Abstract
Late diagnosis and treatment of septic arthritis (SA) after anterior cruciate ligament reconstruction (ACL-R) can lead to graft and cartilage damage. Pathogen eradication time may be the key to preventing the development of osteoarthritis. The purpose of this study was to evaluate the effect of the proposed management of SA after ACL-R on knee function, pathogen eradication time and knee cartilage status on MRI. Five patients with acute knee SA after ACL-R were included in this study. All five patients underwent knee arthroscopic debridement with lavage and flow drainage with physiological saline with vancomycin. All five patients underwent clinical assessment, isokinetic examinations and MRI quantitative cartilage thickness evaluation at two follow-up points: at a mean of 10.9 years and at a mean of 18.1 years. Slight statistical differences in cartilage thickness on the medial femoral condyle were observed between the SA and control groups (2.077 mm and 2.237 mm, respectively; p = 0.021). There were no significant differences in cartilage thickness between the first and last follow-ups in the SA knees. The proposed treatment could lead to a faster eradication of infection, and thus protect against the early development of osteoarthritis. The quadriceps peak torque deficit may persist afterwards at a mean of 18.1 years follow-up.
1. Introduction
Septic arthritis (SA) following anterior cruciate ligament (ACL) reconstruction (ACL-R) is a rare but serious complication, with an incidence rate that ranges from 0.14% to 1.7% [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Early diagnosis is difficult, and late diagnosis and treatment can lead to joint cartilage and graft damage, and to early development of osteoarthritis (OA) [1,3,5,7,8,16,18,19]. The current recommended treatment for SA following ACL-R is arthroscopic debridement and lavage repeated 1–3 times on average, depending on the clinical condition, combined with targeted antibiotic therapy [5,6,20,21]. The aim of repeated knee arthroscopy is the lavage of the joint, leading to removal of the pathogen and proteolytic enzymes it produces, as well as the toxic pro-inflammatory molecules and metalloproteinases responsible for cartilage degradation [22,23]. According to animal studies, a joint infection lasting 7 days leads to the degradation of >50% of glycosaminoglycans and collagen present in the articular cartilage [24]. Recent experimental publications by Zwierzchowski et al. indicate that a 1% loss of cartilage proteoglycans causes a 1.1% decrease in the biomechanical properties of rabbit medial tibial condyle cartilage [25].
The development of OA after SA is multifactorial because its presence is confirmed 10 years after uncomplicated ACL-R, and its severity depends on coexisting pathologies such as meniscus and cartilage damage [26,27,28,29,30]; nevertheless, the results of experimental studies and the long-term results of SA treatment indicate that pathogen eradication time may be the key to preventing the development of OA caused by these factors [24,25,31]. The issue has not, however, been covered in previous publications [3,4,5,13,14,15,16,17,18,19,31,32]. Moreover, despite the passage of time, SA therapeutic recommendations based on meta-analyses have not addressed this issue, and there are no studies in the available literature that quantify the articular cartilage on MRI during the long-term follow-up period after SA treatment following ACL-R [3,4,5,13,14,15,16,17,18,19,31,32]. In addition, the speed of pathogen eradication may affect the cost of treatment and the prevention of graft removal. Recognizing the time of eradication as a key factor in preventing articular cartilage degradation resulting in earlier development of degenerative lesions, the senior author of this study (JF) has, since 2002, been successfully using arthroscopic debridement and lavage with closed flow drainage of the knee until C-reactive protein (CRP) normalization in patients treated for SA after hamstring ACL-R [33].
This study therefore aimed to prospectively evaluate the effect of the applied management of SA after ACL-R on knee function, pathogen eradication time as expressed by CRP normalization time, knee articular cartilage status on MR imaging and treatment costs.
2. Materials and Methods
2.1. Study Design
This case series study was conducted on five patients suffering from unilateral ACL rupture, who developed acute knee SA after ACL-R. The patients were adults (≥18 years old) with no other prior or subsequent operated knee surgical interventions and no other serious diseases such as diabetes, cardiac diseases, autoimmune diseases, cancer, or dysfunction of the opposite healthy limbs. The characteristics of the participants are presented in Table 1:

Table 1.
Baseline characteristic of the patients.
All the patients were consecutively enrolled in this study from the Department of Arthroscopy, Minimal Invasive Surgery, and Sport Traumatology of the Medical University of Lodz (Poland). Written informed consent to their participation in the study was obtained from all the patients. The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval for the study was also obtained from the Bioethics Committee of the Medical University of Lodz (approval number: RNN/651/11/KB from 12 July 2011 and RNN/21/22/KE from 8 February 2022). The main exclusion criteria were history indicating the presence of autoimmune diseases, cancer, dysfunction of the lower limbs caused by past injury, and history of any other surgical operation of the lower limbs.
All the patients were assigned to two groups: study (n = 5) and control (n = 5). The study group consisted of arthritic knees after ACL-R, whereas the control group consisted of the healthy (non-operated) knees of the same patients (Figure 1).

Figure 1.
Participant flow.
2.2. ACL-R and Acute Knee Arthritis Diagnosis
All the patients enrolled in this study developed SA following ACL-R using the hamstring tendon, which was 0.7% of all 714 ACL-Rs performed by the authors of this study between 2002 and 2005. The mean age of the patients at the time of the procedure was 29 (range, 26–31 years). All the patients underwent the same technique: reconstruction with a hamstring using a tourniquet—four with RigidFix J&J fixation system, and one with Endobutton stabilization in the femoral tunnel (Smith & Nephew) and a bio-absorbable screw in the tibial tunnel. One patient had an additional posterior meniscal horn repaired with PDS sutures (inside-out technique), and another patient underwent minimal partial meniscectomy of the posterior horn. Knee SA diagnosis was based on the general and local condition assessment and laboratory tests for elevated serum inflammatory markers such as CRP > 5 mg/L. Clinical examination revealed edema, increased knee temperature, persistent knee pain and limited range of motion; thus, the patients were immediately referred to the hospital and admitted to the ward. After admission, the patients underwent knee arthrocentesis. The collected material was taken to the laboratory for culture and antibiotic sensitivity testing.
2.3. Management and Treatment Protocol of Acute Knee Arthritis after ACL-R
Once the knee SA diagnosis had been made, the patients were administered vancomycin intravenously (1 g, twice a day). Laboratory blood tests performed on the admission day revealed an increased level of leukocytes in two patients, with an average white blood cell count of 11.200/mm3 (from 8.900/mm3 to 14.200/mm3; the normal values, 4.000–10.000 per mm3), an increased level of neutrophils in all of the patients (mean 73.1% (69.1–81.9%); normal range, 43–65%), and an increase in CRP level with a mean 72.5 mg/L (range, 51.4–126.3 mg/L; normal value, 0–5 mg/L) in all patients (Table 1). The mean time from ACL-R to the development of clinical symptoms of infection was 8 days (Table 1). After admission to the ward, the patients underwent knee arthroscopy combined with debridement of the suprapatellar pouch and removal of macroscopically inflamed tissues and fibrin. The knee joint fluid and synovial membrane fragments were sent for bacteriological tests. In all cases, the ACL graft was positively evaluated, with a small amount of fibrin and thrombi, which were removed. The graft with implants used for proper fixation was retained in all patients. In one patient, sutures after medial meniscus repair were also retained. The patients underwent arthroscopic lavage with 15–20 L of physiological saline through draining. The authors used 3 L of physiological saline with 1 g of vancomycin slowly through draining for 24 h.
All the patients had positive joint fluid bacterial growth results. Staphylococcus epidermidis, which is sensitive to vancomycin and rifampicin, was isolated from all patients. Drainage was maintained until the CRP level was within the normal range for an average of 9 days (range, 7–12 days). When the result of bacterial growth was obtained, antibiotic therapy according to an antibiotic sensitivity test was initiated, and vancomycin was still administered, supplemented with oral administration of rifampicin (600 mg/day), which was used for 6 weeks. Over time, antifungal drugs and probiotics were introduced. Every 2 days, blood count and CRP values were tested. Intravenous antibiotic therapy was administered for an average of 16.2 days (Table 1). Body temperature was monitored according to a previously established schedule. There was no increase in CRP levels after normalization in any of the patients during oral administration of antibiotics. CRP tests were repeated weekly. During the hospitalization period, the patients underwent passive motion exercises, patellar gentle mobilization and isometric exercises of the operated limb muscles. Knee joint weight-bearing was applied until the acute clinical symptoms resolved.
2.4. Clinical Examination
The patients enrolled in this study underwent a clinical examination at two points: at a mean of 10.9 years follow-up (first follow-up) and at a mean of 18.1 years follow-up (second follow-up) after acute knee SA treatment post-ACL-R, with particular attention to the detailed knee joint physical examination, including the range of motion, anterior drawer test, Lachman test and pivot-shift tests. Side-to-side differences in ligament laxity were tested using the KT-1000 arthrometer (MEDmetric, San Diego, CA, USA). Additionally, the patients were assessed using the single-leg hop test, the modified Lysholm knee scoring scale [34], and isokinetic testing of the muscle (at the second follow-up only). Isokinetic examinations were performed using a Biodex-3 system (Biodex Multi Joint System-Pro, Biodex Medical Systems, Inc., Shirley, NY, USA) with angular velocities of 60°/s and 180°/s. Analogous values for the intact unaffected extremity were considered as the reference values. The gait analysis was performed with the help of Gait Trainer (Biodex Medical Systems, Inc., Shirley, NY, USA).
2.5. Evaluation of Knee Cartilage Thickness Using MRI
At the first and second follow-ups, cartilage thickness was measured in both knees (study and control group) using MRI, which was performed using a 0.32T O-Scan device designed for knee examination. FSE T2, STIR and 3D-Sharc sequences were obtained (1.9 mm slices). Measurements were performed on the images of the 3D-Sharc sequences. The articular cartilage thickness of each patient was measured in the knee after ACL-R (study group) and in the healthy knee joint (control group). The plane of measurements for femoral and tibial condyles was determined based on a bone reference point at the level of the tendon incision of the popliteus muscle of the lateral femoral condyle and at three reference points in the frontal view: (1) center of the condyle; (2) 5.7 mm medial from the midline; and (3) 5.7 mm lateral to the midline of the condyle (Figure 2 and Figure 3). Cartilage thickness was defined as the shortest distance between the articular surface and the subchondral bone surface of the cartilage.

Figure 2.
The plane and points of measurements for femoral and tibial condyles include three reference points in frontal view: center of the condyle (green line); 5.7 mm medial from the midline (orange line); and 5.7 mm lateral to the midline of the condyle (blue line).
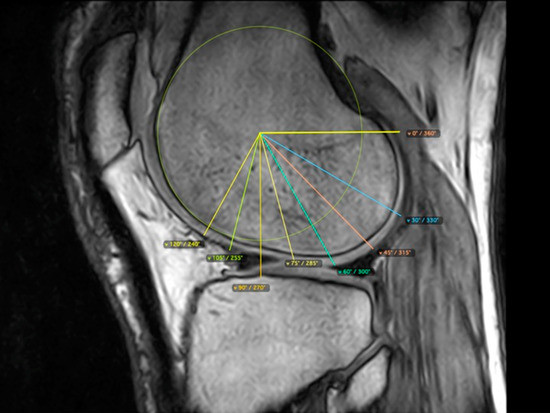
Figure 3.
Eight measurement points for the femoral condyles in the sagittal plane based on a drawn circle tangent to the edge of the condyle and determined angles: 120°, 105°, 90°, 75°, 60°, 45°, 30° and 0°.
2.6. Medial and Lateral Femoral Condyle
The femoral cartilage thickness was measured in the sagittal plane by setting eight measurement points in the condyles based on a drawn circle tangent to the edge of the condyle and determined angles of 120°, 105°, 90°, 75°, 60°, 45°, 30° and 0°. Five points were selected for measurement according to previously established scales: 120°, 90°, 60°, 30° and 0° (Figure 2) [35,36,37,38].
2.7. Medial and Lateral Tibial Condyle
In the tibia, points for measurements were similarly set in three reference locations: in the midline of the condyle and in two lines, 5.7 mm lateral and medial, from the line marking the center of the condyle (Figure 3). The measurements were made in the sagittal plane by dividing the condyle (in the AP dimension of the condyle) into eight equal sections marking seven cartilage measurement points (Figure 4). Five measurement points (points 2–6) were selected for analysis according to previously established scales: 120°, 90°, 60°, 30° and 0° (Figure 3) [35,36,37,38].

Figure 4.
The plane and points of measurements for tibial condyles in the sagittal plane; the condyle was divided (in AP dimension of the condyle) into eight equal sections marking seven cartilage measurement points.
All cartilage dimensions were measured in millimeters. The measurements were taken by two independent radiologists, and the mean values were calculated. The methodology applied for measuring the cartilage in this study was based on previous studies and accepted scales for assessing articular cartilage on MRI [35,36,37,38]. The selected reference and measurement points on the femoral and tibial condyles were based on previously proposed zones for the semiquantitative evaluation of knee joint cartilage in MR images [35,36,37,38].
2.8. Treatment Costs
The total cost of treatment, including arthroscopic debridement and 10 days (average) of hospitalization, was 7000 PLN (2100 PLN surgical procedure + 10 days × 600 PLN = 8100 PLN; 1 PLN = 0.202 USD; 1 PLN = 0.205 EUR).
2.9. Statistical Analysis
The arithmetic mean and standard deviation were calculated from basic position measurements. The Shapiro–Wilk test was used to test data distribution. For mean values with normal distributions, the parametric Student’s t-test was used to identify statistically significant differences between the operated and unoperated limbs. Non-normal variables were analyzed using the non-parametric Mann–Whitney U test and the rank-sum Wilcoxon test. Statistical significance was set at p < 0.05. All calculations were performed with STATISTICA version 10 (StatSoft, Inc. 2011, Tulsa, OK, USA).
3. Results
All the patients were men. In all the patients, the primary ACL-R procedure was performed using a hamstring tendon graft (Table 1). In one case, additional repair of the posterior horn of the medial meniscus was performed using a PDS 2 absorbable suture (Patient 5). In Patient 2, an additional minimal partial medial meniscectomy was performed. Perioperative antibiotic prophylaxis was used in all the patients at the standard dosage. The baseline characteristics of the patients are presented in Table 1.
The mean interval between the primary ACL-R and presentation of symptoms of SA was 8 days (range, 6–12 days). The mean interval between the presentation of symptoms of SA and arthroscopic lavage and drainage was 1.2 days (range, 1–2 days). The mean CRP level at hospital readmission was 72.5 mg/L (range, 51.4–126.3 mg/mL). There was only one single arthroscopic lavage combined with drainage in all cases. In all cases, the ACL graft was preserved. There was no need to remove fixation implants and screws. S. epidermidis was cultured in all the patients. The mean time of intravenous antibiotic administration was 16.2 days (range, 14–21 days). The mean time of CRP level normalization after SA treatment was 9 days (range, 7–12 days).
3.1. Clinical and Functional Results
The mean value of the modified Lysholm score at the first and second follow-up was 90.2 (range, 85–95) and 91.4 (range, 89–92), respectively (Table 2). The KT-1000 examination revealed an abnormal difference in two cases, indicating failure of the reconstructed ligament. One patient (Patient 5) underwent secondary rotational trauma of the operated knee, 4 years before the final follow-up; however, due to good function, the patient declined to undergo revision surgery. The mean side-to-side difference in the KT-1000 arthrometer was 2.36 mm (range, 0–4.6 mm) and 2.44 mm (range, 0–4.7 mm) at the first and last follow-up, respectively (Table 2). Due to the small size of the studied groups, it was not possible to estimate the level of statistical significance (p-value).

Table 2.
Clinical and functional results.
The mean result of single-leg hop tests in the affected knee was 93.8% (range, 85–89) and 91.5% (range, 82.5–101.5) at the first and final follow-up, respectively (Table 2). In two patients (Patients 3 and 5), the results were nearly identical to those on the unaffected side (Table 2). The results were slightly lower in the other three patients (Table 2). Interestingly, there were no significant differences in the results between the first (10.9 years) and last (18.1 years) follow-ups.
All the patients returned to their daily activities and work after the SA treatment. At the time of follow-up, all the patients held relatively sedentary jobs. Patients 3 and 5 reported that they continued some form of occasional sports activity, whereas Patients 1, 2 and 4 denied participation in sports. Regardless of their current level of activity, every patient reported that he was aware of his knee problems, and all of them had modified their lifestyles to avoid any risk to the affected knees.
3.2. Isokinetic Results
Isokinetic evaluation was performed during the last follow-up. The results are presented in Table 3. Isokinetic assessment revealed the persistence of quadriceps strength deficit in all patients at 60° (PT-60-Q) and in three of the four subjects at 180° (PT-180-Q) (Table 3). One patient had an abnormal body mass index (44.8). Virtually all the patients had normalized flexor strength at both velocities (PT-60-H; PT-180-H). None of the patients had normalization of the flexor-to-extensors ratio for either velocity (H/Q) (Table 3). In addition, the quadriceps-force-moment-to-body-weight ratio remained relatively low in all patients (PT/BW) (Table 3). Full normalization of one of the additional parameters was not achieved at time-to-peak torque, peak torque at 30° flexion, angle for peak torque, and peak torque during the first 180 ms (Table 3). The gait distribution was equal, except in Patient 1, whereas the standing distribution in three patients was dominant on the operated side (Table 3).

Table 3.
Isokinetic results.
3.3. MRI Evaluation of the Cartilage
MRI evaluation of knee cartilage thickness was performed at the first and second follow-ups in all the studied patients (Figure 5 and Figure 6, shown as an example). There were only slight statistical differences in cartilage thickness on the medial femoral and tibial condyle (MFC, MTC) between the study and control groups after a mean of 10.9 years follow-up (Table 4). Statistical differences in cartilage thickness on the MFC were observed between the study and control groups (2.077 mm and 2.237 mm, respectively; p = 0.021; Table 4). Interestingly, the articular cartilage on the MTC was slightly thicker in the SA knees than in the uninvolved side (2.478 mm vs. 2.24 mm; p = 0.008, Table 4). There were no significant differences in cartilage thickness in the lateral compartment of the knee at the first follow-up (Table 4). At the final follow-up, slight statistical differences in cartilage thickness were observed only on the lateral femoral condyle. The cartilage in the SA knees was slightly thinner than that on the unaffected side (2.217 mm vs. 2.43 mm; p = 0.041; Table 4). There were no significant differences in cartilage thickness between the first and last follow-ups in the SA-affected knees.

Figure 5.
(A) MRI scan of the SA knee after ACL-R (frontal plane) obtained during the first follow-up (Patient 3); (B) MRI scan of the SA knee after ACL-R (sagittal plane of MFC) obtained during the first follow-up (Patient 3); (C) MRI scan of the control knee (frontal plane) obtained during the first follow-up (Patient 3); (D) MRI scan of the control knee (sagittal plane of MFC) obtained during the first follow-up (Patient 3). MRI, magnetic resonance imaging; SA, septic arthritis; ACL-R, anterior cruciate ligament reconstruction; MFC, medial femoral condyle. 10.9 years follow-up.
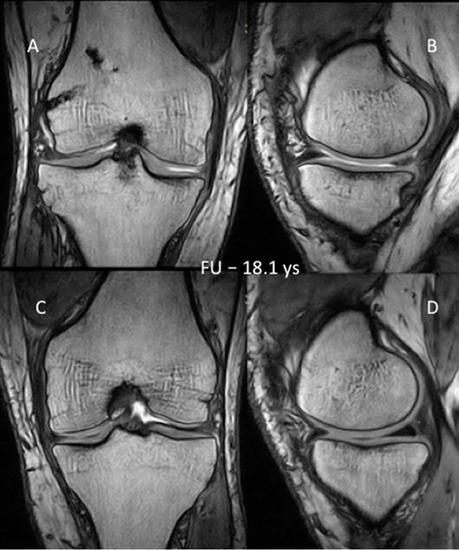
Figure 6.
(A) MRI scan of the SA knee after ACL-R (frontal plane) obtained during the first follow-up (Patient 3); (B) MRI scan of the SA knee after ACL-R (sagittal plane of MFC) obtained during the first follow-up (Patient 3); (C) MRI scan of the control knee (frontal plane) obtained during the first follow-up (Patient 3); (D) MRI scan of the control knee (sagittal plane of MFC) obtained during the first follow-up (Patient 3). MRI, magnetic resonance imaging; SA, septic arthritis; ACL-R, anterior cruciate ligament reconstruction; MFC, medial femoral condyle. 18.1 years follow-up.

Table 4.
Knee joint cartilage thickness in MRI images.
4. Discussion
The chief importance of our study is in documenting a positive correlation between the rate of pathogen eradication time in SA after ACL-R using arthroscopic lavage and debridement combined with close drainage and the preservation of articular cartilage thickness in MRI 18.1 years after surgery. The results of the articular cartilage measurements indicated that there was no obvious damage to the articular cartilage expressed by a change in cartilage thickness during the follow-up period (the first and the second follow-up), except for the measurement of MFC and MTC after the first follow-up; however, this change was not observed at the second follow-up (Table 4). This is even more valuable because, as evidenced by previous work, OA generally develops after post-ACL-R SA after arthroscopic lavage and debridement treatment, and after uncomplicated ACL-R at a minimum of 10 years after surgical treatment [3,13,14,17,31].
Our technique of cartilage thickness measured using MRI is comparable to those presented in Muhlbauer’s study [39]. The technique of cartilage thickness measurement, proposed by us for the first time in this study, concerns the areas of cartilage previously selected for its assessment on a semi-quantitative scale widely accepted in the literature [35,36,37,38]. Thus, our technique can complement the previously mentioned assessment or be used for independent evaluations.
Based on the analysis of radiographs of 68 patients 12 years after ACL-R with a hamstring, Leiter et al. found a narrowing of the joint space in the medial compartment compared to the opposite knee [40]. A completely different observation was made in their study by Bostrom Windhamre et al. based on the analysis of radiographs after a mean follow-up of 66 months [41]. In the available literature, there are two papers in which the cartilage after knee joint infection following ACL-R was assessed by MRI in long-term follow-up [31,42]. Both papers originated from the same research center and were concerned with the evaluation of the same four patients [31,42]. Three years after the eradication of infection, focal chondral defects, diffuse thinning in the medial compartment in two patients, and tricompartmental diffuse thinning in one patient were observed on MRI of all patients [31]. One patient had significant damage to the articular cartilage 42 months after surgery, when compared with preoperative MRI [31]. The same patients were re-evaluated after 17.9 years, and progression of cartilage loss was observed on MRI [42]. In our study, no focal chondral defects, diffuse thinning in the medial compartment, or significant damage to the articular cartilage were found in the patients’ MRIs. A recent study by Meglic et al. showed early signs of OA on MRI scans among patients with SA after ACL-R at a mean of 4 years follow-up [16]. The Boston–Leeds Osteoarthritis Knee Score was used to assess chondral defects [16].
Our results confirmed the previous report by Wipfler et al., which compared MRI of the knee after ACL-R using a hamstring with the opposite knee [43]. This analysis showed no statistically significant articular cartilage damage in the knees after ACL-R at 9 years after surgery [43].
Based on the literature, it is known that the number of arthroscopies required to eradicate infection after ACL-R ranges between 1.3 and 3 [20,21,32,44]. Most authors initially suggest leaving the graft, and performing arthroscopic lavage and debridement combined with intravenous antibiotic therapy [17,32]; however, this initial treatment achieved success in 85.5% of patients affected by this complication [44]. In addition to arthroscopic lavage and debridement combined with intravenous antibiotic therapy, we also used flow drainage with vancomycin (1 g/3 L in 0.9% NaCl) for an average of 9 days, which caused the flushing of pro-inflammatory mediators and bacterial debris and led to faster eradication of the infection. This is evidenced by the faster return of CRP to normal values, which was observed after 9 days (range, 7–12 days). Based on the literature, the fastest CRP return after arthroscopic lavage and debridement combined with intravenous antibiotic therapy alone was observed after an average of 14–21 days [32]. If we objectively compare the arguments for multiple arthroscopy and close drainage, the second option is a more rational choice [21,32,44]. It is also known that postinfectious synovitis persists after SA following an ACL-R [3]: this is because after eradicating the infection, sterile microbial fragments and inflammatory mediators remain in the joint environment for a long time, initiating a continuous inflammatory response, leading to articular cartilage damage and the development of OA [2,3,8,42]. We consider the use of flow drainage, by flushing out sterile microbial fragments and inflammatory mediators, to significantly reduce postinfectious synovitis, as shown in the much faster return of CRP to normal values that we observed in our patients. According to Saper et al., patients who had a single arthroscopic lavage and debridement resulting in the eradication of infection had a higher success rate [44]. When performing arthroscopic irrigation and debridement, we did not perform additional surgical accesses or extensive synovectomy to avoid additional surgical trauma, damaging the natural protective barrier of the synovium and reducing the risk of arthrofibrosis. In fact, synovectomy is unnecessary because in acute infection, the synovial membrane is in a phase of swelling and inflammatory congestion. Moreover, removing fibrous deposits and small adhesions without synovectomy eliminates postoperative bleeding, which can be a source of material blocking the free flow in the drains. The flow drainage we used (1 g of vancomycin in 3 L of 0.9% NaCl administered daily) proved to be completely safe and did not result in any adverse general symptoms. Recent publications also confirm that a vancomycin concentration of 0.3 mg/mL is completely safe for ligament grafting [45].
The faster return of CRP to normal values observed in our study is associated with a shorter duration of hospitalization and a shorter period of intravenous antibiotic administration.
Based on literature data, the length of hospitalization during treatment of this complication is 29.7 ± 11.6 days [32]. Our patients were hospitalized for an average of 16.2 days, the entire period of intravenous antibiotic therapy (Table 1). Our later experience and observations allowed us to reduce this time even further, and patients were discharged from the hospital the day after CRP returned to normal values. In addition, none of our patients required repeated arthroscopy because of failure to eradicate the infection.
The much shorter hospitalization time and lack of need for further arthroscopies significantly reduced the cost of treatment for this complication. Regarding the average length of hospitalization (29.7 days), as reported in the literature, this cost would increase by an additional PLN 11,820 (which is an additional 146% of the original cost) and would amount to PLN 19,920. Each repeated arthroscopic debridement would increase the cost of treatment by another PLN 2100. For comparison, the cost of arthroscopic ACL-R in Poland is PLN 4500 (data from the Polish National Health Insurance Fund).
The etiology of OA after SA following ACL-R is multivariable [24,25,26,31,32]. An overlooked aspect of its etiopathogenesis is the effect of infection on the content of meniscus proteoglycans. Experimental studies have shown that a 1% decrease in their content causes a 1.35% decrease in the biomechanical properties of the meniscus [25]. Moreover, a 1% decrease in the elasticity of the meniscus results in a 0.77% decrease in the elasticity of the underlying medial tibial condyle cartilage. These data indicate that the degradation of 1% of the extracellular matrix proteoglycans of the meniscus results in a total decrease in the shock-absorbing capacity of the meniscus and cartilage together by 2% (1.35% + 0.77%). Assuming a similar decrease in the femoral condyle cartilage, this value approaches 3%. Moreover, experimental studies indicate that a 15% decrease in proteoglycans in the meniscus leads to irreversible changes in cartilage in the form of chondrocyte apoptosis [25,46,47].
Thus, the development of OA post-SA after ACL-R may have a threefold cause: (1) infection that causes degradation of proteoglycans, leading to a decrease in the shock-absorbing capacity of the meniscus and cartilage; (2) damage to the ACL which, despite reconstruction, is associated with the development of degenerative changes at 10 years follow-up; and (3) co-occurrence of pathologies of the meniscus and cartilage. Based on the results presented in this study, it can be concluded that the method we adopted protects the meniscus and articular cartilage from loss of proteoglycans, causing a decrease in the viscoelastic properties of both structures, which has a key effect on the development of degenerative changes.
Apart from the applied treatment of SA after ACL-R, we believe that an extremely important factor in the prevention of knee degenerative changes was convincing the patients to give up competitive sports. This is particularly supported by the correlation of the KT-1000 results with the MRI images.
The observation period (mean 18.1 years) is one of the longest in the current literature. Moreover, our study is prospective because the clinical evaluation of knees after infection was performed twice, at a mean of 10.9 years and a mean of 18.1 years. The clinical results obtained from our patients were similar to those of previous publications [6,16,17,48]. Torres-Claramunt et al. recently published a study assessing the functional outcomes of 15 patients at a mean of 39.3 months after SA following ACL-R. The prevalence of SA was 1.8%. They obtained a mean Lysholm score of 77.7 and an IKDC score of 70.4, and the KT-1000 compared to the non-injured contralateral knee showed a mean difference of 1.3 mm [48]. The Lachman test was positive in four patients and the pivot-shift test in three patients. They also observed early signs of radiographic degeneration (Fairbank grade I) in three patients. In addition to arthroscopic debridement and intravenous, and later oral, antibiotic therapy, ambulatory irrigation of the knee for 4 h/day for an average of 2 days using two spinal needles was used [48]. A similar incidence of SA following ACL-R (1.7%) was observed in her notes by Maria Scholin-Borg [6]. She obtained a mean Lysholm score of 74.9, IKDC score of B-5 patients, C-3 patients, D-2 patients, and a KT-1000 mean difference of 1.4 mm after 3 years follow-up. In the one-leg hop test, seven patients achieved at least 90% motor skill of the contralateral limb, one with 80%, and two who were unable to perform a jump with the reconstructed leg at all. All patients underwent arthroscopic debridement and antibiotic treatment; additionally, most patients (8 patients) were treated with constant irrigation drains left in the joint for 4–7 days, and two patients underwent additional lavage [6]. Radiographic examination showed osteophyte formation in three patients, and joint space narrowing of the medial compartment in two others.
To the best of our knowledge, our study is the first to report complex clinical, isokinetic and MRI evaluations of patients with SA after ACL-R treated with arthroscopic debridement and drainage, where the results were related to the other, healthy knee. To our knowledge, this is the first comprehensive isokinetic study to evaluate knee function after SA following ACL-R treatment in the available literature. Observations indicate that, due to the positive subjective evaluation of the treatment outcome, patients are not willing to engage in intensive and diligent exercise and rehabilitation to fully restore quadriceps and flexor muscle strength, despite a long follow-up period. Furthermore, studies have shown significant deficits in selected isokinetic parameters that may remain non-normalized throughout the patient’s life. However, previous reports by Fabiś indicate that with consistent rehabilitation and isokinetic training, normalization of function and strength of both flexor and quadriceps muscles is entirely possible [49]. The results of gait analysis further revealed that there are differences in dynamic and static loading of the operated limb, which should be considered as an independent risk factor for the early development of degenerative changes.
Our experience indicates the high usefulness of monitoring body temperature, which is a reliable signal of infection development, provided the regime of avoidance of anti-inflammatory drugs that may falsify the measurement results.
There are several limitations to this study that should be considered. The relatively small size of the study group did not allow for more precise statistical analysis. The complications studied are very rare, and it is difficult to collect an appropriate number of patients in a single center. Moreover, for a proper analysis of long-term outcomes, the evaluation should be performed at similar endpoints for each patient. The second limitation of our analysis may be the lack of a control group of patients who underwent primary ACL-R without SA. This would allow for more complete clinical and functional assessments and analyses. However, the main goal of our study was to demonstrate the positive effect of the proposed treatment of SA after ACL-R and its influence on reducing hospitalization time, cost of treatment and cartilage thickness changes in MRI evaluation.
Although the number of patients studied prevented us from drawing far-reaching conclusions, the prospective nature of the study and the extent of comprehensive clinical assessment and MRI may be a valuable addition to our knowledge regarding the therapeutic options for SA after ACL-R and the prognosis of long-term outcomes.
5. Conclusions
- Arthroscopic lavage and debridement combined with flow drainage led to more rapid normalization of CRP values, objectively reflecting the process of infection eradication after ACL-R compared to arthroscopic debridement and lavage alone.
- By faster eradication of infection, the patient can be protected against the early development of degenerative changes, and the treatment costs can be reduced.
- The quantitative evaluation of knee cartilage after SA following ACL-R, proposed for the first time in the present study, may be more accurate for assessing the early symptoms of OA and predicting its development.
- The isokinetic evaluation revealed that despite normalization of hamstring peak torque, deficit of the quadriceps may still persist after a mean of 18.1 years follow-up.
Author Contributions
Conceptualization, M.W. and J.F.; methodology, M.W., J.F., A.F.-S. and K.M.; software, M.W., A.F.-S., A.K.-S. and J.B.; validation, M.W., K.M. and P.B.; formal analysis, M.W., J.F. and J.B.; investigation, M.W., J.F., A.F.-S., A.K.-S. and K.M.; resources, M.W., J.F. and P.B.; data curation, M.W., A.K-S., K.M. and J.B.; writing—original draft preparation, M.W. and J.F.; writing—review and editing, M.W., J.F. and J.B.; visualization, M.W.; supervision, J.F.; project administration, M.W. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by internal fund of the Medical University of Lodz, Lodz, Poland (grant number: 5503/1-040-02/503-51-001-19-00).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (Bioethics Committee) of the Medical University of Lodz Poland (approval number: RNN/651/11/KB from 12 July 2011 and RNN/21/22/KE from 8 February 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demirag, B.; Unal, O.K.; Ozakin, C. Graft retaining debridement in patients with septic arthritis after anterior cruciate ligament reconstruction. Acta Orthop. Traumatol. Turc. 2011, 45, 342–347. [Google Scholar] [PubMed]
- Judd, D.; Bottoni, C.; Kim, D.; Burke, M.; Hooker, S. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy 2006, 22, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Mouzopoulos, G.; Fotopoulos, V.C.; Tzurbakis, M. Septic knee arthritis following ACL reconstruction: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Matava, M.J.; Evans, T.A.; Wright, R.W.; Shively, R.A. Septic arthritis of the knee following anterior cruciate ligament reconstruction: Results of a survey of sports medicine fellowship directors. Arthroscopy 1998, 14, 717–725. [Google Scholar] [CrossRef]
- Wang, C.; Ao, Y.; Wang, J.; Hu, Y.; Cui, G.; Yu, J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: A retrospective analysis of incidence, presentation, treatment, and cause. Arthroscopy 2009, 25, 243–249. [Google Scholar] [CrossRef]
- Schollin-Borg, M.; Michaëlsson, K.; Rahme, H. Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: A case control study. Arthroscopy 2003, 19, 941–947. [Google Scholar] [CrossRef]
- Vallianatos, P.G.; Tilentzoglou, A.C.; Koutsoukou, A.D. Septic arthritis caused by Erysipelothrix rhusiopathiae infection after arthroscopically assisted anterior cruciate ligament reconstruction. Arthroscopy 2003, 19, E26. [Google Scholar]
- Monaco, E.; Maestri, B.; Labianca, L. Clinical and radiological outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. J. Orthop. Sci. 2010, 15, 198–203. [Google Scholar] [CrossRef]
- Fong, S.Y.; Tan, J.L. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Ann. Acad. Med. 2004, 33, 228–234. [Google Scholar]
- Katz, L.M.; Battaglia, T.C.; Patino, P.; Reichmann, W.; Hunter, D.J.; Richmond, J.C. A retrospective comparison of the incidence of bacterial infection following anterior cruciate ligament reconstruction with autograft versus allograft. Arthroscopy 2008, 24, 1330–1335. [Google Scholar] [CrossRef]
- Van Tongel, A.; Stuyck, J.; Bellemans, J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: A retrospective analysis of incidence, management and outcome. Am. J. Sports Med. 2007, 35, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Indelli, P.F.; Dillingham, M.; Fanton, G. Septic arthritis in postoperative anterior cruciate ligament reconstruction. Clin. Orthop. Relat. Res. 2002, 398, 182–188. [Google Scholar] [CrossRef]
- Cadet, E.R.; Makhni, E.C.; Mehran, N.; Schulz, B.M. Management of septic arthritis following anterior cruciate ligament reconstruction: A review of current practices and recommendations. J. Am. Acad. Orthop. Surg. 2013, 21, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Chaurasia, S.; Abhishek, M.; Bulgherhoni, E.; Lane, J. Postoperative infection after anterior cruciate ligament reconstruction. Sports Health 2016, 8, 187–189. [Google Scholar] [CrossRef]
- Lo Presti, M.; Costa, G.G.; Grassi, A.; Cialdella, S.; Agrò, G.; Busacca, M.; Pia Neri, M.; Filardo, G.; Zaffagnini, S. Graft-preserving arthroscopic debridement with hardware removal is effective for septic arthritis after anterior cruciate ligament reconstruction: A clinical, arthrometric, and magnetic resonance imaging evaluation. Am. J. Sports Med. 2020, 48, 1907–1915. [Google Scholar] [CrossRef]
- Meglic, U.; Salapura, V.; Zupanc, O. MRI Findings of Early Osteoarthritis in Patients Who Sustained Septic Arthritis of the Knee After ACL Reconstruction. Orthop. J. Sports Med. 2021, 9, 23259671211052519. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.; Figueroa, D.; Anastasiadis, Z.; Vaisman, A.; Olid, G.; Gili, F.; Valderrama, J.J.; De La Fuente, P. Septic arthritis in ACL reconstruction surgery with hamstring autografts. Eleven years of experience. Knee 2014, 21, 717–720. [Google Scholar] [CrossRef]
- Louboutin, H.; Debarge, R.; Richou, J.; Selmi, T.A.; Donell, S.T.; Neyret, P.; Dubrana, F. Osteoarthritis in patients with anterior cruciate ligament rupture: A review of risk factors. Knee 2009, 16, 239–244. [Google Scholar] [CrossRef]
- Schulz, A.P.; Götze, S.; Schmidt, H.G.; Jürgens, C.; Faschingbauer, M. Septic arthritis of the knee after anterior cruciate ligament surgery: A stage-adapted treatment regimen. Am. J. Sports Med. 2007, 35, 1064–1069. [Google Scholar] [CrossRef]
- Schuster, P.; Schulz, M.; Immendoerfer, M.; Mayer, P.; Schlumberger, M.; Richter, J. Septic Arthritis After Arthroscopic Anterior Cruciate Ligament Reconstruction: Evaluation of an Arthroscopic Graft-Retaining Treatment Protocol. Am. J. Sports Med. 2015, 43, 3005–3012. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.; Radwan, Y.A.; Rizk, A. Multiple arthroscopic debridement and graft retention in septic knee arthritis after ACL reconstruction: A prospective case-control study. Int. Orthop. 2014, 38, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Waszczykowski, M.; Fabiś-Strobin, A.; Bednarski, I.; Lesiak, A.; Narbutt, J.; Fabiś, J. Serum Biomarkers of Inflammation and Turnover of Joint Cartilage Can Help Differentiate Psoriatic Arthritis (PsA) Patients from Osteoarthritis (OA) Patients. Diagnostics 2020, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Waszczykowski, M.; Fabiś-Strobin, A.; Bednarski, I.; Narbutt, J.; Fabiś, J. Serum and synovial fluid concentrations of interleukin-18 and interleukin-20 in patients with osteoarthritis of the knee and their correlation with other markers of inflammation and turnover of joint cartilage. Arch. Med. Sci. 2022, 18, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Kajiyama, G.; Schurman, D.J. Staphylococcal septic arthritis: Antibiotic and nonsteroidal anti-inflammatory drug treatment in a rabbit model. J. Orthop. Res. 1997, 15, 919–926. [Google Scholar] [CrossRef]
- Zwierzchowski, T.J.; Janus, J.; Konecki, W.; Kubiak, G.; Fabiś, J. The quantitative evaluation of the impact of viable medial meniscus graft type on the biochemical and biomechanical properties of the rabbit tibial cartilage. J. Orthop. Surg. Res. 2015, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Zeng, N.; Yan, Z.P.; Li, J.T.; Ni, G.X. Post-traumatic osteoarthritis following ACL injury. Arthritis Res. Ther. 2020, 22, 57. [Google Scholar] [CrossRef]
- Tsoukas, D.; Fotopoulos, V.; Basdekis, G.; Makridis, K.G. No difference in osteoarthritis after surgical and non-surgical treatment of ACL-injured knees after 10 years. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2953–2959. [Google Scholar] [CrossRef]
- Kessler, M.A.; Behrend, H.; Henz, S.; Stutz, G.; Rukavina, A.; Kuster, M.S. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 442–448. [Google Scholar] [CrossRef]
- Delincé, P.; Ghafil, D. Anterior cruciate ligament tears: Conservative or surgical treatment? A critical review of the literature. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 48–61. [Google Scholar] [CrossRef]
- Van Yperen, D.T.; Reijman, M.; van Es, E.M.; Bierma-Zeinstra, S.M.A.; Meuffels, D.E. Twenty-Year Follow-up Study Comparing Operative Versus Nonoperative Treatment of Anterior Cruciate Ligament Ruptures in High-Level Athletes. Am. J. Sports Med. 2018, 46, 1129–1136. [Google Scholar] [CrossRef]
- Schub, D.L.; Schmitz, L.M.; Sakamoto, F.A.; Winalski, C.S.; Parker, R.D. Long-term outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2012, 40, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lee, Y.H.; Siebold, R. Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, G.; Fabiś, J. Evaluation of treatment strategy of acute knee infection after ACL reconstruction with hamstring. Pol. Orthop. Traumatol. 2013, 78, 235–238. [Google Scholar] [PubMed]
- Lysholm, J.; Gillquist, J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am. J. Sports Med. 1982, 10, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Roemer, F.W.; Haugen, I.K.; Crema, M.D.; Hayashi, D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 236–251. [Google Scholar] [CrossRef]
- Li, G.; Park, S.E.; De Frate, L.E.; Schutzer, M.E.; Ji, L.; Gill, T.J.; Rubash, H.E. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin. Biomech. 2005, 20, 736–744. [Google Scholar] [CrossRef]
- Mittal, S.; Pradhan, G.; Singh, S.; Batra, R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol. J. Radiol. 2019, 8, 549–564. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef]
- Mühlbauer, R.; Lukasz, T.S.; Faber, T.S.; Stammberger, T.; Eckstein, F. Comparison of knee joint cartilage thickness in triathletes and physically inactive volunteers based on magnetic resonance imaging and three-dimensional analysis. Am. J. Sports Med. 2000, 28, 541–546. [Google Scholar] [CrossRef]
- Leiter, J.R.; Gourlay, R.; McRae, S.; de Korompay, N.; MacDonald, P.B. Long-term follow-up of ACL reconstruction with hamstring autograft. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1061–1069. [Google Scholar] [CrossRef]
- Boström Windhamre, H.; Mikkelsen, C.; Forssblad, M.; Willberg, L. Postoperative septic arthritis after anterior cruciate ligament reconstruction: Does it affect the outcome? A retrospective controlled study. Arthroscopy 2014, 30, 1100–1109. [Google Scholar] [CrossRef]
- McAllister, D.R.; Parker, R.D.; Cooper, A.E.; Recht, M.P.; Abate, J. Outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am. J. Sports Med. 1999, 27, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, B.; Donner, S.; Zechmann, C.M.; Springer, J.; Siebold, R.; Paessler, H.H. Anterior cruciate ligament reconstruction using patellar tendon versus hamstring tendon: A prospective comparative study with 9-year follow-up. Arthroscopy 2011, 27, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Saper, M.; Stephenson, K.; Heisey, M. Arthroscopic irrigation and debridement in the treatment of septic arthritis after anterior cruciate ligament reconstruction. Arthroscopy 2014, 30, 747–754. [Google Scholar] [CrossRef]
- Banios, K.; Komnos, G.A.; Raoulis, V.; Bareka, M.; Chalatsis, G.; Hantes, M.E. Soaking of autografts with vancomycin is highly effective on preventing postoperative septic arthritis in patients undergoing ACL reconstruction with hamstrings autografts. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Zwierzchowski, T.J.; Stasikowska-Kanicka, O.; Janus, J.; Konecki, W.; Danilewicz, M.; Fabiś, J. Evidence for apoptosis, MMP-1, MMP-3 and TIMP-2 expression and their effect on the mechanical and biochemical properties of fresh viable knee medial meniscal allografts and autografts in the rabbit. Arch. Med. Sci. 2012, 8, 724–732. [Google Scholar] [CrossRef]
- Zwierzchowski, T.J.; Stasikowska-Kanicka, O.; Danilewicz, M.; Fabiś, J. Assessment of apoptosis and MMP-1, MMP-3 and TIMP-2 expression in tibial hyaline cartilage after viable medial meniscus transplantation in the rabbit. Arch. Med. Sci. 2012, 8, 1108–1114. [Google Scholar] [CrossRef]
- Torres-Claramunt, R.; Gelber, P.; Pelfort, X.; Hinarejos, P.; Leal-Blanquet, J.; Pérez-Prieto, D.; Monllau, J.C. Managing septic arthritis after knee ligament reconstruction. Int. Orthop. 2016, 40, 607–614. [Google Scholar] [CrossRef]
- Fabiś, J. The impact of a isokinetic training program on the peak torque of the quadriceps and knee flexors after anterior cruciate ligament reconstruction with hamstrings. Ortop. Traumatol. Rehabil. 2007, 9, 527–531. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).