Antimicrobial Properties of Zinc Oxide Nanoparticles Synthesized from Lavandula pubescens Shoot Methanol Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Lavandula pubescens Shoot Methanol Extract
2.2. GC–MS Analysis of Compounds in Methanol Extract
2.3. Fabrication of ZnO Nanoparticles
2.4. Characterization of ZnO Nanoparticles
2.5. Preparation of Tested Bacteria and Fungi
2.6. In Vitro Antimicrobial Potential of Nanoparticles
2.6.1. Determination of Antimicrobial Activity of ZnO Nanoparticles Loaded with Lavandula pubescens Shoot Methanol Extract (50 and 100 mg) Using Agar Well Diffusion Technique
2.6.2. The Minimum Inhibitory Concentration (MIC) Assay
2.6.3. Minimum Bactericidal/Fungicide Concentration (MBC/MFC) ASSAY
2.7. Morphological Examination of Bacterial Cells
2.8. Statistical Analysis
3. Results and Discussion
3.1. GC–MS Profile of Shoot Methanol Extract of L. pubescens
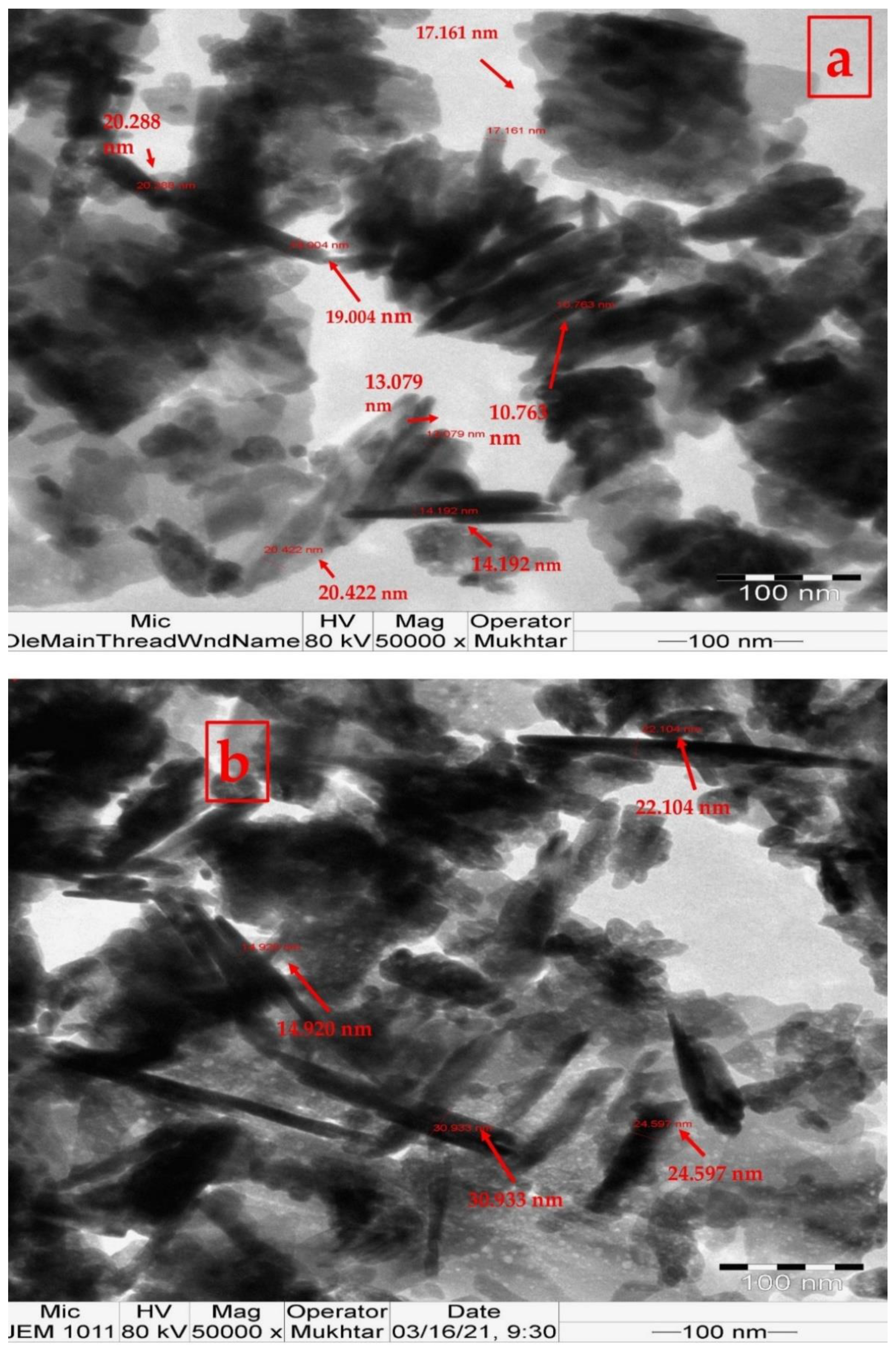
3.2. ZnO Nanoparticle Characterization Analysis
3.3. Antimicrobial Properties of ZnO Nanoparticles Loaded with L. pubescens Shoot Methanol Extract
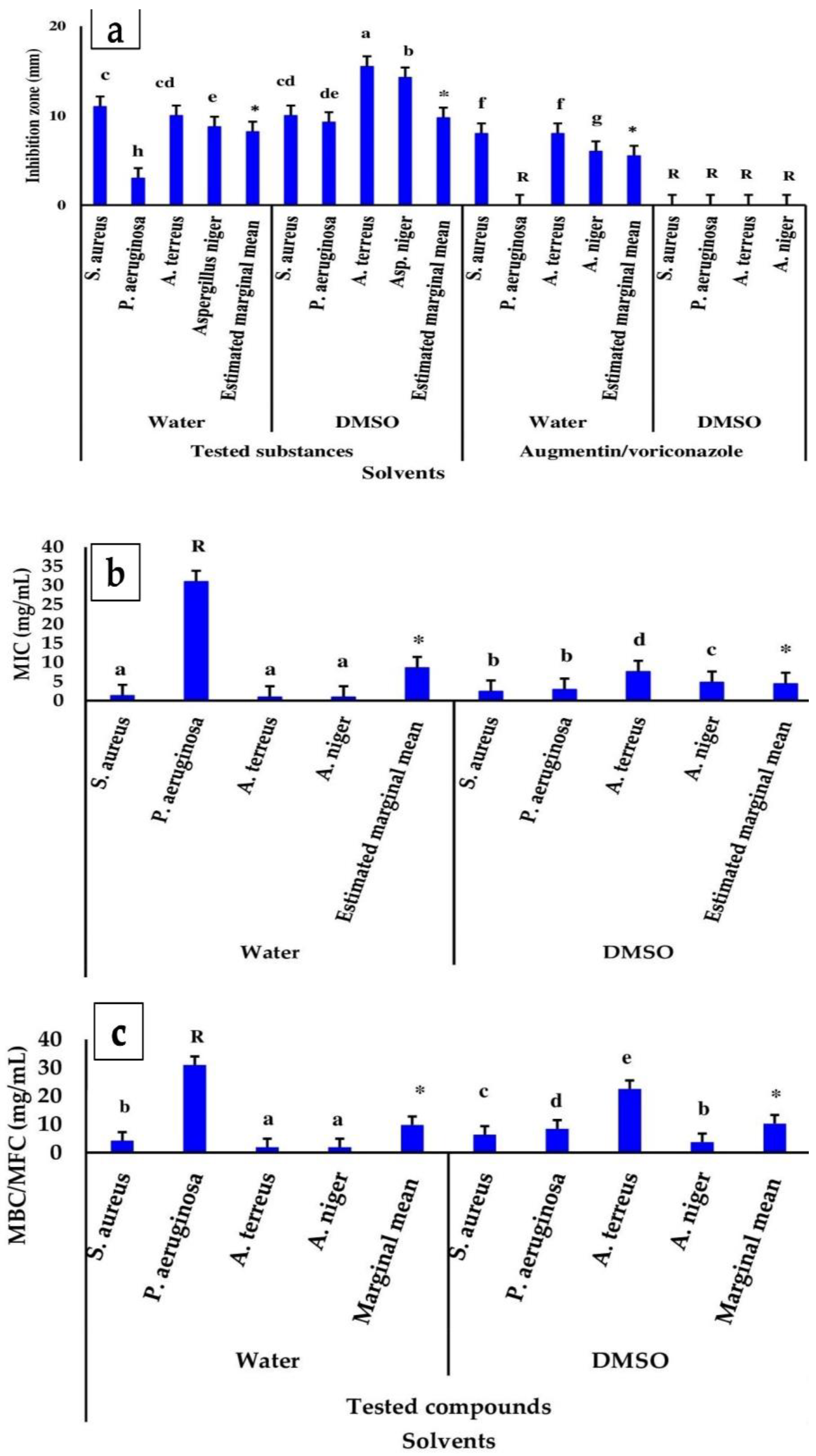
3.3.1. The Zone of Inhibition
3.3.2. Minimum Bactericidal/Fungicide Concentration (MBC/MFC) and Inhibitory Concentration (MIC)
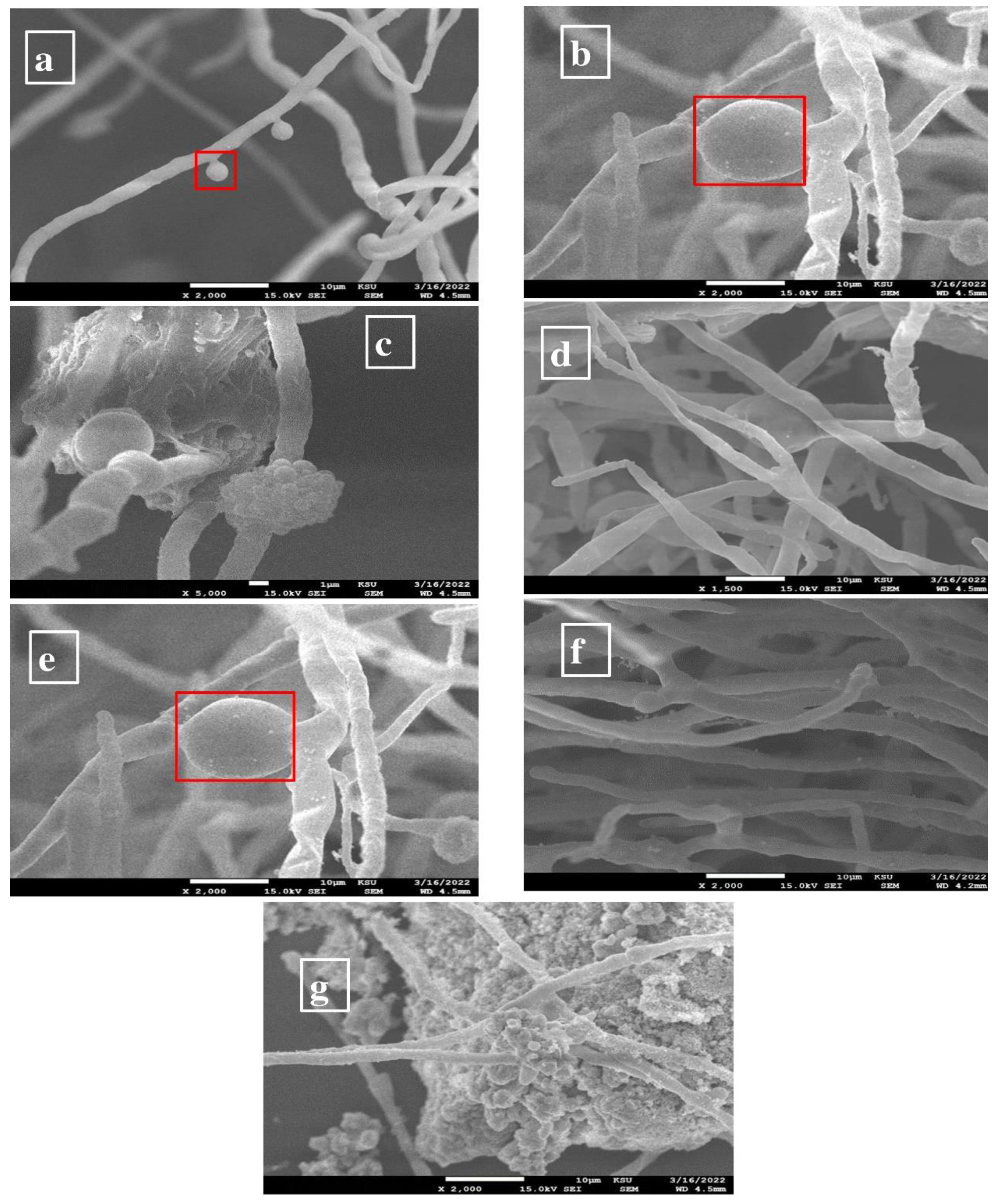
3.3.3. SEM Analysis of ZnO-Nanoparticle-Treated Bacteria and Fungi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasli, N.I.; Basri, H.; Harun, Z. Zinc oxide from aloe vera extract: Two-level factorial screening of biosynthesis parameters. Heliyon 2020, 6, e03156. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO, and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Maret, W. Metals on the move: Zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 2011, 24, 411–418. [Google Scholar] [CrossRef]
- Zare, E.; Pourseyedi, S.; Khatami, M.; Darezereshki, E. Simple biosynthesis of zinc oxide nanoparticles using nature’s source, and it’s in vitro bio-activity. J. Mol. Struct. 2017, 1146, 96–103. [Google Scholar] [CrossRef]
- Fatimah, I.; Pradita, R.Y.; Nurfalinda, A. Plant extract mediated of ZnO nanoparticles by using ethanol extract of Mimosa pudica leaves and coffee powder. Procedia Eng. 2016, 148, 43–48. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Ifeanyichukwu, U.L. Omolola Esther Fayemiand Collins Njie Ateba. Green Synthesis of Zinc Oxide Nanoparticles from Pomegranate (Punica granatum) Extracts and Characterization of Their Antibacterial Activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired Green Synthesis of Chitosan and Zinc Oxide Nanoparticles with Strong Antibacterial Activity against Rice Pathogen Xanthomonas oryzae pv. Oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green Fabrication of Zinc Oxide Nanoparticles Using Phlomis Leaf Extract: Characterization and In Vitro Evaluation of Cytotoxicity and Antibacterial Properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.; Samal, M.; Yun, K. Synthesis of ZnO nanoparticles-decorated spindle-shaped graphene oxide for application in synergistic antibacterial activity. J. Photochem. Photobiol. B Biol. 2018, 183, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef]
- El-Masry, R.M.; Talat, D.; Hassoubah, S.A.; Zabermawi, N.M.; Eleiwa, N.Z.; Sherif, R.M.; Mohammed, A.S.; Abourehab, M.A.S.; Abdel-Sattar, R.M.; Gamal, M.; et al. Evaluation of the Antimicrobial Activity of ZnO Nanoparticles against Enterotoxigenic Staphylococcus aureus. Life 2022, 12, 1662. [Google Scholar] [CrossRef]
- de Lucas-Gil, E.; Leret, P.; Monte-Serrano, M.; Reinosa, J.J.; Enríquez, E.; Del Campo, A.; Cañete, M.; Menéndez, J.; Fernández, J.F.; Rubio-Marcos, F. ZnO Nanoporous Spheres with Broad-Spectrum Antimicrobial Activity by Physicochemical Interactions. ACS Appl. Nano Mater. 2018, 1, 3214–3225. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Shaikh, I.A.; Muddapur, U.M.; Bagewadi, Z.K.; Chiniwal, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alsaikhan, F.; Yaraguppi, D.; Niyonzima, F.N.; More, S.S.; et al. Characterization of Bioactive Compounds from Acacia concinna and Citrus limon, Silver Nanoparticles’ Production by Acacia concinna Extract, and Their Biological Properties. Molecules 2022, 27, 2715. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N.; Pabarja, A.; Abadi, M.F.S.; Tahroudi, M.H.M. Biosynthesis of zinc oxide nanoparticles using Hertia intermedia and evaluation of its cytotoxic and antimicrobial activities. BioNanoScience 2021, 11, 245–255. [Google Scholar] [CrossRef]
- Yilmaz, D.; Fatih, D.; Onmaz, N. Comparison of antimicrobial activity of bio-synthesized silver and zinc oxide nanoparticles using Lavandula stoechas leaf extract. Res. J. Biotechnol. 2019, 14, 24–29. [Google Scholar]
- Rather, G.A.; Nanda, A.; Raj, E.; Mathivanan, N.; Nayak, B. Green synthesis of ZnO nanoparticles using the leaf extract of Lavandula angustifolia and evaluation of their antibacterial activity against human pathogens. Int. J. Health Sci. 2022, 6, 13478–13485. [Google Scholar] [CrossRef]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Turgut, A.C.; Emen, F.M.; Canbay, H.S.; Demirdöğen, R.E.; Çam, N.; Kılıç, D.; Yeşilkaynak, T. Chemical characterization of Lavandula angustifolia Mill. as a phytocosmetic species and investigation of its antimicrobial effect in cosmetic products. J. Turk. Chem. Soc. Sect. A Chem. 2017, 4, 283–298. [Google Scholar] [CrossRef]
- Yassine Ez zoubi, Y.; Bousta, D.; Abdellah Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 9. [Google Scholar] [CrossRef]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid chromatography fingerprint analysis and antioxidant activity of selected lavender species with chemometric calculations. PLoS ONE 2019, 14, e0218974. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, F.; Huang, H.; Ji, T.; Li, C.; Tan, W.; Chen, Y.; Ma, L. Evaluation on bioactivities of total flavonoids from Lavandula angustifolia. Pak. J. Pharm. Sci. 2015, 28, 1245–1251. [Google Scholar]
- Mousa, O.; Gouda, B.; Salama, M.; El-Eraky, W.; Kassem, H. Total phenolic, total flavonoid content, two isolates and bioactivity of Lavandula pubescens Decne. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 254–263. [Google Scholar]
- Divya, B.; Karthikeyan, C.; Rajasimman, M. Chemical synthesis of zinc oxide nanoparticles and its application of dye decolourization. Int. J. Nanosci. Nanotechnol. 2018, 14, 267–275. [Google Scholar]
- Richardson, P.M.; Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. Second Edition. Brittonia 1990, 42, 115. [Google Scholar] [CrossRef]
- Wayne, P. Reference method for broth dilution antifungal susceptibility testing of yeasts. Clin. Lab. Stand. Inst. 2008, 3, M27-A23. [Google Scholar]
- Pereira, F.d.O.; Wanderley, P.A.; Viana, F.A.C.; Lima, R.B.d.; Sousa, F.B.d.; Lima, E.d.O. Growth inhibition and morphological alterations of Trichophyton rubrum induced by essential oil from Cymbopogon winterianus Jowitt ex Bor. Braz. J. Microbiol. 2011, 42, 233–242. [Google Scholar] [CrossRef]
- Alekish, M.; Ismail, Z.B.; Albiss, B.; Nawasrah, S. In vitro antibacterial effects of zinc oxide nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Escherichia coli: An alternative approach for antibacterial therapy of mastitis in sheep. Vet. World 2018, 11, 1428. [Google Scholar] [CrossRef]
- Liu, S.; Long, Q.; Xu, Y.; Wang, J.; Xu, Z.; Wang, L.; Zhou, M.; Wu, Y.; Chen, T.; Shaw, C. Assessment of antimicrobial and wound healing effects of Brevinin-2Ta against the bacterium Klebsiella pneumoniae in dermally-wounded rats. Oncotarget 2017, 8, 111369. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, V.; Pandey, A.K.; Mishra, B.N.; Kulsoom, M.; Dasgupta, N.; Khan, S.; El-Enshasy, H.A.; Haque, S. Preparation and evaluation of the ZnO NP–ampicillin/sulbactam nanoantibiotic: Optimization of formulation variables using RSM coupled GA method and antibacterial activities. Biomolecules 2019, 9, 764. [Google Scholar] [CrossRef]
- Veerasamy, R.; Roy, A.; Karunakaran, R.; Rajak, H. Structure–activity relationship analysis of benzimidazoles as emerging anti-inflammatory agents: An overview. Pharmaceuticals 2021, 14, 663. [Google Scholar] [CrossRef]
- Khedr, F.; Ibrahim, M.K.; Eissa, I.H.; Abulkhair, H.S.; El-Adl, K. Phthalazine-based VEGFR-2 inhibitors: Rationale, design, synthesis, in silico, ADMET profile, docking, and anticancer evaluations. Arch. Pharm. 2021, 354, 2100201. [Google Scholar] [CrossRef]
- Devi, N.; Kaur, K.; Biharee, A.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach. Anti Cancer Agents Med. Chem. 2021, 21, 1802–1824. [Google Scholar] [CrossRef]
- CHEBI:44499. Methyl Nonanoate. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:44499 (accessed on 7 August 2022).
- CHEBI:34429. 3-Methyl-4-Isopropylphenol. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:34429 (accessed on 7 August 2022).
- Arfaie, S.; Zarghi, A. Design, synthesis and biological evaluation of new (E)-and (Z)-1, 2, 3-triaryl-2-propen-1-ones as selective COX-2 inhibitors. Eur. J. Med. Chem. 2010, 45, 4013–4017. [Google Scholar] [CrossRef] [PubMed]
- CHEBI:89188. 2,4-Di-tert-butylphenol. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:89188 (accessed on 7 August 2022).
- Misra, D.; Ghosh, N.N.; Mandal, M.; Mandal, V.; Baildya, N.; Mandal, S. Anti-enteric efficacy and mode of action of tridecanoic acid methyl ester isolated from Monochoria hastata (L.) Solms leaf. Braz. J. Microbiol. 2022, 53, 715–726. [Google Scholar] [CrossRef] [PubMed]
- CHEBI:34698. Diethyl Phthalate. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:34698 (accessed on 7 August 2022).
- CHEBI:64503. 1-Nonadecene. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:64503 (accessed on 7 August 2022).
- Saunier, J.; Mazel, V.; Paris, C.; Yagoubi, N. Polymorphism of Irganox 1076®: Discovery of new forms and direct characterization of the polymorphs on a medical device by Raman microspectroscopy. Eur. J. Pharm. Biopharm. 2010, 75, 443–450. [Google Scholar] [CrossRef] [PubMed]
- CHEBI:77474. 9Z,12Z,15Z-Octadecatrien-1-ol. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:77474 (accessed on 7 August 2022).
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. Int. Sch. Res. Netw. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Ismail, M.; Taha, K.; Modwi, A.; Khezami, L. ZnO nanoparticles: Surface and X-ray profile analysis. J. Ovonic Res. 2018, 14, 381–393. [Google Scholar]
- Dinesh, V.; Biji, P.; Ashok, A.; Dhara, S.; Kamruddin, M.; Tyagi, A.; Raj, B. Plasmon-mediated, highly enhanced photocatalytic degradation of industrial textile dyes using hybrid ZnO@ Ag core–shell nanorods. RSC Adv. 2014, 4, 58930–58940. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Yagoub, A.E.A.; Subash-Babu, P.; Hassan, A.B.; Al-Nouri, D.M.; Mohammed, M.A.; Yahya, M.A.; Elsayim, R. Inhibition of lipid accumulation and adipokine levels in maturing adipocytes by bauhinia rufescens (lam.) stem bark extract loaded titanium oxide nanoparticles. Molecules 2021, 26, 7238. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Yu, Z.; Lin, K.; Yang, S.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Enhanced stability, structural characterization and simulated gastrointestinal digestion of coenzyme Q10 loaded ternary nanoparticles. Food Hydrocoll. 2019, 94, 333–344. [Google Scholar] [CrossRef]
- Belardi, G.; Ballirano, P.; Ferrini, M.; Lavecchia, R.; Medici, F.; Piga, L.; Scoppettuolo, A. Characterization of spent zinc–carbon and alkaline batteries by SEM-EDS, TGA/DTA and XRPD analysis. Thermochim. Acta 2011, 526, 169–177. [Google Scholar] [CrossRef]
- Guan, Z.; Ying, S.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwa, V. eco-friendly greener synthesis of nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef]
- Sorbiun, M.; Mehr, E.S.; Ramazani, A.; Malekzadeh, A.M. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochem. Res. 2018, 3, 1–16. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, H. Lavandula angustifolia L. (Lavender): An important aromatic medicinal shrub and its in vitro micro-propagation for conservation. Int. Agric. Technol. 2013, 9, 691–702. [Google Scholar]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.; Rojas, H.; Navío, J.A.; Hidalgo, M.C. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; RajeshKumar, S. A review on green synthesis of zinc oxide nanoparticles- An ecofriendly approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Abdelkader, D.H.; Negm, W.A.; Elekhnawy, E.; Eliwa, D.; Basmah, N.; Aldosari, B.N.; Alanood, S.; Almurshedi, A.S. Zinc oxide nanoparticles as potential delivery carrier: Green synthesis by Aspergillus niger endophytic fungus, characterization, and in vitro/in vivo antibacterial activity. Pharmaceuticals 2022, 15, 1057. [Google Scholar] [CrossRef]
- Lingaraju, K.; Raja Naika, H.; Manjunath, K.; Basavaraj, R.B.; Nagabhushana, H.; Nagaraju, G.; Suresh, D. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl. Nanosci. 2016, 6, 703–710. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.L.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B. Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, L.; Chang, X.; Guan, D. Biosynthesis and characterization of zinc oxide nanoparticles from Artemisia annuna and investigate their effect on proliferation, osteogenic differentiation of zinc oxide nanoparticles in human osteoblast-like MG-63 cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111652. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Markham, M.C.; Hannan, M.C.; Paternostro, R.M.; Rose, C.B. Oxidation of alcohols catalyzed by zinc oxide and light. J. Am. Chem. Soc. 1958, 80, 5394–5397. [Google Scholar] [CrossRef]
- Baek, Y.-W.; An, Y.-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef]
- Moose, P.J.; Chung, K.; Woessner, D.; Honeggar, M.; Cutler, N.S.; Veranth, J.M. ZnO particulate matter requires cell contact for toxicity in human colon cancer cells. Chem. Res. Toxicol. 2010, 23, 733–739. [Google Scholar] [CrossRef]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Azizi, S.; Tahir, P.M.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. Evid.-Based Complement. Altern. Med. 2015, 2015, 593014. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Majhi, S.; Saha, B.P.; Mukherjee, P.K. Chlorogenic acid–phospholipid complex improve protection against UVA induced oxidative stress. J. Photochem. Photobiol. B Biol. 2014, 130, 293–298. [Google Scholar] [CrossRef]
- Leung, Y.; Chan, C.; Ng, A.; Chan, H.; Chiang, M.; Djurišić, A.; Ng, Y.; Jim, W.; Guo, M.; Leung, F. Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology 2012, 23, 475703. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.; El-Baz, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger, M.-A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. Zno nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of Zno nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mat. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Rokbani, H.; Daigle, F.; Ajji, A. Combined effect of ultrasound stimulations and autoclaving on the enhancement of antibacterial activity of ZnO and SiO2/ZnO nanoparticles. Nanomaterials 2018, 8, 129. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of Zno nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microb. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- d’Água, R.B.; Branquinho, R.; Duarte, M.P.; Maurício, E.; Fernando, A.L.; Martins, R.; Fortunato, E. Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J. Chem. 2018, 42, 1052–1060. [Google Scholar]
- Labrie, S.; Frois-Moniz, K.; Osburne, M.; Kelly, L.; Roggensack, S.; Sullivan, M.; Gearin, G.; Zeng, Q.; Fitzgerald, M.; Henn, M. Genomes of marine cyanopodoviruses reveal multiple origins of diversity. Environ. Microbiol. 2013, 15, 1356–1376. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological effects and mode of action of ZnO nanoparticles against postharvest fungal contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Rasha, E.; Monerah, A.; Manal, A.; Rehab, A.; Mohammed, D.; Doaa, E. Biosynthesis of Zinc Oxide Nanoparticles from Acacia nilotica (L.) Extract to Overcome Carbapenem-Resistant Klebsiella Pneumoniae. Molecules 2021, 26, 1919. [Google Scholar] [CrossRef]
- Eskandari, M.; Haghighi, N.; Ahmadi, V.; Haghighi, F.; Mohammadi, S.R. Growth and investigation of antifungal properties of ZnO nanorod arrays on the glass. Phys. B Condens. Matter 2011, 406, 112–114. [Google Scholar] [CrossRef]


| No. | RT (min) | Peak Area (%) | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Compound Nature | Bioactivity |
|---|---|---|---|---|---|---|---|
| 1 | 7.16 | 0.24 | 2-[2-Amino-4-methoxyphenyl]benzimidazole | C9H11O2 | 165.19 | Cyclic aliphatic ketone | Benzimidazole derivatives are anti-inflammatory and anthelmintic agents [38]. |
| 2 | 8.26 | 21.51 | Indole, 3-(4-nitrophenylamino)- | C14H11N3O2 | 253.26 | Heterocyclic organic compound | Its derivatives have anticancer effects [40] and antibacterial effects [39]. |
| 3 | 12.86 | 0.17 | Nonanoic acid, methyl ester (Methyl pelarigonate) | C10H20O2 | 172.26 | Fatty acid ester | An epitope, antifungal agent, antinematodal drug, and plant metabolite [41]. |
| 4 | 14.611 | 1.76 | 3-Methyl-4-isopropylphenol | C10H14O | 150.22 | Alkylbenzene | It has a synergistic antimicrobial activity against Streptococcus mutans with protamine peptide [42]. |
| 5 | 16.362 | 0.36 | Propenone, 3-(2-benzoxazolylthio)-1-phenyl- | C16H11NO2S | 381.30 | Ketone | Synthetic propen-1-one derivatives possess COX-2 inhibitory activity [43]. |
| 6 | 19.103 | 3.06 | 2,4-Di-tert-butylphenol | C14H22O | 206.32 | Alkylbenzene phenol | A toxic substance and an antioxidant agent [44] |
| 7 | 19.463 | 0.10 | Tridecanoic acid, methyl ester | C14H28O2 | 228.37 | Fatty acid ester | It has antienteric activity against bacteria [45]. |
| 8 | 20.751 | 0.18 | Diethyl Phthalate | C12H14O4 | 222.24 | Phthalate ester | It is a neurotoxin, teratogenic agent, endocrine disrupter, and harmful to the environment [46]. |
| 9 | 24.59 | 4.60 | 1-Nonadecene | C19H38 | 266.5 | Alkene | A bacterial and plant metabolite [47]. |
| 10 | 26.89 | 0.99 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester (Methyl Di-ter-butyl hydroxyhydrocinnamate) | C18H28O3 | 292.4 | Phenolic acid ester | Its derivatives have antioxidant activities [48]. |
| 11 | 29.60 | 1.50 | 9Z,12Z,15Z-Octadecatrien-1-ol | C18H32O | 264.4 | Fatty alcohol | An antibacterial agent [49]. |
| Microorganism | Zone of Inhibition (Diameter (mm)) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPME | ZnO | 50 mg LP–ZnO NPs | 100 mg LP–ZnO NPs | ||||||||||
| Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Estimated Marginal Mean (n = 24) | |
| Staphylococcus aureus | 8.00 ± 0.06 | 4.00 ± 1.00 | 6.00 ± 1.30 | 11.00 ± 0.50 | 12.00 ± 1.00 | 11.50 ± 0.89 | 14.00 ± 0.5 | 12.00 ± 1.00 | 13.00 ± 1.30 | 11.00 ± 0.5 | 12.00 ± 0.50 | 11.00 ± 0.71 | 10.50 ± 3.04 d |
| Pseudomonas aeruginosa | R | 4.00 ± 0.50 | 2.00 ± 0.44 | R | 10.00 ± 1.00 | 5.00 ± 11.52 | 12.00 ± 0.50 | 11.00 ± 0.50 | 11.50 ± 0.71 | R | 12.00 ± 1.00 | 6.00 ± 3.13 | 6.13 ± 4.43 c |
| Aspergillus terreus | R | R | R | 18.00 ± 0.50 | 19.00 ± 0.500 | 18.50 ± 0.71 | 14.00 ± 1.00 | 19.00 ± 1.00 | 16.50 ± 2.88 | 8.00 ± 1.00 | 24.00 ± 1.00 a | 16.00 ± 8.81 | 12.50 ± 7.62 a |
| Aspergillus niger | R | R | R | 19.00 ± 1.00 | 14.00 ± 1.00 | 16.50 ± 2.88 | 7.00 ± 1.00 | 19.00 ± 1.00 | 13.00 ± 6.63 | 9.00 ± 0.50 | 24.00 ± 0.50 | 16.50 ± 8.23 | 11.50 ± 8.73 b |
| Total mean (n = 12) | 2.00.00 ± 0.30 | 2.00 ± 0.21 | 12.00 ± 5.53 | 13.75 ± 3.58 | 11.75 ± 3.06 | 15.25 ± 4.01 | 7.00 ± 4.40 | 18.00 ± 6.30 | |||||
| Estimated marginal mean (n = 24) | 2.00 ± 0.75c | 12.88 ± 6.53 b | 13.50 ± 3.92 a | 12.50 ± 6.20 b | |||||||||
| Grand total mean (n = 96) | 10.22 ± 4.24 (SE = 0.072) | ||||||||||||
| Microorganism | MIC (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPME | ZnO | 50 mg LP–ZnO NPs | 100 mg LP–ZnO NPs | ||||||||||
| Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Estimated Marginal Mean (n = 24) | |
| Staphylococcus aureus | 0.90 ± 0.06 | 7.50 ± 0.80 | 4.20 ± 2.65 | 3.80 ± 0.35 | 1.80 ± 0.15 | 2.80 ± 1.12 | 0.20 ± 0.05 | 0.13 ± 0.03 | 0.17 ± 0.05 | 0.20 ± 0.04 | 0.2 ± 0.00 | 0.20 ± 0.03 | 1.84 ± 1.51 a |
| Pseudomonas aeruginosa | R | 3.80 ± 0.30 | 17.40 ± 3.10 | R | 3.80 ± 0.20 | 17.40 ± 1.08 | R | 3.80 ± 0.20 | 17.40 ± 2.09 | R | 0.2 ± 0.00 | 15.60 ± 0.75 | 16.95 ± 0.80 d |
| Aspergillus terreus | R | R | R | R | 15.00 ± 1.00 | 23.00 ± 3.88 | 1.90 ± 0.10 | 15.00 ± 1.30 | 8.45 ± 1.22 | 1.90 ± 0.22 | 0.2 ± 0.00 | 1.05 ± 0.94 | 15.87 ± 1.15 c |
| Aspergillus niger | R | R | R | R | 15.00 ± 2.00 | 23.00 ± 2.85 | 1.90 ± 0.20 | 3.80 ± 0.4 | 2.85 ± 1.07 | 1.90 ± 0.04 | 0.2 ± 0.00 | 1.05 ± 0.93 | 14.48 ± 1.78 b |
| Total mean (n = 12) | 23.48 ± 1.16 | 18.33 ± 2.61 | 24.20 ± 2.60 | 8.90 ± 3.50 | 8.75 ± 0.64 | 5.68 ± 3.20 | 8.75 ± 1.54 | 0.2 ± 0.00 | |||||
| Estimated marginal mean (n = 24) | 20.90 ± 1.43 d | 16.55 ± 2.09 c | 7.22 ± 2.01 b | 4.48 ± 1.17 a | |||||||||
| Grand mean (n = 96) | 12.29 ± 3.05 (SE = 0.051) | ||||||||||||
| Microorganism | MBC (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPME | ZnO | 50 mg LP–ZnO NPs | 100 mg LP–ZnO NPs | ||||||||||
| Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Water | DMSO | Total Mean (n = 6) | Estimated Marginal Mean (n = 24) | |
| Staphylococcus aureus | 0.50 ± 0.10 | 15.00 ± 1.00 | 7.75 ± 1.90 | 15.00 ± 1.30 | 7.50 ± 0.50 | 11.25 ± 3.42 | 0.50 ± 0.10 | 0.90 ± 0.30 | 0.70 ± 0.30 | 0.90 ± 020 | 1.90 ± 0.26 | 1.40 ± 0.59 | 5.28 ± 4.17 a |
| Pseudomonas aeruginosa | R | 3.80 ± 0.10 | 17.40 ± 1.62 | R | 15.00 ± 0.50 | 23.00 ± 2.77 | R | 7.50 ± 1.90 | 19.25 ± 1.93 | R | 7.50 ± 0.90 | 19.25 ± 2.73 | 19.73 ± 2.30 b |
| Aspergillus terreus | R | R | R | R | 30.00 ± 2.00 | 30.50 ± 1.78 | 3.80 ± 0.00 | 29.67 ± 0.58 | 16.73 ± 4.48 | 3.80 ± 0.20 | 30.00 ± 1.00 | 16.90 ± 4.66 | 23.78 ± 7.15 c |
| Aspergillus niger | R | R | R | R | 0.00 ± 0.00 | 31.00 ± 0.00 | 3.80 ± 0.07 | 7.50 ± 0.50 | 5.65 ± 2.05 | 3.80 ± 0.30 | 7.50 ± 0.30 | 5.65 ± 2.04 | 18.33 ± 1.02 b |
| Total mean (n = 12) | 23.38 ± 3.08 | 20.20 ± 4.31 | 27.00 ± 4.86 | 20.88 ± 4.51 | 9.78 ± 0.88 | 11.39 ± 11.62 | 9.88 ± 1.70 | 11.73 ± 5.29 | |||||
| Estimated marginal mean (n = 24) | 21.79 ± 4.62 c | 23.94 ± 4.35 b | 10.59 ± 2.03 a | 10.81 ± 1.96 a | |||||||||
| Grand mean (n = 96) | 16.78 ± 3.09 (SE = 0.067) | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagoub, A.E.A.; Al-Shammari, G.M.; Al-Harbi, L.N.; Subash-Babu, P.; Elsayim, R.; Mohammed, M.A.; Yahya, M.A.; Fattiny, S.Z.A. Antimicrobial Properties of Zinc Oxide Nanoparticles Synthesized from Lavandula pubescens Shoot Methanol Extract. Appl. Sci. 2022, 12, 11613. https://doi.org/10.3390/app122211613
Yagoub AEA, Al-Shammari GM, Al-Harbi LN, Subash-Babu P, Elsayim R, Mohammed MA, Yahya MA, Fattiny SZA. Antimicrobial Properties of Zinc Oxide Nanoparticles Synthesized from Lavandula pubescens Shoot Methanol Extract. Applied Sciences. 2022; 12(22):11613. https://doi.org/10.3390/app122211613
Chicago/Turabian StyleYagoub, Abu ElGasim A., Ghedeir M. Al-Shammari, Laila Naif Al-Harbi, Pandurangan Subash-Babu, Rasha Elsayim, Mohammed A. Mohammed, Mohammed Abdo Yahya, and Sndos Z. A. Fattiny. 2022. "Antimicrobial Properties of Zinc Oxide Nanoparticles Synthesized from Lavandula pubescens Shoot Methanol Extract" Applied Sciences 12, no. 22: 11613. https://doi.org/10.3390/app122211613
APA StyleYagoub, A. E. A., Al-Shammari, G. M., Al-Harbi, L. N., Subash-Babu, P., Elsayim, R., Mohammed, M. A., Yahya, M. A., & Fattiny, S. Z. A. (2022). Antimicrobial Properties of Zinc Oxide Nanoparticles Synthesized from Lavandula pubescens Shoot Methanol Extract. Applied Sciences, 12(22), 11613. https://doi.org/10.3390/app122211613







