic procedure for microtia patients using 3D printing technology without reducing hearing gain [10].
Baha is preferred in patients with bilateral hearing loss, with bone conduction hearing levels of 55 dB HL or less in at least one ear, and with difficult or inadequate hearing improvement using either air, bone, or cartilage conduction hearing aids [11]. The qualifications for Baha are external auditory canal atresia, continuous otorrhea from the external and middle ear, and cases in which hearing improvement cannot be expected or cannot be obtained with appropriate surgery. It is also an option for those in which the contralateral side is deaf or has severe hearing loss, so as to avoid the risk of complications of hearing loss due to surgery. Baha requires surgery for placement of the implant in the temporal bone, however, patients can try the device to know how it hears without surgery using a sound arc.
ADHEAR is a new bone conduction hearing device consisting of a thin adhesive adapter and a sound processor. The adapter sticks to the skin behind the ear and the sound processor clicks onto it. Because ADHEAR is a non-implanted device, it does not require surgery. There is work recommending ADHEAR to patients who cannot or do not want to undergo surgery [12]. It also overcomes the problems of aesthetics and comfortability associated with conventional bone conduction hearing aids. Furthermore, ADHEAR is small and can be discreetly placed behind the ear where it does not exert pressure on the skin. Additionally, ADHEAR’s volume can be changed by turning the dial. Although it is simple, there is the problem that fine adjustments of the hearing gain is not possible in Japan compared to other devices.
There are many overlaps in the characteristics for conduction hearing aid devices, and it is difficult for otologists and audiologists to objectively distinguish the indications based on patient-side factors such as hearing levels and disease conditions. In our department, we conducted a comparative trial of CC-HA, Baha, and ADHEAR, and provided patients with an opportunity to select conduction hearing aids, based on hearing gain and subjective evaluation. In this study, we evaluated the performance of these devices, the likelihood of subjectively reported improvement and examined the necessity of a comparative trial.
2. Materials and Methods
The data of 6 patients who underwent comparative trials in our department between October 2021 and August 2022 were retrospectively examined. This study was approved by the Institutional Review Board of Keio University (Ethics Number 20200033).
The diagnoses of the enrolled patients included external auditory canal obstruction (two cases), external auditory canal stenosis (one case), external middle ear malformation (one case), superior canal dehiscence syndrome (one case), and postoperative chronic otitis media (one case). Four of these cases were unilateral while the remaining two cases were bilateral.
CC-HA optimally adjusted the gain according to the patient’s hearing ability. This procedure was similar to those used for AC-HA [13]. ADHEAR was used in default mode (directivity of the microphone, howling suppression, and noise suppression) without any further fitting changes. Patients were able to adjust the volume of ADHEAR devices by turning the dial. The volume was adjusted individually to the most comfortable level for each patient. Baha was programmed using specific fitting software provided by the Cochlea Corporation according to each patient’s direct bone conduction hearing thresholds obtained through the BC Direct function.
CC-HA was affixed using double-sided adhesive tape to the skin of the tragus or auricular cartilage (Figure 1). ADHEAR was snugly attached to the skin of the mastoid with no hair (Figure 2). Sweat made the device easy to peel off, and therefore, the adhesion part of the seal was wiped off before its application. After confirming the hearing gain of CC-HA and ADHEAR by the sound field threshold test, the trial was performed for a month. During this time, sound localization tests, and evaluations of quality of life (QOL) using the Glasgow Benefit Inventory (GBI) were performed. Baha was worn using a sound arc for the trial (Figure 3). After confirming the hearing gain by a sound field threshold test, patients tried the device in the hospital for about an hour, then the same evaluation was performed.
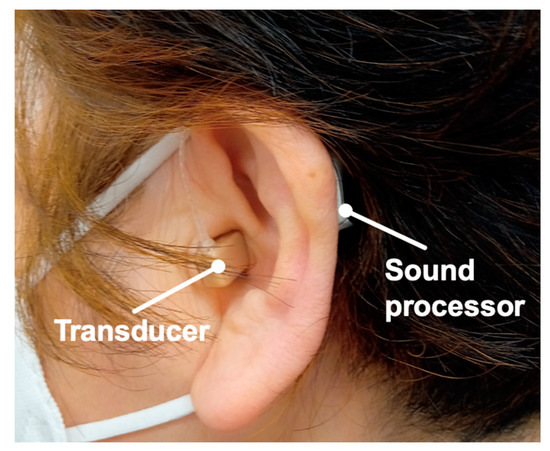
Figure 1.
Example of ear with a cartilage conduction hearing aid (CC-HA). Profile views (left side) of a patient fitted with a CC-HA. Transducer and sound processor components of CC-HA are attached to the skin using double-sided adhesive tape.
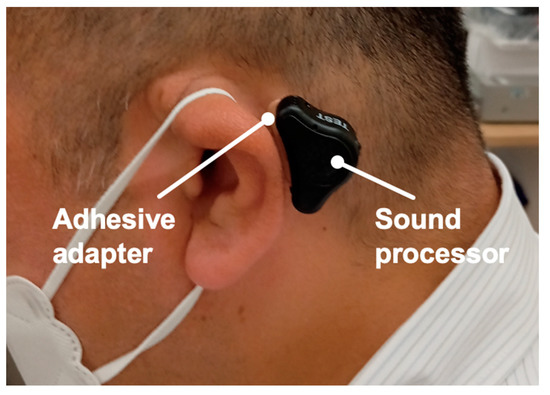
Figure 2.
Example of an ear with an ADHEAR. Profile views (left side) of a patient fitted with an ADHEAR. Sound processor is attached to the skin with an adhesive adapter.
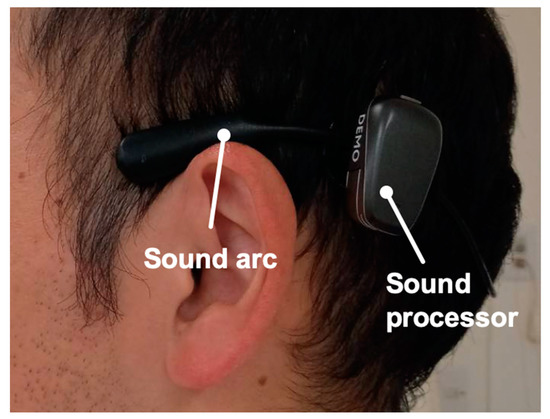
Figure 3.
Example of an ear with a Bone anchored hearing aid (Baha). Profile views (left side) of a patient fitted with a Baha. The sound arc is designed to sit above the ears and be worn behind the head with a sound processor.
Sound field audiometry was performed according to the Guidelines for Hearing Aid Fit Testing (2010), which are advocated by the Japan Audiological Society [14]. Patients were seated 1 m from the sound source speaker in a soundproof room, and the height of the speaker was the same height to the center of the patient’s head. The speaker and the patient were kept at least 1 m away from the wall. A hearing aid was worn on the affected ear and the unaffected ear was masked with narrowband noise during sound field audiometry.
The sound localization test was performed according to Kitoh’s report [15]. Nine speakers were installed at equal intervals (22.5°) on a semicircle 1 m in front of the patient and from the left end, No. 1 (−90°) to No. 9 (90°). CCITT (Comite Consultatif International Telegraphique et Telephonique) noise was used as the sound source and it was presented twice at 60, 65, and 70 dB SPL, for a total of 54 times. The numbers answered by the patients, denoting the speakers from which they thought the sounds were presented, were recorded. Localization accuracy was evaluated using the root mean square (RMS) score. A lower score indicated a more accurate sense of direction. The sound localization test was performed only in cases of unilateral primary disease.
GBI is a questionnaire that evaluates the extent to which the QOL of patients improves in association with intervention on a scale from −100 to 100 [16]. A score greater than 0 indicated that the intervention improved their QOL, whereas a score less than 0 indicated that it negatively affected their QOL. The higher the score, the greater the degree of improvement in QOL. The original GBI was developed in English, and therefore we translated it into Japanese, as follows, before they werecompleted by the patients. First, we translated the original GBI into Japanese. Second, it was retranslated into English by a translator who was bilingual in English and Japanese. Finally, it was compared with the original GBI and certified by a translator as having the same meaning.
Based on the results of comparative trial within each case, the usefulness of each device was examined.
3. Results
Table 1 shows the results of the comparative trials of CC-HA, Baha, and ADHEAR for each case.

Table 1.
Results of the comparative trials of CC-HA, Baha and ADHEAR in each case.
3.1. Case 1
The patient was a 58-year-old male with left superior canal dehiscence syndrome and middle ear malformation. He used an AC-HA on the left, but was not satisfied with the effect, so he requested another device to try and participated in this trial. His air conduction hearing thresholds are 25 (125 Hz)–30 (250 Hz)–35 (500 Hz)–25 (1 kHz)–10 (2 kHz)–25 (4 kHz)–60 (8 kHz) dB HL on the right and 75 (125 Hz)–75 (250 Hz)–80 (500 Hz)–70 (1 kHz)–40 (2 kHz)–50 (4 kHz)–90 (8 kHz) dB HL on the left. His bone conduction hearing thresholds are 25 (250 Hz)–30 (500 Hz)–25 (1 kHz)–5 (2 kHz)–20 (4 kHz) dB HL on the right and 30 (250 Hz)–45 (500 Hz)–25 (1 kHz)–25 (2 kHz)–40 (4 kHz) dB HL on the left. The hearing thresholds of the ADHEAR device was larger than that of the other two devices at all frequencies. The hearing thresholds with CC-HA and Baha both showed approximately 30 dB HL at the middle frequencies, however, the thresholds with Baha were smaller than those with CC-HA at low frequencies. Regarding the sound localization test, RMS increased with CC-HA, decreased slightly with Baha and ADHEAR, and was the lowest with ADHEAR (Figure 4 and Figure 5). GBI was the same and positive for all devices (Figure 6).
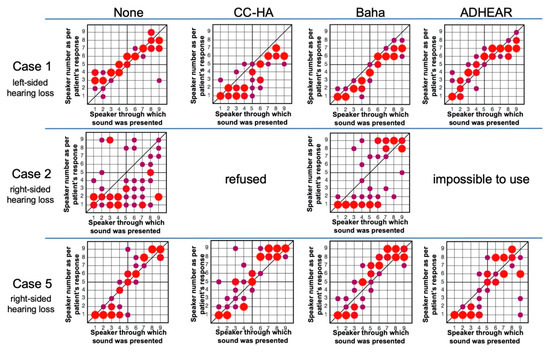
Figure 4.
Results of Sound Localization Tests.
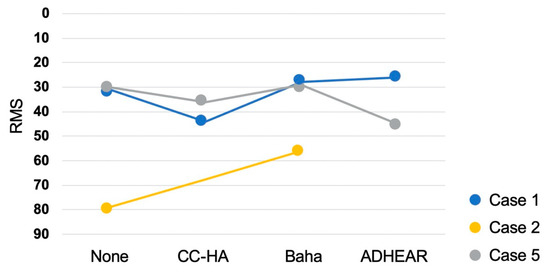
Figure 5.
The root mean square (RMS) value of each device.
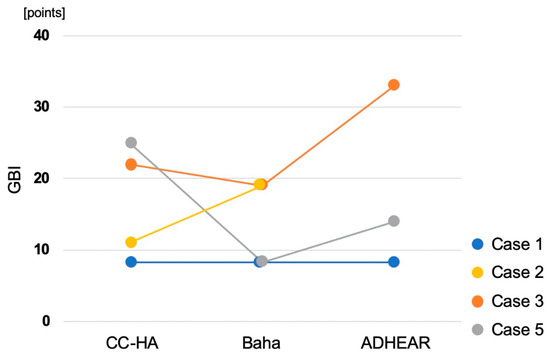
Figure 6.
Results of Glasgow Benefit Inventory (GBI).
This graph shows the results of the sound localization test without hearing aids and with cartilage conduction hearing aids (CC-HA), Baha, and ADHEAR. The horizontal axis represents the number of the speaker sounded, and the vertical axis represents the speaker number as per the patient’s response. The size of the circles in the graph is proportional to the number of times the patient responded with the number of each speaker. The circles on the slanted line indicate correct answers. The larger the circle is from the slanted line, the more times the patient answered the incorrect speaker.
A lower score indicates a more accurate sense of direction. In case 1, the score worsened with CC-HA, but improved in Baha and ADHEAR. In case 2, the score improved with Baha. In case 5, the score worsened with CC-HA and ADHEAR, whereas Baha showed little change, albeit slight improvement.
In case 1, the score indicated that all devices improved the QOL to the same extent. In case 2, Baha showed greater improvement than CC-HA. In case 3, improvement was observed in the order of ADHEAR, CC-HA, and Baha. In case 5, improvement was observed in the order of CC-HA, ADHEAR, and Baha.
3.2. Case 2
The patient was a 56-year-old female with right chronic otitis media. She used an AC-HA on the right, but was unable to continue using it due to otorrhea and participated in this trial. Her air conduction hearing thresholds are 75 (125 Hz)–85 (250 Hz)–100 (500 Hz)–110 (1 kHz)–105 (2 kHz)–115 (4 kHz)–105 (8 kHz) dB HL on the right and 30 (125 Hz)–25 (250 Hz)–25 (500 Hz)–35 (1 kHz)–45 (2 kHz)–55 (4 kHz)–50 (8 kHz) dB HL on the left. Her bone conduction hearing thresholds are 30 (250 Hz)–35 (500 Hz)–50 (1 kHz)–65 (2 kHz)–60 (4 kHz) dB HL on the right and 20 (250 Hz)–20 (500 Hz)–30 (1 kHz)–50 (2 kHz)–50 (4 kHz) dB HL on the left. She could not attempt using ADHEAR because the device immediately peeled off from her skin. The hearing thresholds with Baha were almost the same at middle-to-high frequencies as the thresholds with CC-HA but were smaller at low frequencies. Regarding the sound localization test, RMS decreased with Baha (Figure 4 and Figure 5). The patient refused the later sound localization test with CC-HA because she experienced severe difficulty when performing the test with Baha and she was discouraged to continue the test. GBI was higher in Baha than in CC-HA (Figure 6).
3.3. Case 3
The patient was a 45-year-old female with bilateral malformation of the external and middle ears. She was unable to use AC-HAs because of the malformation and participated in this trial. Her air conduction hearing thresholds are 65 (125 Hz)–75 (250 Hz)–75 (500 Hz)–60 (1 kHz)–50 (2 kHz)–115 (4 kHz)–105 (8 kHz) dB HL on the right and 60 (125 Hz)–60 (250 Hz)–55 (500 Hz)–35 (1 kHz)–30 (2 kHz)–45 (4 kHz)–75 (8 kHz) dB HL on the left. Her bone conduction hearing thresholds are 45 (250 Hz)–45 (500 Hz)–30 (1 kHz)–50 (2 kHz)–50 (4 kHz) dB HL on the right and 25 (250 Hz)–25 (500 Hz)–30 (1 kHz)–15 (2 kHz)–30 (4 kHz) dB HL on the left. She used devices on her left ear. The hearing thresholds with ADHEAR were almost the same as the thresholds with CC-HA and Baha at high frequencies but were larger at low and middle frequencies. There was no significant difference between the thresholds with CC-HA and Baha at any frequency. GBI increased in the order of ADHEAR, CC-HA, and Baha (Figure 6).
3.4. Case 4
The patient was a 49-year-old female with left aural atresia. She was unable to use an AC-HA because of atresia and participated in this trial. Her air conduction hearing thresholds are 25 (125 Hz)–20 (250 Hz)–20 (500 Hz)–25 (1 kHz)–15 (2 kHz)–20 (4 kHz)–20 (8 kHz) dB HL on the right and 50 (125 Hz)–50 (250 Hz)–65 (500 Hz)–70 (1 kHz)–65 (2 kHz)–50 (4 kHz)–65 (8 kHz) dB HL on the left. Her bone conduction hearing thresholds are 25 (250 Hz)–25 (500 Hz)–25 (1 kHz)–25 (2 kHz)–25 (4 kHz) dB HL on the left. She could not attempt ADHEAR because the device immediately peeled off from her skin. She did not attempt using Baha because she did not want to undergo surgery. The hearing level improved at middle frequencies, however, there was little gain at 250 Hz and 4000 Hz with CC-HA. GBI was positive for CC-HA.
3.5. Case 5
The patient was a 30-year-old male with right aural atresia. He was unable to use an AC-HA because of atresia and participated in this trial. His air conduction hearing thresholds are 75 (125 Hz)–90 (250 Hz)–80 (500 Hz)–70 (1 kHz)–70 (2 kHz)–70 (4 kHz)–100 (8 kHz) dB HL on the right and 15 (125 Hz)–15 (250 Hz)–10 (500 Hz)–10 (1 kHz)–5 (2 kHz)–0 (4 kHz)–15 (8 kHz) dB HL on the left. His bone conduction hearing thresholds are 15 (250 Hz)–15 (500 Hz)–10 (1 kHz)–10 (2 kHz)–5 (4 kHz) dB HL on the right. The hearing levels improved with all three devices. The thresholds with Baha were smaller than those of CC-HA and ADHEAR at low frequencies. There was almost no difference between the thresholds for CC-HA and ADHEAR. Regarding the sound localization test, RMS increased with CC-HA and Baha, but slightly decreased with ADHEAR. (Figure 4 and Figure 5). GBI increased in the order of CC-HA, ADHEAR, and Baha (Figure 6).
3.6. Case 6
The patient was a 73-year-old male with bilateral ear canal stenosis. He was not satisfied with the effect and participated in this trial. His air conduction hearing thresholds are 70 (125 Hz)–55 (250 Hz)–40 (500 Hz)–40 (1 kHz)–60 (2 kHz)–75 (4 kHz)–105 (8 kHz) dB HL on the right and 65 (125 Hz)–60 (250 Hz)–30 (500 Hz)–45 (1 kHz)–65 (2 kHz)–70 (4 kHz)–100 (8 kHz) dB HL on the left. His bone conduction hearing thresholds are 10 (250 Hz)–10 (500 Hz)–10 (1 kHz)–55 (2 kHz)–45 (4 kHz) dB HL on the right and 15 (250 Hz)–10 (500 Hz)–20 (1 kHz)–60 (2 kHz)–40 (4 kHz) dB HL on the left. He used devices on his right ear. The hearing thresholds with CC-HA were smaller than those with ADHEAR at low and middle frequencies. He did not attempt using Baha because he did not want to undergo surgery.
4. Discussion
Advances in medical technology have led to the development of several devices that can be used in patients with hearing loss. Selecting devices for patients has become more difficult, as more devices are available for patients with conductive or mixed hearing loss. Although, AC-HA remains the most commonly used device, and CC-HA, Baha, and ADHEAR are also indicated when AC-HA is difficult to use. There are many overlaps in hearing levels and indicated diseases for each device. Since factors other than patient factors can determine which device is deemed suitable for each patient by an otologist and audiologist, we examined the results using a comparative trial.
The hearing gains of Baha and CC-HA tended to be greater than those of ADHEAR, particularly at low and middle frequencies. There was a report comparing ADHEAR with another bone conduction implant, Bonebridge (MED-EL, Innsbruck, Austria). In Dahm’s study, the gain of Bonebridge is larger than that of ADHEAR [17], meaning that, the gain of Baha, one of a transcutaneous bone conduction implant, is more likely to be larger than that of ADHEAR. Although, it is difficult to determine the cause of the lower hearing gain observed with ADHEAR, but several mechanisms are conceived. First, ADHEAR uses less pressure than conventional bone conduction hearing aids. Increased comfort is associated with ADHEAR usage, however, it diminishes low-frequency hearing gains by compressing the temporal bone. Second, the gain of ADHEAR is set in a readymade manner that cannot be adjusted or fine-tuned to the patient’s hearing level in Japan. On the other hand, the other two hearing devices (CC-HA and Baha) can fine-tune the hearing gain and accelerate the low-frequency hearing function. In a previous report by Dahm, it was suspected that amplification is smaller with ADHEAR, as working principles were considered. The manufacturer suggests that cases with bone conduction hearing thresholds within 25 dB HL are good candidates for ADHEAR [18], so the limits of ADHEAR’s gain might be smaller than those of the other two devices. However, we believe that it would be meaningful for patients who cannot use AC-HAs for some reason, such as ear discharge or itchy ears, to experience the ADHEAR device, which does not need to be placed in the ear, and is noninvasive. Hearing thresholds at 4 kHz in each type of hearing aid are worse than without in cases 1, 3, and 4. Since the sound sources used in sound field audiometry are not pure-tone, but a warble-tone, this difference may have caused a measurement error for high frequencies. Although no subjective hearing loss was observed in any of the cases, we believe that we need to pay attention to this difference.
Regarding the device fixation, ADHEAR immediately peeled off and could not be tested in two out of the six patients, suggesting that the condition of the skin limits ADHEAR usage in some patients. Baha was not tested in two out of the six patients because they had negative reactions to the operation. Even so, all patients attempted to use CC-HA, which is thought to be advantageous because it is easy to use in daily life and does not require surgery.
Regarding the results of the sound localization test, there was no clear advantage for any of the hearing devices. In cases where the score worsened, patients reported that it was because the sound from all speakers stood out equally, and claimed that it felt like all the sounds were coming from the side with the hearing aid. Previous studies have also reported that wearing Baha does not improve the sense of sound localization. Similar to the results of this study, some cases improved while others worsened [19]. To date, there have been few reports on the improvements in sound localization using ADHEAR or CC-HA. We believe that in order to make accurate evaluations for these devices, a few issues will need to be clarified, such as the setting of the presentation sound pressure level in examinations.
Regarding the results of GBI, all three devices contributed to the improvement of QOL in all cases. There have been studies on ADHEAR and QOL [20,21], though the method of QOL evaluation was different, and they also reported that the use of ADHEAR improved QOL. There are examples in the literature where GBI is used for QOL evaluation, and that also acknowledge the improvement of QOL due to using Baha. In our study, the GBI score for Baha was relatively lower to those reported in previous studies (38 on average), but it is considered that factors such as race, hearing level, and the duration of using the device were different [22,23]. Additionally, there have been works in the literature comparing ADHEAR with Baha on the point of QOL, but there have been no reports comparing the degree of QOL improvement using these three devices. In the work comparing ADHEAR with Baha, there was no significant difference between two devices [17], and in this study, no clear relationship was found among the three devices in the QOL evaluation, and the results of the devices differed for each case. The degree of improvement in QOL is often inconsistent with the results of objective tests such as sound field audiometry and sound localization tests. Various subjective factors, such as comfortability and sound quality, are thought to be related to improvements in QOL.
Baha may be suitable for patients who desire a large gain in hearing. However, this is not an option for patients who refuse surgery.
CC-HA and ADHEAR are options suitable for patients who refuse surgery. ADHEAR claims simplicity as its merit, however, its gain tends to be smaller than that of CC-HA. In addition, comfort in wearing the device and its aesthetics are said to be advantages of using ADHEAR. In this study, however, the device easily peeled off from the skin of a few patients, and it was unexpectedly noticeable compared to others. We concluded that the decision of which device is most satisfactory depends on the patient’s impression, regardless of the gain and score of the sound source localization test. Therefore, we believe that it is necessary to provide an opportunity for comparative trials and consultations with each patient in the process of device selection.
5. Limitations
In this study, the number of cases were relatively small, therefore statistical examination was not performed. In addition, in some cases not all the devices were tested, but we consider not only the gain and QOL improvement, but also the reason why Baha or ADHEAR was not selected as an important point in this study, and hence, we included these cases. If these cases are excluded, we suspect that selection bias will occur for the purpose of this paper, so we advocate to include them as cases. In the future, we would like to solve these problems by increasing the number of cases.
6. Conclusions
The gains of Baha and CC-HA were larger than those of ADHEAR. Depending on the condition of the skin, ADHEAR may peel off easily and not be available. Some patients may avoid Baha because they do not desire to undergo surgery. In the sound localization test, there were cases in which the score worsened while using the device, however, some patients were able to hear from the affected side. No clear relationship was found in the degree of improvement in QOL among the three devices. Furthermore, QOL was not only influenced by hearing gain but also by the patient’s impression, thus, comparative trials are suggested for device selection.
Author Contributions
Conceptualization, T.K., T.N. and N.O.; methodology, T.N.; formal analysis, T.K.; investigation, T.K., T.N., K.I., T.W., M.N.S., M.H., H.O. and N.O.; resources, T.N., H.O. and N.O.; data curation, T.K.; writing—original draft preparation, T.K.; writing—review and editing, T.N. and N.O.; visualization, T.K.; supervision, N.O.; project administration, T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Grant-in-Aid for Early-Career Scientists (19K18744) from the Japan Society for the Promotion of Science and Takeda Science Foundation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board Keio University (Ethics Number 20200033 27 April 2020).
Informed Consent Statement
The details of this clinical research study are displayed in a consultation room and on our website (https://ent-otol.med.keio.ac.jp/, accessed on 14 October 2022). According to the Japan Ethical Guidelines for Medical and Health Research Involving Human Subjects, informed consent for observational studies is not required. We notified the research participants or made public information concerning the research, including the purpose of collecting and using the research information. We also informed the participants that they could refuse participation at any time or request that their data be removed from the study after commencement.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We acknowledge the support of the Department of Otorhinolaryngology, Keio University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nishimura, T.; Hosoi, H.; Shimokura, R.; Morimoto, C.; Kitahara, T. Cartilage Conduction Hearing and Its Clinical Application. Audiol. Res. 2021, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Cywka, K.B.; Krol, B.; Skarzynski, P.H. Effectiveness of Bone Conduction Hearing Aids in Young Children with Congenital Aural Atresia and Microtia. Med. Sci. Monit. 2021, 27, e933915. [Google Scholar] [CrossRef] [PubMed]
- O’Niel, M.B.; Runge, C.L.; Friedland, D.R.; Kerschner, J.E. Patient Outcomes in Magnet-Based Implantable Auditory Assist Devices. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 513–520. [Google Scholar] [CrossRef]
- Hakansson, B.; Tjellstrom, A.; Rosenhall, U.; Carlsson, P. The bone-anchored hearing aid. Principal design and a psychoacoustical evaluation. Acta Otolaryngol. 1985, 100, 229–239. [Google Scholar] [CrossRef]
- Hosoi, H. Cartilage Conduction Hearing Aids: The Third Pathway for Sound Transmission and Its Application. ENT and Audiology News. 2020. Available online: https://www.entandaudiologynews.com/features/audiology-features/post/cartilage-conduction-hearing-aids-the-third-pathway-for-sound-transmission-and-its-application (accessed on 14 October 2022).
- Hosoi, H. Approach in the use of cartilage conduction speaker. Japanese Patent Number 4541111, 17 November 2004. [Google Scholar]
- Nishimura, T.; Hosoi, H.; Saito, O.; Miyamae, R.; Shimokura, R.; Matsui, T.; Yamanaka, T.; Levitt, H. Is cartilage conduction classified into air or bone conduction? Laryngoscope 2014, 124, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Oishi, N.; Ogawa, K. Who are good adult candidates for cartilage conduction hearing aids? Eur. Arch Otorhinolaryngol. 2021, 278, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Oishi, N.; Ogawa, K. Efficacy of cartilage conduction hearing aids in children. Int. J. Pediatr. Otorhinolaryngol. 2021, 142, 110628. [Google Scholar] [CrossRef]
- Nishiyama, T.; Hayashi, S.; Oishi, N. A novel auricular prosthesis which incorporates a cartilage conduction hearing aid based on 3D data processing technique: A preclinical evaluation. Eur. Arch. Otorhinolaryngol. 2022, 279, 3741–3744. [Google Scholar] [CrossRef]
- Wade, P.S.; Halik, J.J.; Werger, J.P.; Vosu, L.K. Ten-year experience with percutaneous bone-anchored hearing aids: A 3- to 10-year follow-up Markham Stouffville Hospital, 1990 to 2000. J. Otolaryngol. 2002, 31, 80–84. [Google Scholar] [CrossRef]
- Skarzynski, P.H.; Ratuszniak, A.; Osinska, K.; Koziel, M.; Krol, B.; Cywka, K.B.; Skarzynski, H. A Comparative Study of a Novel Adhesive Bone Conduction Device and Conventional Treatment Options for Conductive Hearing Loss. Otol. Neurotol. 2019, 40, 858–864. [Google Scholar] [CrossRef]
- Scollie, S.; Seewald, R.; Cornelisse, L.; Moodie, S.; Bagatto, M.; Laurnagaray, D.; Beaulac, S.; Pumford, J. The Desired Sensation Level multistage input/output algorithm. Trends Amplif. 2005, 9, 159–197. [Google Scholar] [CrossRef] [PubMed]
- Kodera, K.; Hosoi, H.; Manabe, T.; Kanda, Y.; Shiraishi, K.; Sugiuchi, T.; Suzuki, K.; Tauchi, H.; Nishimura, T.; Matsuhira, T. The guideline for adaptation tests to hearing aids. Audiol. Jpn. 2010, 53, 708–726. [Google Scholar]
- Kitoh, R.; Moteki, H.; Nishio, S.; Shinden, S.; Kanzaki, S.; Iwasaki, S.; Ogawa, K.; Usami, S. The effects of cochlear implantation in Japanese single-sided deafness patients: Five case reports. Acta Otolaryngol. 2016, 136, 460–464. [Google Scholar] [CrossRef]
- Robinson, K.; Gatehouse, S.; Browning, G.G. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann. Otol. Rhinol. Laryngol. 1996, 105, 415–422. [Google Scholar] [CrossRef]
- Dahm, V.; Traxler, S.; Liepins, R.; Auinger, A.B.; Sterrer, E.; Kaider, A.; Riss, D.; Arnoldner, C. Performance With an Adhesive Bone Conduction Device in Active Transcutaneous Bone Conduction Implant Users. Otol. Neurotol. 2021, 42, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Amanda, O.; Uta, S.; Charlotte, D. ADHEAR System by MED-EL: An Overview of the Research and Clinical Experiences from the Field. AudiologyOnline 2019, 25825. Available online: https://www.audiologyonline.com/articles/adhear-system-by-med-el-25825 (accessed on 14 October 2022).
- Battista, R.A.; Mullins, K.; Wiet, R.M.; Sabin, A.; Kim, J.; Rauch, V. Sound localization in unilateral deafness with the Baha or TransEar device. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 64–70. [Google Scholar] [CrossRef]
- Favoreel, A.; Heuninck, E.; Mansbach, A.L. Audiological benefit and subjective satisfaction of children with the ADHEAR audio processor and adhesive adapter. Int. J. Pediatr. Otorhinolaryngol. 2020, 129, 109729. [Google Scholar] [CrossRef]
- Almuhawas, F.; Alzhrani, F.; Saleh, S.; Alsanosi, A.; Yousef, M. Auditory Performance and Subjective Satisfaction with the ADHEAR System. Audiol. Neurootol. 2021, 26, 1–10. [Google Scholar] [CrossRef]
- Sanchez-Camon, I.; Lassaletta, L.; Castro, A.; Gavilan, J. Quality of life of patients with BAHA. Acta Otorrinolaringol. Esp. 2007, 58, 316–320. [Google Scholar] [CrossRef]
- Lekue, A.; Lassaletta, L.; Sanchez-Camon, I.; Perez-Mora, R.; Gavilan, J. Quality of life in patients implanted with the BAHA device depending on the aetiology. Acta Otorrinolaringol. Esp. 2013, 64, 17–21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).